Impact of mRNA and Inactivated COVID-19 Vaccines on Ovarian Reserve
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Declaration
2.2. Experimental Animals and Care
2.3. Vaccination Procedure
2.4. Tissue Collection
2.5. Histological Procedure
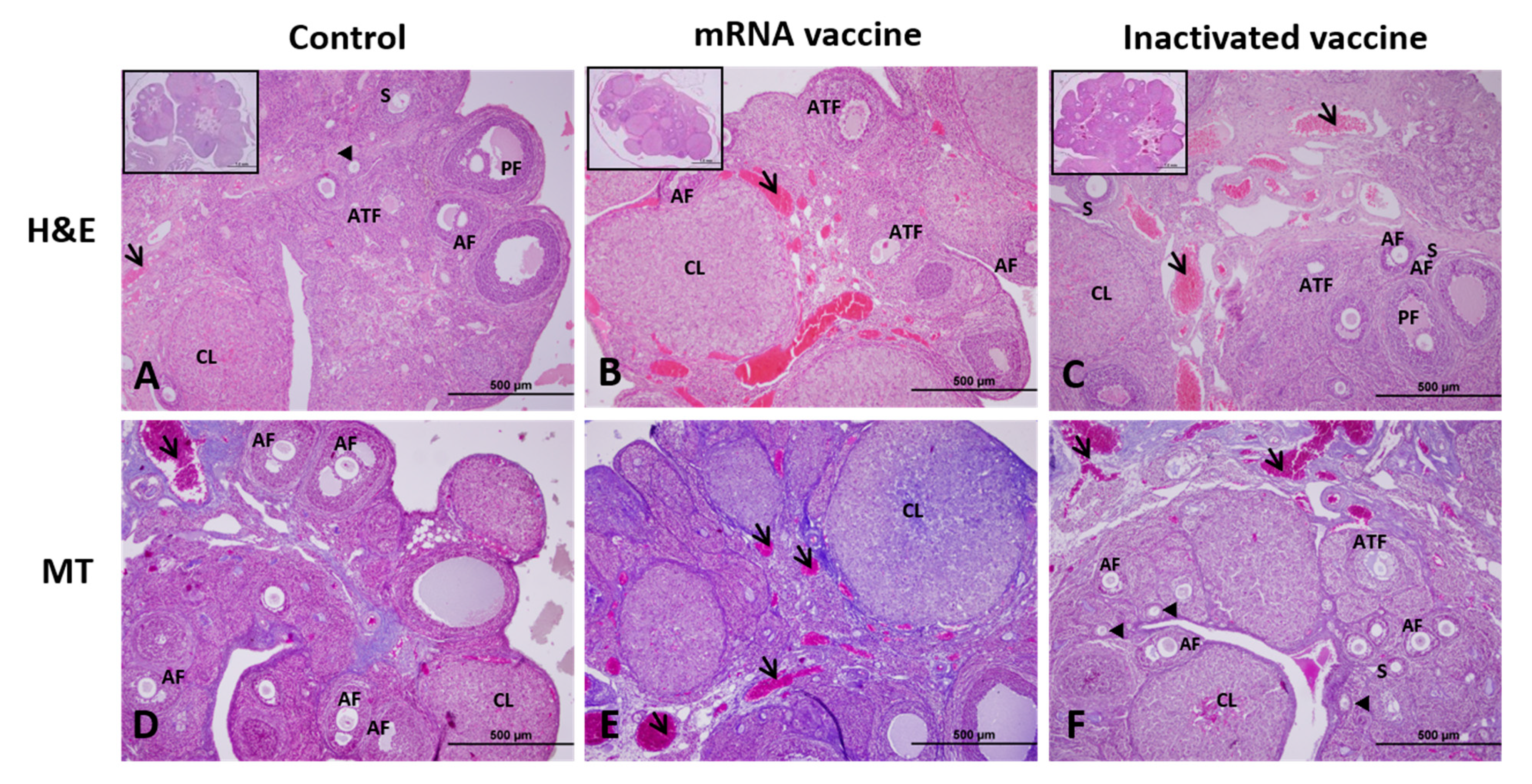
2.6. Histological Analysis and Follicle Classification
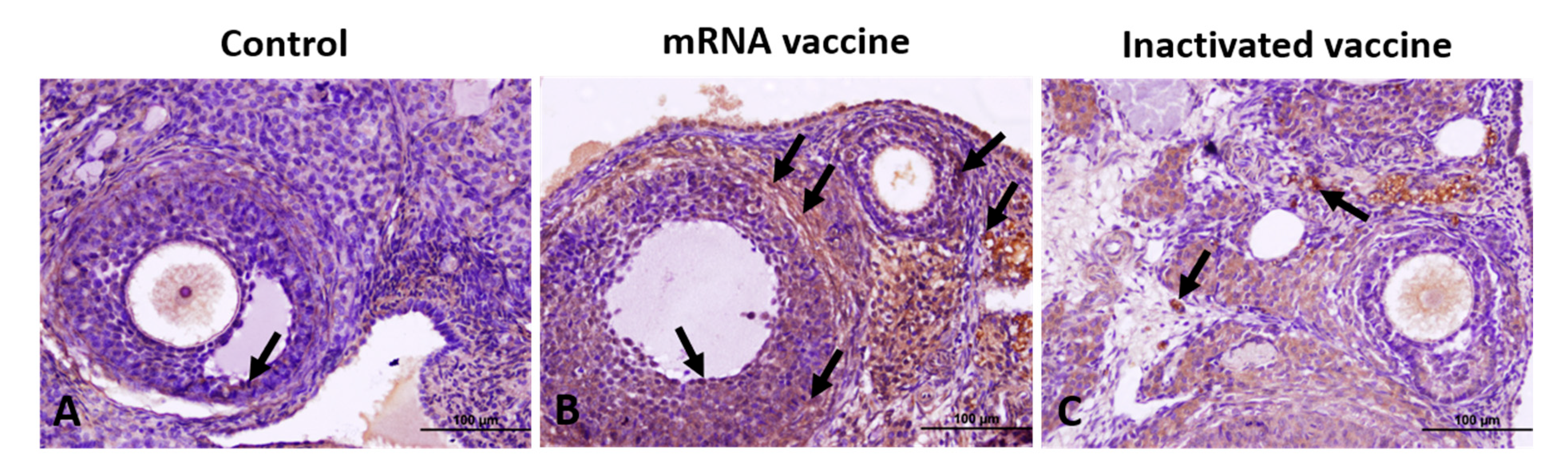
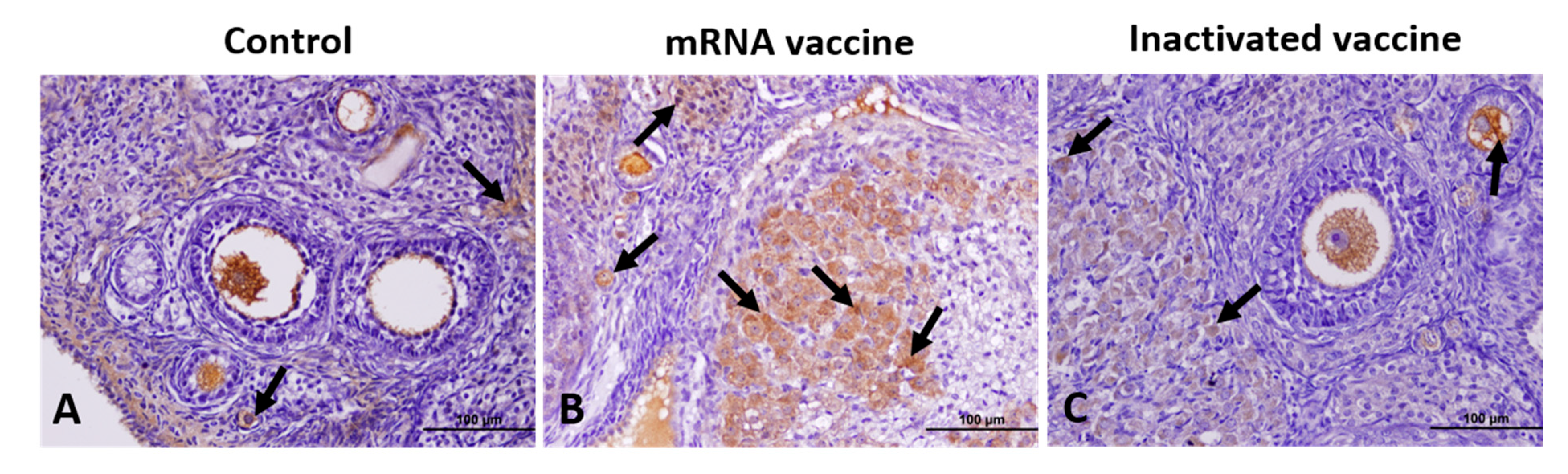
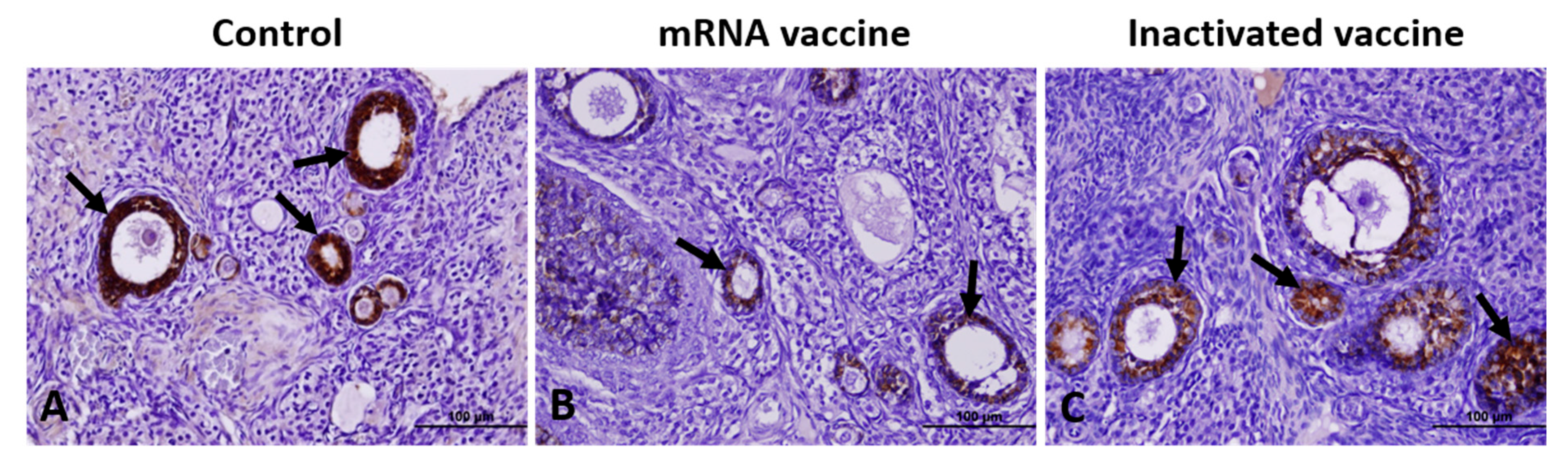
2.7. Immunohistochemical Analysis
2.8. Measurement of Serum AMH Levels
2.9. Statistical Analysis
3. Results
3.1. TGF-β1, VEGF, Caspase-3, and AMH Expression
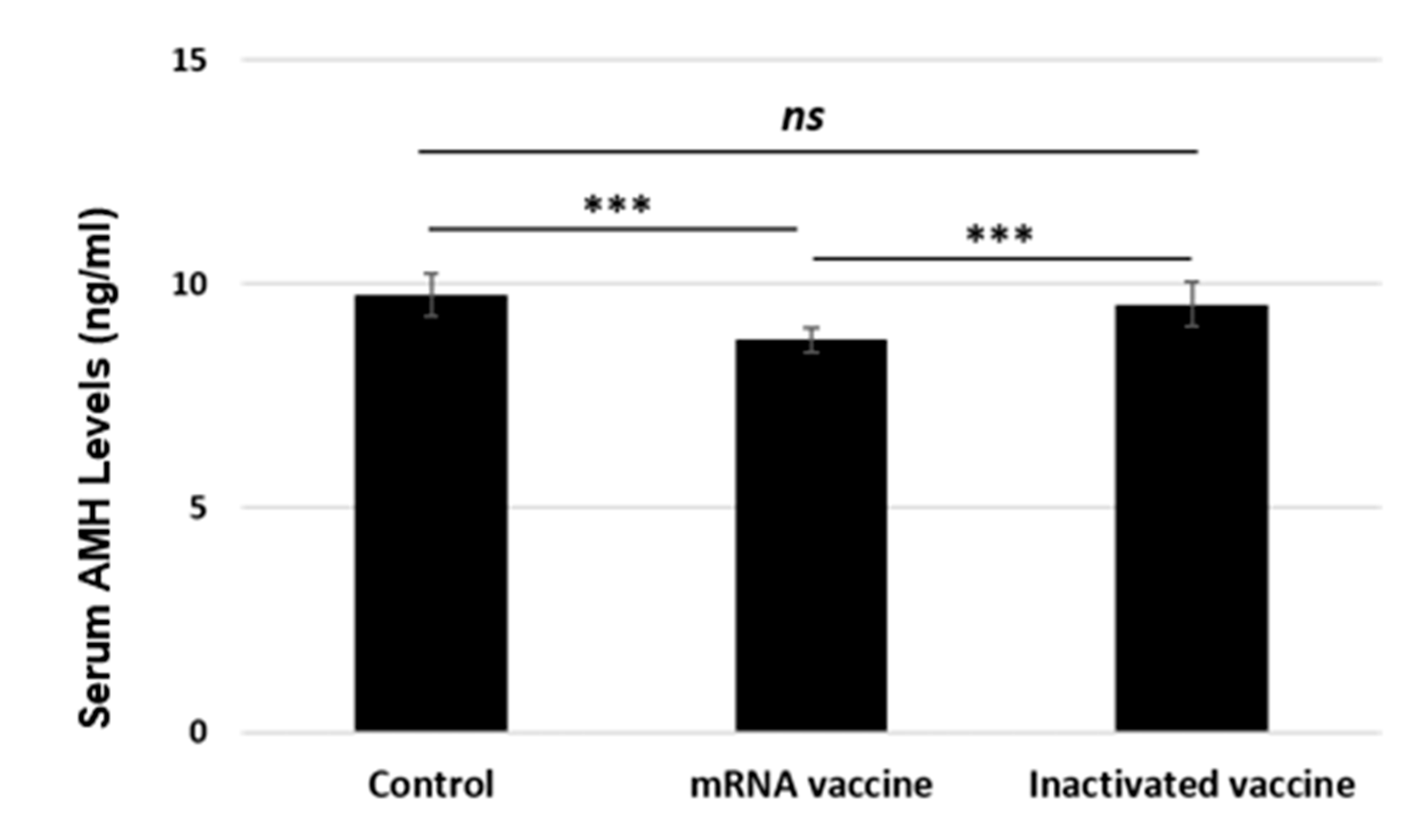
3.2. Serum AMH Levels
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. COVID-19 Dashboard. COVID-19 Cases, World. Available online: https://data.who.int/dashboards/covid19/cases (accessed on 8 September 2024).
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [PubMed]
- WHO. COVID-19 Vaccine Tracker. Available online: https://covid19.trackvaccines.org/agency/who/ (accessed on 8 September 2024).
- Li, S.; Liu, H.; Li, D.; Chen, F. Female reproductive health during the COVID-19 pandemic: Latest evidence and understanding. Arch. Gynecol. Obstet. 2023, 308, 1691–1696. [Google Scholar] [PubMed]
- GOV.UK. Coronavirus Vaccine—Summary of Yellow Card Reporting. Available online: https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting (accessed on 8 September 2024).
- Muhaidat, N.; Alshrouf, M.A.; Azzam, M.I.; Karam, A.M.; Al-Nazer, M.; Al-Ani, A. Menstrual Symptoms After COVID-19 Vaccine: A Cross-Sectional Investigation in the MENA Region. Int. J. Womens Health 2022, 14, 395–404. [Google Scholar]
- Saçıntı, K.G.; Oruç, G.; Şükür, Y.E.; Koç, A. COVID-19 vaccine has no impact on the menstrual cycle. J. Obstet. Gynaecol. 2022, 42, 3403–3404. [Google Scholar] [PubMed]
- Odeh-Natour, R.; Shapira, M.; Estrada, D.; Freimann, S.; Tal, Y.; Atzmon, Y.; Bilgory, A.; Aslih, N.; Abu-Raya, Y.S.; Shalom-Paz, E. Does mRNA SARS-CoV-2 vaccine in the follicular fluid impact follicle and oocyte performance in IVF treatments? Am. J. Reprod. Immunol. 2022, 87, e13530. [Google Scholar]
- Bentov, Y.; Beharier, O.; Moav-Zafrir, A.; Kabessa, M.; Godin, M.; Greenfield, C.S.; Ketzinel-Gilad, M.; Ash Broder, E.; Holzer, H.E.G.; Wolf, D.; et al. Ovarian follicular function is not altered by SARS-CoV-2 infection or BNT162b2 mRNA COVID-19 vaccination. Hum. Reprod. 2021, 36, 2506–2513. [Google Scholar]
- Requena, A.; Vergara, V.; González-Ravina, C.; Ruiz, M.E.; Cruz, M. The type of SARS-CoV-2 vaccine does not affect ovarian function in assisted reproduction cycle. Fertil. Steril. 2023, 119, 618–623. [Google Scholar]
- Yang, L.; Neal, S.; Lee, T.; Chou, A.; Schutt, A.K.; Gibbons, W. Comparison of Female Ovarian Reserve Before vs. After COVID-19 Vaccination. JAMA Netw. Open 2023, 6, e2318804. [Google Scholar]
- Cruz, G.; Fernandois, D.; Paredes, A.H. Ovarian function and reproductive senescence in the rat: Role of ovarian sympathetic innervation. Reproduction 2017, 153, R59–R68. [Google Scholar]
- Gougeon, A. Regulation of ovarian follicular development in primates: Facts and hypotheses. Endocr. Rev. 1996, 17, 121–155. [Google Scholar]
- Li, L.; Shi, X.; Shi, Y.; Wang, Z. The signaling pathways involved in ovarian follicle development. Front. Physiol. 2021, 12, 730196. [Google Scholar]
- Kerr, J.B.; Myers, M.; Anderson, R.A. The dynamics of the primordial follicle reserve. Reproduction 2013, 146, R205–R215. [Google Scholar]
- Matsuda, F.; Inoue, N.; Manabe, N.; Ohkura, S. Follicular growth and atresia in mammalian ovaries: Regulation by survival and death of granulosa cells. J. Reprod. Dev. 2012, 58, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Nagaraju, G.; Liu, Z.; Liu, K. Functional roles of the phosphatidylinositol 3-kinases (PI3Ks) signaling in the mammalian ovary. Mol. Cell. Endocrinol. 2012, 356, 24–30. [Google Scholar] [CrossRef]
- McGee, E.A.; Hsueh, A.J. Initial and cyclic recruitment of ovarian follicles. Endocr. Rev. 2000, 21, 200–214. [Google Scholar]
- Robinson, R.S.; Woad, K.J.; Hammond, A.J.; Laird, M.; Hunter, M.G.; Mann, G.E. Angiogenesis and vascular function in the ovary. Reproduction 2009, 138, 869–881. [Google Scholar] [CrossRef]
- Yang, M.Y.; Fortune, J.E. Vascular endothelial growth factor stimulates the primary to secondary follicle transition in bovine follicles in vitro. Mol. Reprod. Dev. 2007, 74, 1095–1104. [Google Scholar]
- Patton, B.K.; Madadi, S.; Pangas, S.A. Control of ovarian follicle development by TGF-β family signaling. Curr. Opin. Endocr. Metab. Res. 2021, 18, 102–110. [Google Scholar] [PubMed]
- Durlinger, A.L.; Gruijters, M.J.; Kramer, P.; Karels, B.; Ingraham, H.A.; Nachtigal, M.W.; Uilenbroek, J.T.; Grootegoed, J.A.; Themmen, A.P. Anti-Müllerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology 2002, 143, 1076–1084. [Google Scholar] [CrossRef]
- Durlinger, A.L.; Gruijters, M.J.; Kramer, P.; Karels, B.; Kumar, T.R.; Matzuk, M.M.; Rose, U.M.; de Jong, F.H.; Uilenbroek, J.T.J.; Grootegoed, J.A.; et al. Anti-Mullerian hormone attenuates the effects of FSH on follicle development in the mouse ovary. Endocrinology 2001, 142, 4891–4899. [Google Scholar] [CrossRef]
- Wang, Z.P.; Mu, X.Y.; Guo, M.; Wang, Y.J.; Teng, Z.; Mao, G.P.; Niu, W.B.; Feng, L.Z.; Zhao, L.H.; Xia, G.L. Transforming growth factor-β signaling participates in the maintenance of the primordial follicle pool in the mouse ovary. J. Biol. Chem. 2014, 289, 8299–8311. [Google Scholar] [CrossRef]
- Boone, D.L.; Tsang, B.K. Caspase-3 in the rat ovary: Localization and possible role in follicular atresia and luteal regression. Biol. Reprod. 1998, 58, 1533–1539. [Google Scholar]
- Ding, T.; Wang, T.; Zhang, J.; Cui, P.; Chen, Z.; Zhou, S.; Yuan, S.; Ma, W.; Zhang, M.; Rong, Y.; et al. Analysis of ovarian injury associated with COVID-19 disease in reproductive-aged women in Wuhan, China: An observational study. Front. Med. 2021, 8, 635255. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Fang, Z.; Liu, Y.; Xing, C.; Huang, L.; Mao, J.; Chen, H.; Huang, Z.; Xia, L.; Tang, L.; et al. Effect of female coronavirus disease 2019 vaccination on assisted reproductive outcomes: A systematic review and meta-analysis. Fertil. Steril. 2023, 119, 772–783. [Google Scholar]
- Food and Drug Administration (FDA). Guidance for Industry-Considerations for Developmental Toxicity Studies for Preventive and Therapeutic Vaccines for Infectious Disease Indications. 2006. Available online: https://www.fda.gov/media/73986/download (accessed on 3 March 2024).
- World Health Organization (WHO). Guidelines on the Non-Clinical Evaluation of Vaccine Adjuvants and Adjuvanted Vaccines, WHO Technical Report Series, TRS 987, Annex 2. 2014. Available online: https://cdn.who.int/media/docs/default-source/biologicals/vaccine-standardization/trs_987_annex2.pdf?sfvrsn=ea91caca_3&download=true (accessed on 3 March 2024).
- European Medicines Agency (EMA). ICH Guideline S5 (R3) on Reproductive Toxicology: Detection of Toxicity to Reproduction for Human Pharmaceuticals. 2020. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-s5-r3-detection-toxicity-reproduction-human-pharmaceuticals-step-5_en.pdf (accessed on 3 March 2024).
- Karaman, E.; Onder, G.O.; Goktepe, O.; Karakas, E.; Mat, O.C.; Bolat, D.; Koseoglu, E.; Tur, K.; Baran, M.; Ermis, M.; et al. Protective effects of boric acid taken in different ways on experimental ovarian ischemia and reperfusion. Biol. Trace Elem. Res. 2024, 202, 2730–2743. [Google Scholar] [PubMed]
- Myers, M.; Britt, K.L.; Wreford, N.G.M.; Ebling, F.J.; Kerr, J.B. Methods for quantifying follicular numbers within the mouse ovary. Reproduction 2004, 127, 569–580. [Google Scholar] [PubMed]
- Karaman, E.; Yavuz, A.; Ayan, D.; Bayram, E.; Yay, F.; Sakallı, E.; Temur, I.; Sag, I.; Yesim, Y.; Ayag, M.E.; et al. Hormonal and histological effects of the inactivated SARS-CoV-2 (TURKOVAC™) vaccine on ovarian reserve. Flora 2024, 29, 516–525. [Google Scholar]
- Pangas, S.A.; Matzuk, M.M. Genetic models for transforming growth factor β superfamily signaling in ovarian follicle development. Mol. Cell. Endocrinol. 2004, 225, 83–91. [Google Scholar]
- Harlow, C.R.; Davidson, L.; Burns, K.H.; Yan, C.; Matzuk, M.M.; Hillier, S.G. FSH and TGF-β superfamily members regulate granulosa cell connective tissue growth factor gene expression in vitro and in vivo. Endocrinology 2002, 143, 3316–3325. [Google Scholar]
- Wang, E.Y.; Chen, H.; Sun, B.Q.; Wang, H.; Qu, H.Q.; Liu, Y.; Sun, X.Z.; Qu, J.; Fang, Z.F.; Tian, L.; et al. Serum levels of the IgA isotype switch factor TGF-β1 are elevated in patients with COVID-19. FEBS Lett. 2021, 595, 1819–1824. [Google Scholar]
- El-Derany, M.O.; Said, R.S.; El-Demerdash, E. Bone marrow-derived mesenchymal stem cells reverse radiotherapy-induced premature ovarian failure: Emphasis on signal integration of TGF-β, Wnt/β-catenin and hippo pathways. Stem Cell Rev. Rep. 2021, 17, 1429–1445. [Google Scholar]
- Nakayama, T. An inflammatory response is essential for the development of adaptive immunity—Immunogenicity and immunotoxicity. Vaccine 2016, 34, 5815–5818. [Google Scholar] [PubMed]
- Cossigny, D.A.F. The Role of TGF-β Superfamily Members: TGF-β1 and Activin A in Early Folliculogenesis. Ph.D. Thesis, Monash University, Melbourne, Australia, 2017. [Google Scholar] [CrossRef]
- Guzmán, A.; Hernández-Coronado, C.G.; Gutiérrez, C.G.; Rosales-Torres, A.M. The vascular endothelial growth factor (VEGF) system as a key regulator of ovarian follicle angiogenesis and growth. Mol. Reprod. Dev. 2023, 90, 201–217. [Google Scholar]
- Irusta, G.; Abramovich, D.; Parborell, F.; Tesone, M. Direct survival role of vascular endothelial growth factor (VEGF) on rat ovarian follicular cells. Mol. Cell. Endocrinol. 2010, 325, 93–100. [Google Scholar]
- Abramovich, D.; Celin, A.R.; Hernandez, F.; Tesone, M.; Parborell, F. Spatiotemporal analysis of the protein expression of angiogenic factors and their related receptors during folliculogenesis in rats with and without hormonal treatment. Reproduction 2009, 137, 309–320. [Google Scholar]
- Zhang, X.; Niu, Y.; Huang, Y. Melatonin inhibits cell proliferation in a rat model of breast hyperplasia by mediating the PTEN/AKT pathway. Oncol. Rep. 2021, 45, 66. [Google Scholar] [PubMed]
- Al-Shahat, A.; Hulail, M.A.E.; Soliman, N.M.M.; Khamis, T.; Fericean, L.M.; Arisha, A.H.; Moawad, R.S. Melatonin mitigates cisplatin-induced ovarian dysfunction via altering steroidogenesis, inflammation, apoptosis, oxidative stress, and PTEN/PI3K/Akt/mTOR/AMPK signaling pathway in female rats. Pharmaceutics 2022, 14, 2769. [Google Scholar] [CrossRef]
- Caglayan, C.; Kandemir, F.M.; Yıldırım, S.; Kucukler, S.; Kılınc, M.A.; Saglam, Y.S. Zingerone ameliorates cisplatin-induced ovarian and uterine toxicity via suppression of sex hormone imbalances, oxidative stress, inflammation and apoptosis in female Wistar rats. Biomed. Pharmacother. 2018, 102, 517–530. [Google Scholar]
- Penzias, A.; Azziz, R.; Bendikson, K.; Falcone, T.; Hansen, K.; Hill, M.; Hurd, W.; Jindal, S.; Kalra, S.; Mersereau, J.; et al. Testing and interpreting measures of ovarian reserve: A committee opinion. Fertil. Steril. 2020, 114, 1151–1157. [Google Scholar]
- Visser, J.A.; Themmen, A.P. Anti-Müllerian hormone and folliculogenesis. Mol. Cell. Endocrinol. 2005, 234, 81–86. [Google Scholar]
- Gruijters, M.J.; Visser, J.A.; Durlinger, A.L.; Themmen, A.P. Anti-Müllerian hormone and its role in ovarian function. Mol. Cell. Endocrinol. 2003, 211, 85–90. [Google Scholar] [PubMed]
- Kuyucu, Y.; Tap, Ö. The effects of the anti-Müllerian hormone on folliculogenesis in rats: Light and electron microscopic evaluation. Ultrastruct. Pathol. 2021, 45, 59–70. [Google Scholar]
- Durlinger, A.; Visser, J.; Themmen, A. Regulation of ovarian function: The role of anti-Müllerian hormone. Reproduction 2002, 124, 601–609. [Google Scholar]
- Durlinger, A.L.; Kramer, P.; Karels, B.; de Jong, F.H.; Uilenbroek, J.T.; Grootegoed, J.A.; Themmen, A.P. Control of primordial follicle recruitment by anti-Müllerian hormone in the mouse ovary. Endocrinology 1999, 140, 5789–5796. [Google Scholar] [PubMed]
- Visser, J.A.; Durlinger, A.L.; Peters, I.J.; van den Heuvel, E.R.; Rose, U.M.; Kramer, P.; de Jong, F.H.; Themmen, A.P. Increased oocyte degeneration and follicular atresia during the estrous cycle in anti-Müllerian hormone null mice. Endocrinology 2007, 148, 2301–2308. [Google Scholar] [PubMed]
- Orvieto, R.; Noach-Hirsh, M.; Segev-Zahav, A.; Haas, J.; Nahum, R.; Aizer, A. Does mRNA SARS-CoV-2 vaccine influence patients’ performance during IVF-ET cycle? Reprod. Biol. Endocrinol. 2021, 19, 69. [Google Scholar]
- Aharon, D.; Lederman, M.; Ghofranian, A.; Hernandez-Nieto, C.; Canon, C.; Hanley, W.; Gounko, D.; Lee, J.A.; Stein, D.; Buyuk, E.; et al. In vitro fertilization and early pregnancy outcomes after coronavirus disease 2019 (COVID-19) vaccination. Obstet. Gynecol. 2022, 139, 490–497. [Google Scholar]
- Huang, J.; Xia, L.; Lin, J.; Liu, B.; Zhao, Y.; Xin, C.; Ai, X.; Cao, W.; Zhang, X.; Tian, L.; et al. No effect of inactivated SARS-CoV-2 vaccination on in vitro fertilization outcomes: A propensity score-matched study. J. Inflamm. Res. 2022, 15, 839–849. [Google Scholar]
- Avraham, S.; Kedem, A.; Zur, H.; Youngster, M.; Yaakov, O.; Yerushalmi, G.M.; Gat, I.; Gidoni, Y.; Hochberg, A.; Baum, M.; et al. Coronavirus disease 2019 vaccination and infertility treatment outcomes. Fertil. Steril. 2022, 117, 1291–1299. [Google Scholar]
- Huang, J.; Xia, L.; Tian, L.; Fan, H.; Xu, D.; Ai, X.; Wu, X.; Chen, J.; Xing, G.; Huang, L.; et al. Impact of inactivated SARS-CoV-2 vaccination on embryo ploidy: A retrospective cohort study of 133 PGT-A cycles in China. Biol. Res. 2022, 55, 26. [Google Scholar]
- Karavani, G.; Chill, H.H.; Dick, A.; Meirman, C.; Gutman-Ido, E.; Herzberg, S.; Ben-Meir, A.; Imbar, T. Pfizer SARS-CoV-2 BNT162b2 mRNA vaccination (BNT162b2) has no adverse effect on elective oocyte cryopreservation outcomes. Reprod. Biomed. Online 2022, 45, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Ghaemi, M.; Hantoushzadeh, S.; Shafiee, A.; Gargari, O.K.; Fathi, H.; Eshraghi, N.; Razavi, J.; Habibi, G.R.; Jafarabady, K. The effect of COVID-19 and COVID-19 vaccination on serum anti-Müllerian hormone: A systematic review and meta-analysis. Immun. Inflamm. Dis. 2024, 12, e1136. [Google Scholar] [CrossRef] [PubMed]
- Bahl, K.; Senn, J.J.; Yuzhakov, O.; Bulychev, A.; Brito, L.A.; Hassett, K.J.; Laska, M.E.; Smith, M.; Almarsson, Ö.; Thompson, J.; et al. Preclinical and clinical demonstration of immunogenicity by mRNA vaccines against H10N8 and H7N9 influenza viruses. Mol. Ther. 2017, 25, 1316–1327. [Google Scholar] [CrossRef]
- Vogel, A.B.; Kanevsky, I.; Che, Y.; Swanson, K.A.; Muik, A.; Vormehr, M.; Kranz, L.M.; Walzer, K.C.; Hein, S.; Güler, A.; et al. BNT162b vaccines protect rhesus macaques from SARS-CoV-2. Nature 2021, 592, 283–289. [Google Scholar] [CrossRef]
- Laczkó, D.; Hogan, M.J.; Toulmin, S.A.; Hicks, P.; Lederer, K.; Gaudette, B.T.; Castaño, D.; Amanat, F.; Muramatsu, H.; Oguin, T.H., 3rd; et al. A single immunization with nucleoside-modified mRNA vaccines elicits strong cellular and humoral immune responses against SARS-CoV-2 in mice. Immunity 2020, 53, 724–732.e7. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Huang, B.; Deng, W.; Quan, Y.; Wang, W.; Xu, W.; Zhao, Y.; Li, N.; Zhang, J.; et al. Development of an inactivated vaccine candidate, BBIBP-CorV, with potent protection against SARS-CoV-2. Cell 2020, 182, 713–721.e9. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [PubMed]
- Cagigi, A.; Loré, K. Immune responses induced by mRNA vaccination in mice, monkeys, and humans. Vaccines 2021, 9, 61. [Google Scholar] [CrossRef]
- Gao, Q.; Bao, L.; Mao, H.; Wang, L.; Xu, K.; Yang, M.; Li, Y.; Zhu, L.; Wang, N.; Lv, Z.; et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science 2020, 369, 77–81. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Cain, L.; Chatterjee, S.; Collins, T.J. In vitro folliculogenesis of rat preantral follicles. Endocrinology 1995, 136, 3369–3377. [Google Scholar] [PubMed]
- Kuo, S.W.; Ke, F.C.; Chang, G.D.; Lee, M.T.; Hwang, J.J. Potential role of follicle-stimulating hormone (FSH) and transforming growth factor (TGF-β1) in the regulation of ovarian angiogenesis. J. Cell Physiol. 2011, 226, 1608–1619. [Google Scholar] [PubMed]

| Parameter | Control Group | mRNA Vaccine Group | Inactivated Vaccine Group | p |
|---|---|---|---|---|
| Number of Follicles | ||||
| Primordial | 106.70 ± 5.33 a | 42.40 ± 4.96 b | 70.10 ± 12.04 c | 0.001 |
| Primary | 45.20 ± 4.21 a | 29.40 ± 4.57 b | 38.30 ± 5.41 c | 0.001 |
| Secondary | 39.60 ± 6.09 a | 25.30 ± 2.86 b | 32.70 ± 8.07 c | 0.001 |
| Antral | 32.80 ± 5.59 a | 26.20 ± 3.01 b | 29.40 ± 4.30 ab | 0.010 |
| Preovulatory | 18.20 ± 1.87 a | 15.00 ± 3.12 b | 16.20 ± 3.04 ab | 0.045 |
| Atretic | 13.90 ± 2.07 a | 26.40 ± 5.06 b | 17.10 ± 1.96 a | 0.001 |
| Parameter | Control Group | mRNA Vaccine Group | Inactivated Vaccine Group | p |
|---|---|---|---|---|
| Follicular AMH | ||||
| Primary | 129.09 ± 10.30 a | 100.35 ± 20.88 b | 111.43 ± 12.06 c | 0.001 |
| Secondary | 119.23 ± 10.09 a | 97.91 ± 9.71 b | 106.20 ± 13.67 c | 0.001 |
| Antral | 104.77 ± 14.00 a | 88.68 ± 10.38 b | 94.70 ± 11.31 b | 0.001 |
| TGF-β1 | 85.08 ± 7.87 a | 130.78 ± 14.58 b | 120.63 ± 10.30 c | 0.001 |
| VEGF | 91.40 ± 5.57 a | 145.15 ± 7.36 b | 113.46 ± 5.57 c | 0.001 |
| Caspase-3 | 82.78 ± 6.80 a | 138.09 ± 7.94 b | 98.85 ± 8.69 c | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karaman, E.; Yavuz, A.; Karakas, E.; Balcioglu, E.; Karaca, B.; Doganay, H.N.; Sacinti, K.G.; Yildiz, O. Impact of mRNA and Inactivated COVID-19 Vaccines on Ovarian Reserve. Vaccines 2025, 13, 345. https://doi.org/10.3390/vaccines13040345
Karaman E, Yavuz A, Karakas E, Balcioglu E, Karaca B, Doganay HN, Sacinti KG, Yildiz O. Impact of mRNA and Inactivated COVID-19 Vaccines on Ovarian Reserve. Vaccines. 2025; 13(4):345. https://doi.org/10.3390/vaccines13040345
Chicago/Turabian StyleKaraman, Enes, Adem Yavuz, Erol Karakas, Esra Balcioglu, Busra Karaca, Hande Nur Doganay, Koray Gorkem Sacinti, and Orhan Yildiz. 2025. "Impact of mRNA and Inactivated COVID-19 Vaccines on Ovarian Reserve" Vaccines 13, no. 4: 345. https://doi.org/10.3390/vaccines13040345
APA StyleKaraman, E., Yavuz, A., Karakas, E., Balcioglu, E., Karaca, B., Doganay, H. N., Sacinti, K. G., & Yildiz, O. (2025). Impact of mRNA and Inactivated COVID-19 Vaccines on Ovarian Reserve. Vaccines, 13(4), 345. https://doi.org/10.3390/vaccines13040345





