Vaccination and Platelet Biology: Unraveling the Immuno-Hemostatic Interplay
Abstract
1. Introduction
1.1. Alpha Granules
1.2. Dense Granules
1.3. Lysosomes
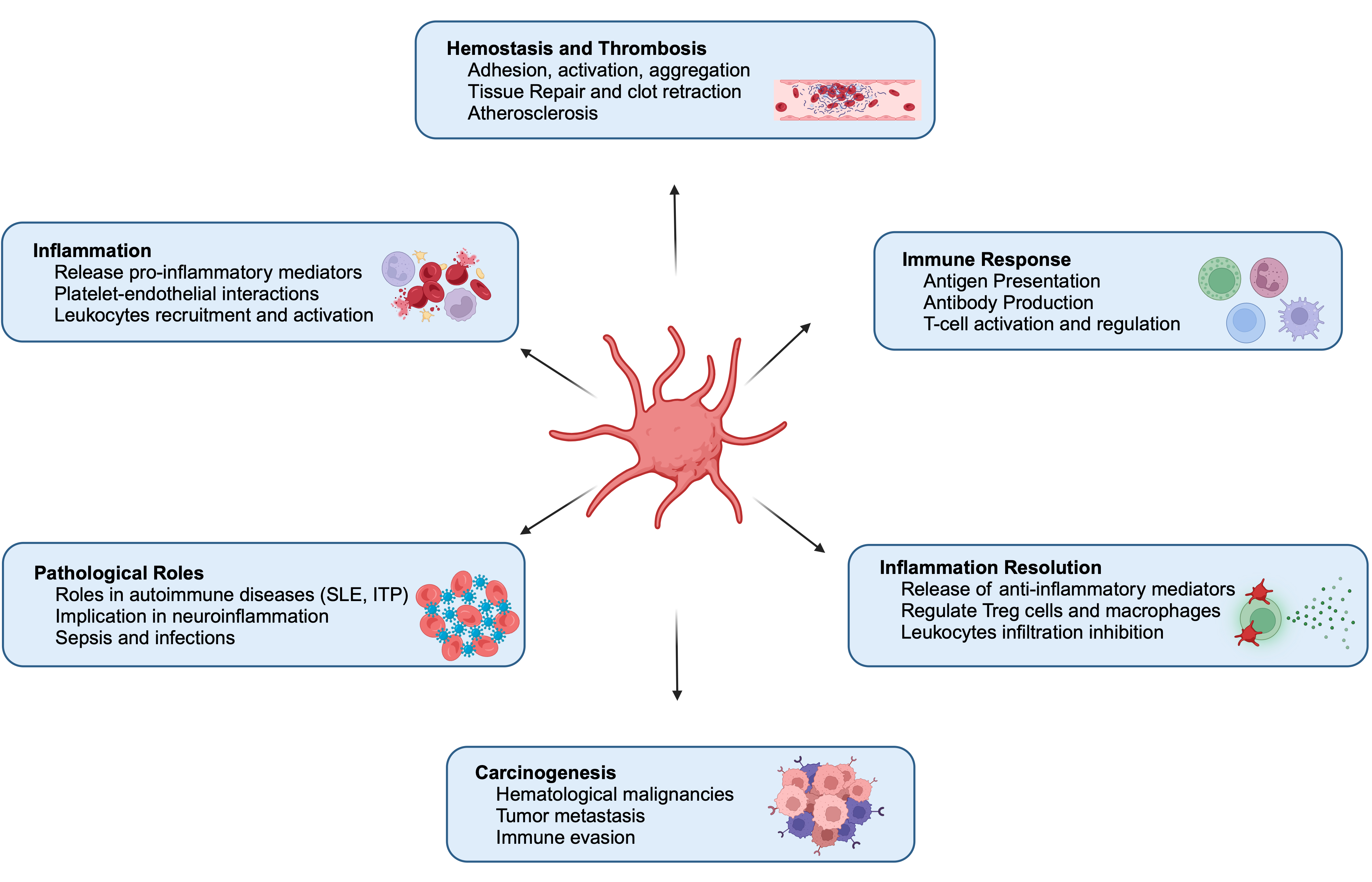
2. Multifunctional Roles of Platelets in Human Physiology
3. Synergy of Platelets and Macrophages in Immune Regulation
4. Platelets as Mediators of Innate Immunity
5. Platelets as Adaptive Immune Regulators
6. Platelets in Vaccine-Induced Immune Thrombotic Thrombocytopenia
7. Therapeutic Implications of Platelets
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 5-HIAA | 5-Hydroxy indoleacetic acid |
| ADP | Adenosine diphosphate |
| AG | Alpha Granules |
| ATP | Adenosine triphosphate |
| BLOC | Biogenesis of lysosome-related organelles complex |
| cGAS | Cyclic GMP-AMP synthase |
| CLRs | C-type lectin receptors |
| DAPT | Dual antiplatelet therapy |
| DCs | Dendritic cells |
| DG | Dense Granules |
| EGFR | Epidermal growth factor receptor |
| EPCR | Endothelial protein C receptor |
| HIT | Heparin-Induced Thrombotic Thrombocytopenia |
| IVIG | Intravenous immunoglobulin |
| MK | Megakaryocytes |
| MVB | Multivesicular bodies |
| NBEAL2 | Neurobeachin-like 2 |
| NET | Neutrophil extracellular traps |
| NLRs | NOD-like receptors |
| PAR | Protease-Activated Receptor |
| PDGF | Platelet-derived growth factor |
| PF4 | Platelet factor 4 |
| PRP | Platelet-rich plasma |
| PRR | Pattern recognition receptors |
| SLE | Systemic lupus erythematosus |
| SNAP23 | Synaptosome-associated protein 23 |
| STIM | Stromal Interaction Molecule |
| STING | Stimulator of interferon genes |
| TGF-β | Transforming growth factor-beta |
| TGN | Trans-Golgi network |
| TLRs | Toll-like receptors |
| TPO | Thrombopoietin |
| VAMP | Vesicle-associated membrane protein |
| VEGF | vascular endothelial growth factor |
| VITT | Vaccine-induced immune thrombotic thrombocytopenia |
| VPS16B | Vacuolar Protein Sorting 16B |
| VPS33B | Vacuolar Protein Sorting 33B |
| vWF | von Willebrand factor |
References
- Scherlinger, M.; Richez, C.; Tsokos, G.C.; Boilard, E.; Blanco, P. The role of platelets in immune-mediated inflammatory diseases. Nat. Rev. Immunol. 2023, 23, 495–510. [Google Scholar] [CrossRef]
- Maouia, A.; Rebetz, J.; Kapur, R.; Semple, J.W. The Immune Nature of Platelets Revisited. Transfus. Med. Rev. 2020, 34, 209–220. [Google Scholar] [CrossRef]
- Franco, A.T.; Corken, A.; Ware, J. Platelets at the interface of thrombosis, inflammation, and cancer. Blood 2015, 126, 582–588. [Google Scholar] [CrossRef]
- Machlus, K.R.; Italiano, J.E., Jr. The incredible journey: From megakaryocyte development to platelet formation. J. Cell Biol. 2013, 201, 785–796. [Google Scholar] [CrossRef]
- Bergmeier, W.; Stefanini, L. Platelet ITAM signaling. Curr. Opin. Hematol. 2013, 20, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yuan, Y.; Li, W. Sorting machineries: How platelet-dense granules differ from alpha-granules. Biosci. Rep. 2018, 38, BSR20180458. [Google Scholar] [CrossRef]
- Chen, C.H.; Lo, R.W.; Urban, D.; Pluthero, F.G.; Kahr, W.H. alpha-granule biogenesis: From disease to discovery. Platelets 2017, 28, 147–154. [Google Scholar] [CrossRef]
- Flaumenhaft, R. alpha-granules: A story in the making. Blood 2012, 120, 4908–4909. [Google Scholar] [CrossRef]
- Sharda, A.; Flaumenhaft, R. The life cycle of platelet granules. F1000Res 2018, 7, 236. [Google Scholar] [CrossRef]
- Smith, C.W. Release of alpha-granule contents during platelet activation. Platelets 2022, 33, 491–502. [Google Scholar] [CrossRef]
- Golebiewska, E.M.; Poole, A.W. Platelet secretion: From haemostasis to wound healing and beyond. Blood Rev. 2015, 29, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Etulain, J.; Martinod, K.; Wong, S.L.; Cifuni, S.M.; Schattner, M.; Wagner, D.D. P-selectin promotes neutrophil extracellular trap formation in mice. Blood 2015, 126, 242–246. [Google Scholar] [CrossRef]
- Ambrosio, A.L.; Boyle, J.A.; Di Pietro, S.M. Mechanism of platelet dense granule biogenesis: Study of cargo transport and function of Rab32 and Rab38 in a model system. Blood 2012, 120, 4072–4081. [Google Scholar] [CrossRef]
- King, S.M.; McNamee, R.A.; Houng, A.K.; Patel, R.; Brands, M.; Reed, G.L. Platelet dense-granule secretion plays a critical role in thrombosis and subsequent vascular remodeling in atherosclerotic mice. Circulation 2009, 120, 785–791. [Google Scholar] [CrossRef]
- Thon, J.N.; Italiano, J.E. Platelets: Production, morphology and ultrastructure. Handb. Exp. Pharmacol. 2012, 3–22. [Google Scholar] [CrossRef]
- Heijnen, H.; van der Sluijs, P. Platelet secretory behaviour: As diverse as the granules... or not? J. Thromb. Haemost. 2015, 13, 2141–2151. [Google Scholar] [CrossRef]
- Rendu, F.; Brohard-Bohn, B. The platelet release reaction: Granules’ constituents, secretion and functions. Platelets 2001, 12, 261–273. [Google Scholar] [CrossRef]
- Italiano, J.E., Jr.; Battinelli, E.M. Selective sorting of alpha-granule proteins. J. Thromb. Haemost. 2009, 7 (Suppl. 1), 173–176. [Google Scholar] [CrossRef] [PubMed]
- Silvestre-Roig, C.; Braster, Q.; Ortega-Gomez, A.; Soehnlein, O. Neutrophils as regulators of cardiovascular inflammation. Nat. Rev. Cardiol. 2020, 17, 327–340. [Google Scholar] [CrossRef]
- Smyth, S.S.; Reis, E.D.; Zhang, W.; Fallon, J.T.; Gordon, R.E.; Coller, B.S. Beta(3)-integrin-deficient mice but not P-selectin-deficient mice develop intimal hyperplasia after vascular injury: Correlation with leukocyte recruitment to adherent platelets 1 hour after injury. Circulation 2001, 103, 2501–2507. [Google Scholar] [CrossRef]
- Morrell, C.N.; Aggrey, A.A.; Chapman, L.M.; Modjeski, K.L. Emerging roles for platelets as immune and inflammatory cells. Blood 2014, 123, 2759–2767. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, N.; Hilger, A.; Zarbock, A.; Rossaint, J. Platelets at the Crossroads of Pro-Inflammatory and Resolution Pathways during Inflammation. Cells 2022, 11, 1957. [Google Scholar] [CrossRef]
- Hou, Y.; Carrim, N.; Wang, Y.; Gallant, R.C.; Marshall, A.; Ni, H. Platelets in hemostasis and thrombosis: Novel mechanisms of fibrinogen-independent platelet aggregation and fibronectin-mediated protein wave of hemostasis. J. Biomed. Res. 2015, 29, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhong, H.; Zhao, Y.; Luo, X.; Gao, W. Role of platelet biomarkers in inflammatory response. Biomark. Res. 2020, 8, 28. [Google Scholar] [CrossRef]
- Kisucka, J.; Butterfield, C.E.; Duda, D.G.; Eichenberger, S.C.; Saffaripour, S.; Ware, J.; Ruggeri, Z.M.; Jain, R.K.; Folkman, J.; Wagner, D.D. Platelets and platelet adhesion support angiogenesis while preventing excessive hemorrhage. Proc. Natl. Acad. Sci. USA 2006, 103, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Wu, H.; Fang, X.; He, J.; Zhu, F. Platelet, a key regulator of innate and adaptive immunity. Front. Med. 2023, 10, 1074878. [Google Scholar] [CrossRef]
- Ho-Tin-Noe, B. The multifaceted roles of platelets in inflammation and innate immunity. Platelets 2018, 29, 531–532. [Google Scholar] [CrossRef]
- Contursi, A.; Tacconelli, S.; Di Berardino, S.; De Michele, A.; Patrignani, P. Platelets as crucial players in the dynamic interplay of inflammation, immunity, and cancer: Unveiling new strategies for cancer prevention. Front. Pharmacol. 2024, 15, 1520488. [Google Scholar] [CrossRef]
- Denis, C.V.; Lenting, P.J. von Willebrand factor: At the crossroads of bleeding and thrombosis. Int. J. Hematol. 2012, 95, 353–361. [Google Scholar] [CrossRef]
- Sopova, K.; Tatsidou, P.; Stellos, K. Platelets and platelet interaction with progenitor cells in vascular homeostasis and inflammation. Curr. Vasc. Pharmacol. 2012, 10, 555–562. [Google Scholar] [CrossRef]
- Ho-Tin-Noe, B.; Boulaftali, Y.; Camerer, E. Platelets and vascular integrity: How platelets prevent bleeding in inflammation. Blood 2018, 131, 277–288. [Google Scholar] [CrossRef]
- Bordon, Y. Innate immunity: Platelets on the prowl. Nat. Rev. Immunol. 2018, 18, 3. [Google Scholar] [CrossRef]
- Nording, H.; Langer, H.F. Complement links platelets to innate immunity. Semin. Immunol. 2018, 37, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Semple, J.W.; Freedman, J. Platelets and innate immunity. Cell Mol. Life Sci. 2010, 67, 499–511. [Google Scholar] [CrossRef]
- Kapur, R.; Semple, J.W. Platelets as immune-sensing cells. Blood Adv. 2016, 1, 10–14. [Google Scholar] [CrossRef]
- Lam, F.W.; Vijayan, K.V.; Rumbaut, R.E. Platelets and Their Interactions with Other Immune Cells. Compr. Physiol. 2015, 5, 1265–1280. [Google Scholar] [CrossRef]
- Rolfes, V.; Ribeiro, L.S.; Hawwari, I.; Bottcher, L.; Rosero, N.; Maasewerd, S.; Santos, M.L.S.; Prochnicki, T.; Silva, C.M.S.; Wanderley, C.W.S.; et al. Platelets Fuel the Inflammasome Activation of Innate Immune Cells. Cell Rep. 2020, 31, 107615. [Google Scholar] [CrossRef]
- Speth, C.; Loffler, J.; Krappmann, S.; Lass-Florl, C.; Rambach, G. Platelets as immune cells in infectious diseases. Future Microbiol. 2013, 8, 1431–1451. [Google Scholar] [CrossRef]
- von Hundelshausen, P.; Weber, C. Platelets as immune cells: Bridging inflammation and cardiovascular disease. Circ. Res. 2007, 100, 27–40. [Google Scholar] [CrossRef]
- Bo, Y.; Lu, Q.; Li, B.; Sha, R.; Yu, H.; Miao, C. The role of platelets in central hubs of inflammation: A literature review. Medicine 2024, 103, e38115. [Google Scholar] [CrossRef]
- Cleary, S.J.; Conrad, C. Investigating and imaging platelets in inflammation. Int. J. Biochem. Cell Biol. 2023, 157, 106373. [Google Scholar] [CrossRef]
- Rayes, J.; Bourne, J.H.; Brill, A.; Watson, S.P. The dual role of platelet-innate immune cell interactions in thrombo-inflammation. Res. Pract. Thromb. Haemost. 2020, 4, 23–35. [Google Scholar] [CrossRef]
- Vulliamy, P.; Armstrong, P.C. Platelets in Hemostasis, Thrombosis, and Inflammation After Major Trauma. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Carestia, A.; Mena, H.A.; Olexen, C.M.; Ortiz Wilczynski, J.M.; Negrotto, S.; Errasti, A.E.; Gomez, R.M.; Jenne, C.N.; Carrera Silva, E.A.; Schattner, M. Platelets Promote Macrophage Polarization toward Pro-inflammatory Phenotype and Increase Survival of Septic Mice. Cell Rep. 2019, 28, 896–908.e5. [Google Scholar] [CrossRef]
- Yip, J.Y.; Kanneganti, A.; Binte Ahmad, N.; Lim, M.X.K.; Chew, S.L.S.; Huang, Z. Optimizing intrauterine insemination and spontaneous conception in women with unilateral hydrosalpinx or tubal pathology: A systematic review and narrative synthesis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2023, 286, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, R.; Toyoda, E.; Maehara, M.; Wasai, S.; Omura, H.; Watanabe, M.; Sato, M. Effect of Platelet-Rich Plasma on M1/M2 Macrophage Polarization. Int. J. Mol. Sci. 2021, 22, 2336. [Google Scholar] [CrossRef] [PubMed]
- De Giovanni, M.; Tam, H.; Valet, C.; Xu, Y.; Looney, M.R.; Cyster, J.G. GPR35 promotes neutrophil recruitment in response to serotonin metabolite 5-HIAA. Cell 2022, 185, 1103–1104. [Google Scholar] [CrossRef]
- Revenstorff, J.; Ludwig, N.; Hilger, A.; Mersmann, S.; Lehmann, M.; Grenzheuser, J.C.; Kardell, M.; Bone, J.; Kotting, N.M.; Marx, N.C.; et al. Role of S100A8/A9 in Platelet-Neutrophil Complex Formation during Acute Inflammation. Cells 2022, 11, 3944. [Google Scholar] [CrossRef]
- Schrottmaier, W.C.; Kral-Pointner, J.B.; Salzmann, M.; Mussbacher, M.; Schmuckenschlager, A.; Pirabe, A.; Brunnthaler, L.; Kuttke, M.; Maier, B.; Heber, S.; et al. Platelet p110beta mediates platelet-leukocyte interaction and curtails bacterial dissemination in pneumococcal pneumonia. Cell Rep. 2022, 41, 111614. [Google Scholar] [CrossRef]
- Shang, Y.; Sun, J.; Wu, X.; Wang, Q. Activated platelet membrane nanovesicles recruit neutrophils to exert the antitumor efficiency. Front. Chem. 2022, 10, 955995. [Google Scholar] [CrossRef]
- Clark, S.R.; Ma, A.C.; Tavener, S.A.; McDonald, B.; Goodarzi, Z.; Kelly, M.M.; Patel, K.D.; Chakrabarti, S.; McAvoy, E.; Sinclair, G.D.; et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 2007, 13, 463–469. [Google Scholar] [CrossRef]
- de Stoppelaar, S.F.; Claushuis, T.A.; Jansen, M.P.; Hou, B.; Roelofs, J.J.; van ’t Veer, C.; van der Poll, T. The role of platelet MyD88 in host response during gram-negative sepsis. J. Thromb. Haemost. 2015, 13, 1709–1720. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, S.; Hu, L.; Zhai, L.; Xue, R.; Ye, J.; Chen, L.; Cheng, G.; Mruk, J.; Kunapuli, S.P.; et al. Nucleotide-binding oligomerization domain 2 receptor is expressed in platelets and enhances platelet activation and thrombosis. Circulation 2015, 131, 1160–1170. [Google Scholar] [CrossRef]
- Brown, G.T.; McIntyre, T.M. Lipopolysaccharide signaling without a nucleus: Kinase cascades stimulate platelet shedding of proinflammatory IL-1beta-rich microparticles. J. Immunol. 2011, 186, 5489–5496. [Google Scholar] [CrossRef]
- Aggrey, A.A.; Srivastava, K.; Ture, S.; Field, D.J.; Morrell, C.N. Platelet induction of the acute-phase response is protective in murine experimental cerebral malaria. J. Immunol. 2013, 190, 4685–4691. [Google Scholar] [CrossRef]
- Denis, M.M.; Tolley, N.D.; Bunting, M.; Schwertz, H.; Jiang, H.; Lindemann, S.; Yost, C.C.; Rubner, F.J.; Albertine, K.H.; Swoboda, K.J.; et al. Escaping the nuclear confines: Signal-dependent pre-mRNA splicing in anucleate platelets. Cell 2005, 122, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Boylan, B.; Gao, C.; Rathore, V.; Gill, J.C.; Newman, D.K.; Newman, P.J. Identification of FcgammaRIIa as the ITAM-bearing receptor mediating alphaIIbbeta3 outside-in integrin signaling in human platelets. Blood 2008, 112, 2780–2786. [Google Scholar] [CrossRef]
- Zhi, H.; Rauova, L.; Hayes, V.; Gao, C.; Boylan, B.; Newman, D.K.; McKenzie, S.E.; Cooley, B.C.; Poncz, M.; Newman, P.J. Cooperative integrin/ITAM signaling in platelets enhances thrombus formation in vitro and in vivo. Blood 2013, 121, 1858–1867. [Google Scholar] [CrossRef]
- Arman, M.; Krauel, K.; Tilley, D.O.; Weber, C.; Cox, D.; Greinacher, A.; Kerrigan, S.W.; Watson, S.P. Amplification of bacteria-induced platelet activation is triggered by FcgammaRIIA, integrin alphaIIbbeta3, and platelet factor 4. Blood 2014, 123, 3166–3174. [Google Scholar] [CrossRef] [PubMed]
- Boilard, E.; Pare, G.; Rousseau, M.; Cloutier, N.; Dubuc, I.; Levesque, T.; Borgeat, P.; Flamand, L. Influenza virus H1N1 activates platelets through FcgammaRIIA signaling and thrombin generation. Blood 2014, 123, 2854–2863. [Google Scholar] [CrossRef]
- Karas, S.P.; Rosse, W.F.; Kurlander, R.J. Characterization of the IgG-Fc receptor on human platelets. Blood 1982, 60, 1277–1282. [Google Scholar] [CrossRef]
- Sun, D.; Popescu, N.I.; Raisley, B.; Keshari, R.S.; Dale, G.L.; Lupu, F.; Coggeshall, K.M. Bacillus anthracis peptidoglycan activates human platelets through FcgammaRII and complement. Blood 2013, 122, 571–579. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Benezech, C.; Nayar, S.; Finney, B.A.; Withers, D.R.; Lowe, K.; Desanti, G.E.; Marriott, C.L.; Watson, S.P.; Caamano, J.H.; Buckley, C.D.; et al. CLEC-2 is required for development and maintenance of lymph nodes. Blood 2014, 123, 3200–3207. [Google Scholar] [CrossRef]
- Hitchcock, J.R.; Cook, C.N.; Bobat, S.; Ross, E.A.; Flores-Langarica, A.; Lowe, K.L.; Khan, M.; Dominguez-Medina, C.C.; Lax, S.; Carvalho-Gaspar, M.; et al. Inflammation drives thrombosis after Salmonella infection via CLEC-2 on platelets. J. Clin. Investig. 2015, 125, 4429–4446. [Google Scholar] [CrossRef] [PubMed]
- Al Hawas, R.; Ren, Q.; Ye, S.; Karim, Z.A.; Filipovich, A.H.; Whiteheart, S.W. Munc18b/STXBP2 is required for platelet secretion. Blood 2012, 120, 2493–2500. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Jiang, H.; Ding, C.; Zhang, L.; Ding, N.; Li, G.; Zhang, F.; Wang, J.; Deng, L.; Liu, J.; et al. STING activation in platelets aggravates septic thrombosis by enhancing platelet activation and granule secretion. Immunity 2023, 56, 1013–1026.e6. [Google Scholar] [CrossRef]
- Hassan, A.; Bagu, E.T.; Levesque, M.; Patten, S.A.; Benhadjeba, S.; Edjekouane, L.; Villemure, I.; Tremblay, A.; Moldovan, F. The 17beta-estradiol induced upregulation of the adhesion G-protein coupled receptor (ADGRG7) is modulated by ESRalpha and SP1 complex. Biol. Open 2019, 8, 037390. [Google Scholar] [CrossRef]
- Bhagirath, V.C.; Dwivedi, D.J.; Liaw, P.C. Comparison of the Proinflammatory and Procoagulant Properties of Nuclear, Mitochondrial, and Bacterial DNA. Shock 2015, 44, 265–271. [Google Scholar] [CrossRef]
- Boudreau, L.H.; Duchez, A.C.; Cloutier, N.; Soulet, D.; Martin, N.; Bollinger, J.; Pare, A.; Rousseau, M.; Naika, G.S.; Levesque, T.; et al. Platelets release mitochondria serving as substrate for bactericidal group IIA-secreted phospholipase A2 to promote inflammation. Blood 2014, 124, 2173–2183. [Google Scholar] [CrossRef]
- Melki, I.; Allaeys, I.; Tessandier, N.; Levesque, T.; Cloutier, N.; Laroche, A.; Vernoux, N.; Becker, Y.; Benk-Fortin, H.; Zufferey, A.; et al. Platelets release mitochondrial antigens in systemic lupus erythematosus. Sci. Transl. Med. 2021, 13, eaav5928. [Google Scholar] [CrossRef]
- Puhm, F.; Boilard, E.; Machlus, K.R. Platelet Extracellular Vesicles: Beyond the Blood. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 87–96. [Google Scholar] [CrossRef]
- Tavukcuoglu, Z.; Butt, U.; Faria, A.V.S.; Oesterreicher, J.; Holnthoner, W.; Laitinen, S.; Palviainen, M.; Siljander, P.R. Platelet-derived extracellular vesicles induced through different activation pathways drive melanoma progression by functional and transcriptional changes. Cell Commun. Signal 2024, 22, 601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Itagaki, K.; Hauser, C.J. Mitochondrial DNA is released by shock and activates neutrophils via p38 map kinase. Shock 2010, 34, 55–59. [Google Scholar] [CrossRef]
- Nish, S.; Medzhitov, R. Host defense pathways: Role of redundancy and compensation in infectious disease phenotypes. Immunity 2011, 34, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Nicolai, L.; Pekayvaz, K.; Massberg, S. Platelets: Orchestrators of immunity in host defense and beyond. Immunity 2024, 57, 957–972. [Google Scholar] [CrossRef]
- Ali, R.A.; Wuescher, L.M.; Worth, R.G. Platelets: Essential components of the immune system. Curr. Trends Immunol. 2015, 16, 65–78. [Google Scholar]
- Hirosue, S.; Dubrot, J. Modes of Antigen Presentation by Lymph Node Stromal Cells and Their Immunological Implications. Front. Immunol. 2015, 6, 446. [Google Scholar] [CrossRef] [PubMed]
- Tsukiji, N.; Suzuki-Inoue, K. Impact of Hemostasis on the Lymphatic System in Development and Disease. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 1747–1754. [Google Scholar] [CrossRef]
- Han, P.; Hanlon, D.; Arshad, N.; Lee, J.S.; Tatsuno, K.; Robinson, E.; Filler, R.; Sobolev, O.; Cote, C.; Rivera-Molina, F.; et al. Platelet P-selectin initiates cross-presentation and dendritic cell differentiation in blood monocytes. Sci. Adv. 2020, 6, eaaz1580. [Google Scholar] [CrossRef]
- Ramanujam, M.; Steffgen, J.; Visvanathan, S.; Mohan, C.; Fine, J.S.; Putterman, C. Phoenix from the flames: Rediscovering the role of the CD40-CD40L pathway in systemic lupus erythematosus and lupus nephritis. Autoimmun. Rev. 2020, 19, 102668. [Google Scholar] [CrossRef]
- Hess, P.R.; Rawnsley, D.R.; Jakus, Z.; Yang, Y.; Sweet, D.T.; Fu, J.; Herzog, B.; Lu, M.; Nieswandt, B.; Oliver, G.; et al. Platelets mediate lymphovenous hemostasis to maintain blood-lymphatic separation throughout life. J. Clin. Investig. 2014, 124, 273–284. [Google Scholar] [CrossRef]
- Cognasse, F.; Hamzeh-Cognasse, H.; Lafarge, S.; Chavarin, P.; Cogne, M.; Richard, Y.; Garraud, O. Human platelets can activate peripheral blood B cells and increase production of immunoglobulins. Exp. Hematol. 2007, 35, 1376–1387. [Google Scholar] [CrossRef]
- Greinacher, A.; Schonborn, L.; Siegerist, F.; Steil, L.; Palankar, R.; Handtke, S.; Reder, A.; Thiele, T.; Aurich, K.; Methling, K.; et al. Pathogenesis of vaccine-induced immune thrombotic thrombocytopenia (VITT). Semin. Hematol. 2022, 59, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Mendonca, S.A.; Lorincz, R.; Boucher, P.; Curiel, D.T. Adenoviral vector vaccine platforms in the SARS-CoV-2 pandemic. NPJ Vaccines 2021, 6, 97. [Google Scholar] [CrossRef]
- Roytenberg, R.; Garcia-Sastre, A.; Li, W. Vaccine-induced immune thrombotic thrombocytopenia: What do we know hitherto? Front. Med. 2023, 10, 1155727. [Google Scholar] [CrossRef] [PubMed]
- Thiele, T.; Schwarz, S.L.; Handtke, S. Platelet size as a mirror for the immune response after SARS-CoV-2 vaccination. J. Thromb. Haemost. 2022, 20, 818–820. [Google Scholar] [CrossRef] [PubMed]
- Grassle, S.; Huck, V.; Pappelbaum, K.I.; Gorzelanny, C.; Aponte-Santamaria, C.; Baldauf, C.; Grater, F.; Schneppenheim, R.; Obser, T.; Schneider, S.W. von Willebrand factor directly interacts with DNA from neutrophil extracellular traps. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1382–1389. [Google Scholar] [CrossRef]
- Johnston, I.; Sarkar, A.; Hayes, V.; Koma, G.T.; Arepally, G.M.; Chen, J.; Chung, D.W.; Lopez, J.A.; Cines, D.B.; Rauova, L.; et al. Recognition of PF4-VWF complexes by heparin-induced thrombocytopenia antibodies contributes to thrombus propagation. Blood 2020, 135, 1270–1280. [Google Scholar] [CrossRef]
- Laridan, E.; Martinod, K.; De Meyer, S.F. Neutrophil Extracellular Traps in Arterial and Venous Thrombosis. Semin. Thromb. Hemost. 2019, 45, 86–93. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Brodsky, R.A.; McCrae, K.R. Complement in the Pathophysiology of the Antiphospholipid Syndrome. Front. Immunol. 2019, 10, 449. [Google Scholar] [CrossRef]
- Skendros, P.; Mitsios, A.; Chrysanthopoulou, A.; Mastellos, D.C.; Metallidis, S.; Rafailidis, P.; Ntinopoulou, M.; Sertaridou, E.; Tsironidou, V.; Tsigalou, C.; et al. Complement and tissue factor-enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. J. Clin. Investig. 2020, 130, 6151–6157. [Google Scholar] [CrossRef]
- Gavriilaki, E.; Brodsky, R.A. Complementopathies and precision medicine. J. Clin. Investig. 2020, 130, 2152–2163. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, J.; Uzun, G.; Zlamal, J.; Singh, A.; Bakchoul, T. Platelet-neutrophil interaction in COVID-19 and vaccine-induced thrombotic thrombocytopenia. Front. Immunol. 2023, 14, 1186000. [Google Scholar] [CrossRef] [PubMed]
- Puschel, V.A.A.; Fhon, J.R.S.; Nogueira, L.S.; Poveda, V.B.; Oliveira, L.B.; Salvetti, M.G.; Lemos, C.S.; Bruna, C.Q.M.; Lima, F.R.; Silva, A.; et al. Factors associated with infection and hospitalization due to COVID-19 in Nursing professionals: A cross-sectional study. Rev. Lat. Am. Enferm. 2022, 30, e3571. [Google Scholar] [CrossRef]
- Sakr, Y.; Giovini, M.; Leone, M.; Pizzilli, G.; Kortgen, A.; Bauer, M.; Tonetti, T.; Duclos, G.; Zieleskiewicz, L.; Buschbeck, S.; et al. Pulmonary embolism in patients with coronavirus disease-2019 (COVID-19) pneumonia: A narrative review. Ann. Intensive Care 2020, 10, 124. [Google Scholar] [CrossRef]
- Ostrowski, S.R.; Sogaard, O.S.; Tolstrup, M.; Staerke, N.B.; Lundgren, J.; Ostergaard, L.; Hvas, A.M. Inflammation and Platelet Activation After COVID-19 Vaccines—Possible Mechanisms Behind Vaccine-Induced Immune Thrombocytopenia and Thrombosis. Front. Immunol. 2021, 12, 779453. [Google Scholar] [CrossRef]
- Ferro, J.M.; de Sousa, D.A.; Coutinho, J.M.; Martinelli, I. European stroke organization interim expert opinion on cerebral venous thrombosis occurring after SARS-CoV-2 vaccination. Eur. Stroke J. 2021, 6, CXVI–CXXI. [Google Scholar] [CrossRef]
- Gresele, P.; Marietta, M.; Ageno, W.; Marcucci, R.; Contino, L.; Donadini, M.P.; Russo, L.; Tiscia, G.L.; Palareti, G.; Tripodi, A.; et al. Management of cerebral and splanchnic vein thrombosis associated with thrombocytopenia in subjects previously vaccinated with Vaxzevria (AstraZeneca): A position statement from the Italian Society for the Study of Haemostasis and Thrombosis (SISET). Blood Transfus. 2021, 19, 281–283. [Google Scholar] [CrossRef]
- Jacobson, B.F.; Schapkaitz, E.; Mer, M.; Louw, S.; Haas, S.; Buller, H.R.; Brenner, B.; Abdool-Carrim, A.T.O.; De Jong, P.; Hsu, P.; et al. Recommendations for the diagnosis and management of vaccine-induced immune thrombotic thrombocytopenia. S Afr. Med. J. 2021, 111, 535–537. [Google Scholar]
- Tutwiler, V.; Madeeva, D.; Ahn, H.S.; Andrianova, I.; Hayes, V.; Zheng, X.L.; Cines, D.B.; McKenzie, S.E.; Poncz, M.; Rauova, L. Platelet transactivation by monocytes promotes thrombosis in heparin-induced thrombocytopenia. Blood 2016, 127, 464–472. [Google Scholar] [CrossRef]
- Stolla, M.; Kapur, R.; Semple, J.W. New Emerging Developments of Platelets in Transfusion Medicine. Transfus. Med. Rev. 2020, 34, 207–208. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, C.H.; DomBourian, M.G.; Millward, P.A. Platelet transfusion for patients with cancer. Cancer Control 2015, 22, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Zhao, J.; Ren, Q.; Ma, Y.; Duan, P.; Huang, Y.; Wang, S. Clinical application of platelet rich plasma to promote healing of open hand injury with skin defect. Regen. Ther. 2024, 26, 308–314. [Google Scholar] [CrossRef]
- van der Meijden, P.E.J.; Heemskerk, J.W.M. Platelet biology and functions: New concepts and clinical perspectives. Nat. Rev. Cardiol. 2019, 16, 166–179. [Google Scholar] [CrossRef]
- Jones, W.S.; Mulder, H.; Wruck, L.M.; Pencina, M.J.; Kripalani, S.; Munoz, D.; Crenshaw, D.L.; Effron, M.B.; Re, R.N.; Gupta, K.; et al. Comparative Effectiveness of Aspirin Dosing in Cardiovascular Disease. N. Engl. J. Med. 2021, 384, 1981–1990. [Google Scholar] [CrossRef]
- Alagna, G.; Mazzone, P.; Contarini, M.; Ando, G. Dual Antiplatelet Therapy with Parenteral P2Y(12) Inhibitors: Rationale, Evidence, and Future Directions. J. Cardiovasc. Dev. Dis. 2023, 10, 0163. [Google Scholar] [CrossRef] [PubMed]
- Halvorsen, S.; Andreotti, F.; ten Berg, J.M.; Cattaneo, M.; Coccheri, S.; Marchioli, R.; Morais, J.; Verheugt, F.W.; De Caterina, R. Aspirin therapy in primary cardiovascular disease prevention: A position paper of the European Society of Cardiology working group on thrombosis. J. Am. Coll. Cardiol. 2014, 64, 319–327. [Google Scholar] [CrossRef]
- McFadyen, J.D.; Schaff, M.; Peter, K. Current and future antiplatelet therapies: Emphasis on preserving haemostasis. Nat. Rev. Cardiol. 2018, 15, 181–191. [Google Scholar] [CrossRef]
- Olie, R.H.; van der Meijden, P.E.J.; Ten Cate, H. The coagulation system in atherothrombosis: Implications for new therapeutic strategies. Res. Pract. Thromb. Haemost. 2018, 2, 188–198. [Google Scholar] [CrossRef]
- Patrono, C.; Morais, J.; Baigent, C.; Collet, J.P.; Fitzgerald, D.; Halvorsen, S.; Rocca, B.; Siegbahn, A.; Storey, R.F.; Vilahur, G. Antiplatelet Agents for the Treatment and Prevention of Coronary Atherothrombosis. J. Am. Coll. Cardiol. 2017, 70, 1760–1776. [Google Scholar] [CrossRef]
- Armstrong, P.C.; Leadbeater, P.D.; Chan, M.V.; Kirkby, N.S.; Jakubowski, J.A.; Mitchell, J.A.; Warner, T.D. In the presence of strong P2Y12 receptor blockade, aspirin provides little additional inhibition of platelet aggregation. J. Thromb. Haemost. 2011, 9, 552–561. [Google Scholar] [CrossRef]
- Best, M.G.; Vancura, A.; Wurdinger, T. Platelet RNA as a circulating biomarker trove for cancer diagnostics. J. Thromb. Haemost. 2017, 15, 1295–1306. [Google Scholar] [CrossRef]
- Xiang, Y.Z.; Xia, Y.; Gao, X.M.; Shang, H.C.; Kang, L.Y.; Zhang, B.L. Platelet activation, and antiplatelet targets and agents: Current and novel strategies. Drugs 2008, 68, 1647–1664. [Google Scholar] [CrossRef]
- Duley, L.; Meher, S.; Hunter, K.E.; Seidler, A.L.; Askie, L.M. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst. Rev. 2019, 2019, CD004659. [Google Scholar] [CrossRef]
- Safouris, A.; Magoufis, G.; Tsivgoulis, G. Emerging agents for the treatment and prevention of stroke: Progress in clinical trials. Expert. Opin. Investig. Drugs 2021, 30, 1025–1035. [Google Scholar] [CrossRef]
- Gelbenegger, G.; Jilma, B. Clinical pharmacology of antiplatelet drugs. Expert Rev. Clin. Pharmacol. 2022, 15, 1177–1197. [Google Scholar] [CrossRef]
| Alpha Granule Soluble Factors | |
|---|---|
| Function | Examples |
| Thrombosis | Fibrinogen, Thrombospondin, Coagulation Factors, Angiostatin, Laminin, Plasminogen |
| Inflammation | CCL2, CCL3, CCL5, CXCL4, CXL5, CXCL7, CXCL8, CD62P, CD40L, Complement Components (C3a, C5a) |
| Wound Healing | Endostatin, Thymosin beta 4, VEGF, TGF-beta, PDGF Family |
| Alpha Granule Membrane Proteins | |
| Protein Type | Examples |
| Surface Markers | CD9, CD36, CD63, Septin5, Siglec7 |
| Integrins | Integrin αIIbβ3, alpha 6 |
| Receptors | Fc gamma RIIA, CD62P, DC-SIGN, |
| Membrane Proteins | VAMP 2, 3, 7, 8, Syntaxin 2, Syntaxin 4, 8, 11, Munc18-2, SNAP23 |
| Dense Granules | |
| Component | Examples |
| Nucleotides | ADP, ATP |
| Ions | Calcium, Magnesium, Potassium |
| Small Molecules | Epinephrine, Serotonin, Histamine |
| Polyphosphates | Polyphosphates, Pyrophosphate |
| Proteins | STIM1, STIM2 |
| Lysosome | |
| Enzyme | Examples |
| Glycosidases | α-galactosidase, β-galactosidase |
| Proteases | Cathepsin A, Hyaluronidase-2 (HYAL2) |
| Lipases | Glucosylceramidase (GBA), ASAHL |
| Other Enzymes | Catalase, Lactate Dehydrogenase, Hexosaminidase A/B, Heparinase |
| Molecule | Target | Inhibitory Mechanism |
|---|---|---|
| Aspirin and NSAIDs | Cyclooxygenase1 | Blocks TXA2 formation |
| Clopidogrel | P2Y12 | Irreversibly inhibits ADP receptors |
| Ticagrelor | P2Y12 | Reversibly inhibits ADP receptors |
| Cangrelor | P2Y12 | Reversibly inhibits ADP receptors |
| Prasugrel | P2Y12 | Irreversibly inhibits ADP receptors |
| Tirofiban | αIIbβ3 | Blocks integrin |
| Eptifibatide | αIIbβ3 | Blocks integrin |
| Abciximab | αIIbβ3 | Blocks integrin |
| Vorapaxar | PAR1 | Blocked thrombin receptors |
| Iloprost | PGI2 analogue | Increases platelet cAMP levels, thus acting as an intravenous reversible antiplatelet agent |
| Cilostazol | PDE3A | Inhibits adenosine cellular uptake, increases intraplatelet levels of cyclic AMP |
| Dipyridamole | PDE3/5 | Scavenge peroxy radicals and increase interstitial adenosine levels, increases intraplatelet levels of cyclic AMP |
| Revacept | CLEC2/GPVI | Competes with platelet GPVI for binding to collagen |
| Quercetin | PDI | PI3K/Akt inactivation, cAMP elevation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratnapriya, S.; Yabaji, S.M. Vaccination and Platelet Biology: Unraveling the Immuno-Hemostatic Interplay. Vaccines 2025, 13, 403. https://doi.org/10.3390/vaccines13040403
Ratnapriya S, Yabaji SM. Vaccination and Platelet Biology: Unraveling the Immuno-Hemostatic Interplay. Vaccines. 2025; 13(4):403. https://doi.org/10.3390/vaccines13040403
Chicago/Turabian StyleRatnapriya, Sneha, and Shivraj M. Yabaji. 2025. "Vaccination and Platelet Biology: Unraveling the Immuno-Hemostatic Interplay" Vaccines 13, no. 4: 403. https://doi.org/10.3390/vaccines13040403
APA StyleRatnapriya, S., & Yabaji, S. M. (2025). Vaccination and Platelet Biology: Unraveling the Immuno-Hemostatic Interplay. Vaccines, 13(4), 403. https://doi.org/10.3390/vaccines13040403






