Dynamics of SARS-CoV-2 IgG in Nursing Home Residents in Belgium Throughout Three BNT162b2 Vaccination Rounds: 19-Month Follow-Up
Abstract
1. Background
2. Materials and Methods
2.1. Study Design, Population, and Sample Size

2.2. Ethics
2.3. Data Collection
2.3.1. Clinical Sample Collection
2.3.2. Questionnaires
2.3.3. Quantification of SARS-CoV-2 S1RBD IgG Antibodies
2.3.4. Detection of SARS-CoV-2 S1RBD IgG Antibodies
2.3.5. Statistical Analysis
3. Results
3.1. Participant Characteristics
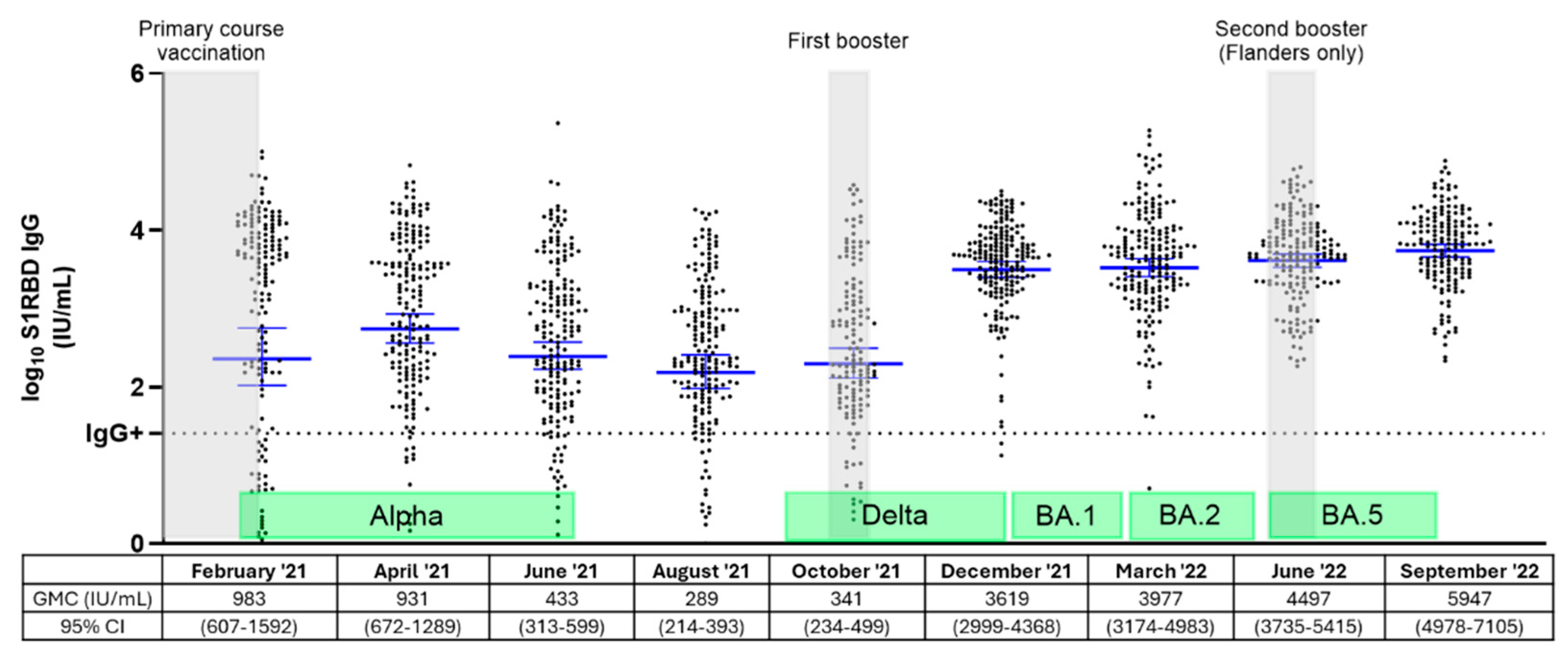
3.2. Dynamics of S1RBD IgG Levels Among Belgian NHRs Throughout Three Vaccination Rounds and Different Epidemiological Waves
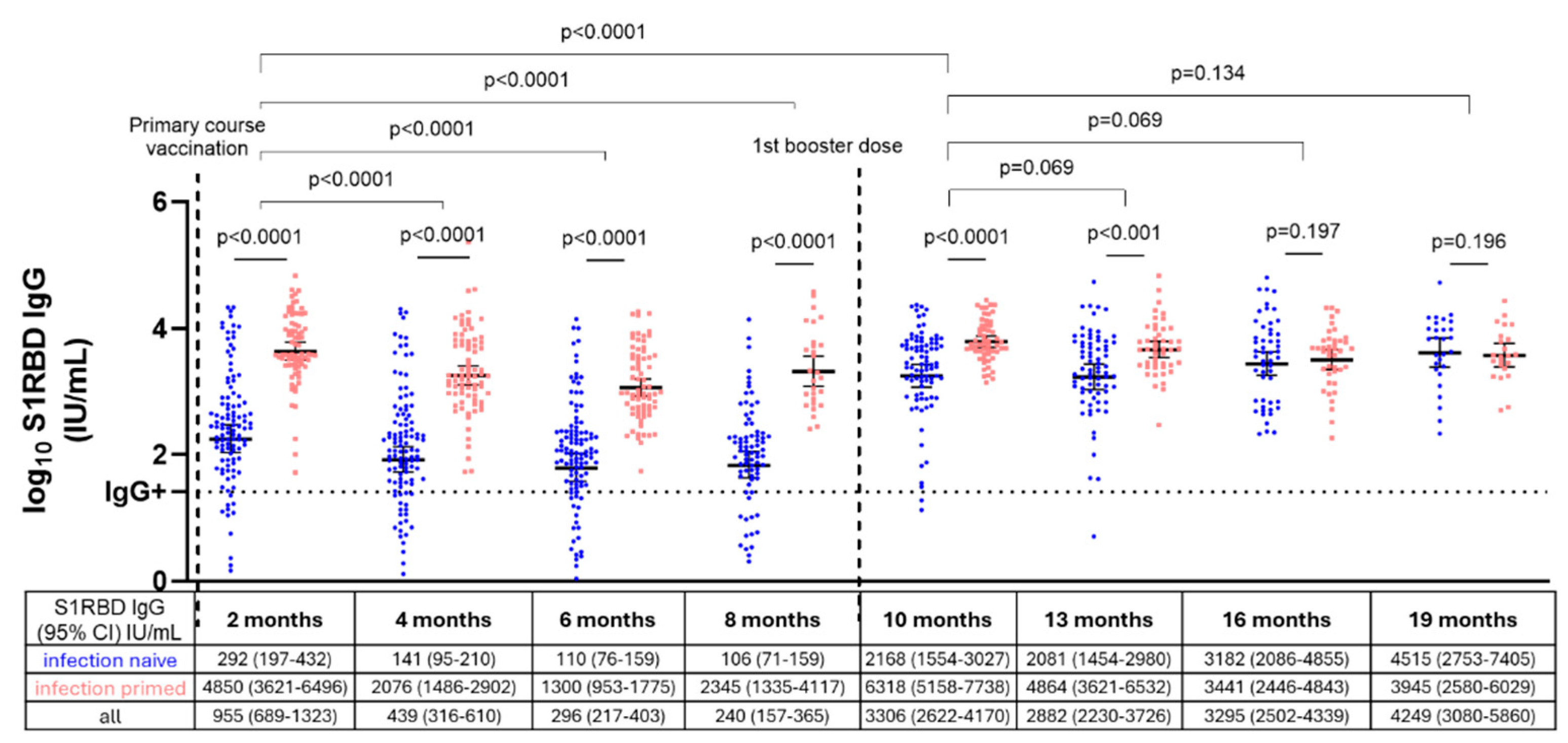
3.3. S1RBD IgG Antibody Levels in Infection-Naive and Infection-Primed NHRs After Primary Course and First Booster Vaccination
3.4. SARS-CoV-2 Antibody Response After First Booster in Primary Course Non-Responders Versus Responders
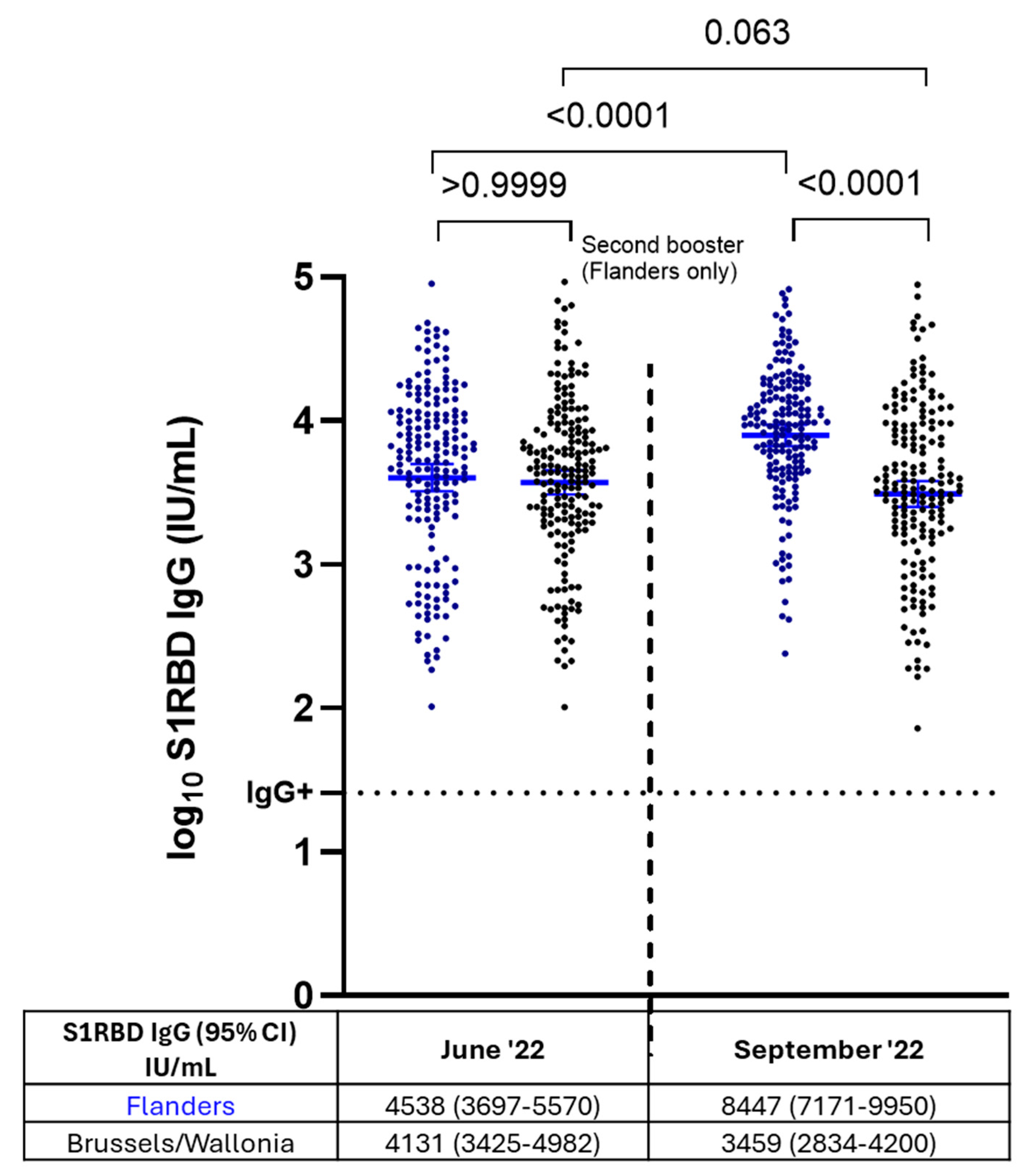
3.5. S1RBD IgG Antibody Levels After Administration of Two Booster Doses Versus a Single Booster Dose in NHRs (Flanders vs. Brussels/Wallonia)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.; Dou, Q.; Zhang, W.; Wang, C.; Xie, X.; Yang, Y.; Zeng, Y. Frailty as a Predictor of All-Cause Mortality Among Older Nursing Home Residents: A Systematic Review and Meta-analysis. J. Am. Med. Dir. Assoc. 2019, 20, 657–663.e654. [Google Scholar] [CrossRef] [PubMed]
- Leece, P.; Whelan, M.; Costa, A.P.; Daneman, N.; Johnstone, J.; McGeer, A.; Rochon, P.; Schwartz, K.L.; Brown, K.A. Nursing home crowding and its association with outbreak-associated respiratory infection in Ontario, Canada before the COVID-19 pandemic (2014-19): A retrospective cohort study. Lancet Healthy Longev. 2023, 4, e107–e114. [Google Scholar] [CrossRef]
- Catteau, D.S.; Haarhuis, L.; Vandael, F.; Stouten, E.; Litzroth, V.; Wyndham, A.; Sciensano, T.C. Surveillance van de COVID-19 Vaccinatie in Belgische Woonzorgcentra. Resultaten tot 24 Maart 2021; Sciensano: Brussels, Belgium, 2021. [Google Scholar]
- Meyers, E.; De Rop, L.; Engels, F.; Gioveni, C.; Coen, A.; De Burghgraeve, T.; Digregorio, M.; Van Ngoc, P.; De Clercq, N.; Buret, L.; et al. Follow-Up of SARS-CoV-2 Antibody Levels in Belgian Nursing Home Residents and Staff Two, Four and Six Months after Primary Course BNT162b2 Vaccination. Vaccines 2024, 12, 951. [Google Scholar] [CrossRef]
- Kolarić, B.; Ambriović-Ristov, A.; Tabain, I.; Vilibić-Čavlek, T. Waning immunity six months after BioNTech/Pfizer COVID-19 vaccination among nursing home residents in Zagreb, Croatia. Croat. Med. J. 2021, 62, 630–633. [Google Scholar] [CrossRef]
- Canaday, D.H.; Oyebanji, O.A.; Keresztesy, D.; Payne, M.; Wilk, D.; Carias, L.; Aung, H.; St. Denis, K.; Lam, E.C.; Rowley, C.F.; et al. Significant Reduction in Vaccine-Induced Antibody Levels and Neutralization Activity Among Healthcare Workers and Nursing Home Residents 6 Months Following Coronavirus Disease 2019 BNT162b2 mRNA Vaccination. Clin. Infect. Dis. 2021, 75, e884–e887. [Google Scholar] [CrossRef]
- Blain, H.; Tuaillon, E.; Gamon, L.; Pisoni, A.; Miot, S.; Picot, M.-C. Strong Decay of SARS-CoV-2 Spike Antibodies after 2 BNT162b2 Vaccine Doses and High Antibody Response to a Third Dose in Nursing Home Residents. J. Am. Med. Dir. Assoc. 2022, 23, 750–753. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, W.; Gerlo, S.; De Smet, E.; Wejda, M.; Acar, D.; Callens, S.; Heytens, S.; Padalko, E.; Vercruysse, H.; Cools, P.; et al. Humoral and Cellular Responses to COVID-19 Vaccination Indicate the Need for Post-Vaccination Testing in Frail Population. Vaccines 2022, 10, 260. [Google Scholar] [CrossRef] [PubMed]
- Fulop, T.; Pawelec, G.; Castle, S.; Loeb, M. Immunosenescence and vaccination in nursing home residents. Clin. Infect. Dis. 2009, 48, 443–448. [Google Scholar] [CrossRef]
- Meyers, E.; Deschepper, E.; Duysburgh, E.; De Rop, L.; De Burghgraeve, T.; Van Ngoc, P.; Digregorio, M.; Delogne, S.; Coen, A.; De Clercq, N.; et al. Declining Prevalence of SARS-CoV-2 Antibodies among Vaccinated Nursing Home Residents and Staff Six Months after the Primary BNT162b2 Vaccination Campaign in Belgium: A Prospective Cohort Study. Viruses 2022, 14, 2361. [Google Scholar] [CrossRef]
- Stouten, V.; Billuart, M.; Van Evercooren, I.; Hubin, P.; Nasiadka, L.; Catteau, L.; Rygaert, X.; Braeye, T.; Vermeiren, E.; Van Loenhout, J. Thematisch Rapport: Vaccinatiegraad en Epidemiologische Impact van de COVID-19-Vaccinatiecampagne bij Personen met Onderliggende Gezondheidsaandoeningen in België; Sciensano: Brussels, Belgium, 2023. [Google Scholar]
- Maerevoet, E. Bewoners van Woonzorgcentra en Thuiswonende 80-Plussers Krijgen Tweede Boosterprik Aangeboden; Vrt: Brussels, Belgium, 2022. [Google Scholar]
- Belgisch Centrum voor Farmacotherapeutische Informatie. COVID-19: Herfstcampagne Voor Tweede Booster Tegen COVID-19: Prioritaire Doelgroepen en Praktische Info. Available online: https://www.bcfi.be/nl/gows/3906 (accessed on 17 February 2025).
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Jurcevic, J.; Ekelson, R.; Nganda, S.; Bustos Sierra, N.; Vernemmen, C. Epidemiology of COVID 19 Mortality in Belgium, from Wave 1 to Wave 7 (March 2020–11 September 2022); Sciensano: Brussels, Belgium, 2023; Available online: https://www.sciensano.be/sites/default/files/epidemiology_of_covid-19_mortality_in_belgium_from_wave_1_to_7_1.pdf (accessed on 18 February 2025).
- Sciensano. Belgium COVID-19 Epidemiological Situation. Available online: https://lookerstudio.google.com/embed/reporting/c14a5cfc-cab7-4812-848c-0369173148ab/page/cUWaB (accessed on 1 September 2023).
- Meyers, E.; Coen, A.; Padalko, E.; Cools, P. Short- and long-term stability of SARS-CoV-2 antibodies on dried blood spots under different storage conditions. Microbiol. Spectr. 2024, 12, e01113–e01124. [Google Scholar] [CrossRef] [PubMed]
- Shelkey, M.; Wallace, M. Katz Index of Independence in Activities of Daily Living. Home Healthc. Now 2001, 19, 323–324. [Google Scholar] [CrossRef]
- Meyers, E.; Coen, A.; De Sutter, A.; Padalko, E.; Callens, S.; Vandekerckhove, L.; Witkowski, W.; Heytens, S.; Cools, P. Diagnostic performance of the SARS-CoV-2 S1RBD IgG ELISA (ImmunoDiagnostics) for the quantitative detection of SARS-CoV-2 antibodies on dried blood spots. J. Clin. Virol. 2022, 155, 105270. [Google Scholar] [CrossRef] [PubMed]
- Eggink, D.; Andeweg, S.P.; Vennema, H.; van Maarseveen, N.; Vermaas, K.; Vlaemynck, B.; Schepers, R.; van Gageldonk-Lafeber, A.B.; van den Hof, S.; Reusken, C.B.; et al. Increased risk of infection with SARS-CoV-2 Omicron BA.1 compared with Delta in vaccinated and previously infected individuals, the Netherlands, 22 November 2021 to 19 January 2022. Eurosurveillance 2022, 27, 2101196. [Google Scholar] [CrossRef]
- Braeye, T.; Catteau, L.; Brondeel, R.; van Loenhout, J.A.F.; Proesmans, K.; Cornelissen, L.; Van Oyen, H.; Stouten, V.; Hubin, P.; Billuart, M.; et al. Vaccine effectiveness against transmission of alpha, delta and omicron SARS-COV-2-infection, Belgian contact tracing, 2021–2022. Vaccine 2023, 41, 3292–3300. [Google Scholar] [CrossRef]
- Esper, F.P.; Adhikari, T.M.; Tu, Z.J.; Cheng, Y.W.; El-Haddad, K.; Farkas, D.H.; Bosler, D.; Rhoads, D.; Procop, G.W.; Ko, J.S.; et al. Alpha to Omicron: Disease Severity and Clinical Outcomes of Major SARS-CoV-2 Variants. J. Infect. Dis. 2023, 227, 344–352. [Google Scholar] [CrossRef]
- Kuhlmann, C.; Mayer, C.K.; Claassen, M.; Maponga, T.; Burgers, W.A.; Keeton, R.; Riou, C.; Sutherland, A.D.; Suliman, T.; Shaw, M.L.; et al. Breakthrough infections with SARS-CoV-2 omicron despite mRNA vaccine booster dose. Lancet 2022, 399, 625–626. [Google Scholar] [CrossRef]
- Pannus, P.; Neven, K.Y.; De Craeye, S.; Heyndrickx, L.; Vande Kerckhove, S.; Georges, D.; Michiels, J.; Francotte, A.; Van Den Bulcke, M.; Zrein, M.; et al. Poor Antibody Response to BioNTech/Pfizer Coronavirus Disease 2019 Vaccination in Severe Acute Respiratory Syndrome Coronavirus 2–Naive Residents of Nursing Homes. Clin. Infect. Dis. 2022, 75, e695–e704. [Google Scholar] [CrossRef]
- Gallichotte Emily, N.; Nehring, M.; Stromberg, S.; Young Michael, C.; Snell, A.; Daniels, J.; Pabilonia Kristy, L.; VandeWoude, S.; Ehrhart, N.; Ebel Gregory, D. Impact of Prior Infection on SARS-CoV-2 Antibody Responses in Vaccinated Long-Term Care Facility Staff. mSphere 2022, 7, e00169-22. [Google Scholar] [CrossRef]
- Canaday, D.H.; Carias, L.; Oyebanji, O.A.; Keresztesy, D.; Wilk, D.; Payne, M.; Aung, H.; St Denis, K.; Lam, E.C.; Rowley, C.F.; et al. Reduced BNT162b2 Messenger RNA Vaccine Response in Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)-Naive Nursing Home Residents. Clin. Infect. Dis. 2021, 73, 2112–2115. [Google Scholar] [CrossRef]
- Alidjinou, E.K.; Demaret, J.; Corroyer-Simovic, B.; Labreuche, J.; Goffard, A.; Trauet, J.; Lupau, D.; Miczek, S.; Vuotto, F.; Dendooven, A.; et al. Immunogenicity of BNT162b2 vaccine booster against SARS-CoV-2 Delta and Omicron variants in nursing home residents: A prospective observational study in older adults aged from 68 to 98 years. Lancet Reg. Health–Eur. 2022, 17, 100385. [Google Scholar] [CrossRef] [PubMed]
- Tut, G.; Lancaster, T.; Krutikov, M.; Sylla, P.; Bone, D.; Spalkova, E.; Bentley, C.; Amin, U.; Jadir, A.; Hulme, S.; et al. Strong peak immunogenicity but rapid antibody waning following third vaccine dose in older residents of care homes. Nat. Aging 2023, 3, 93–104. [Google Scholar] [CrossRef]
- Gravenstein, S.; DeVone, F.; Oyebanji, O.A.; Abul, Y.; Cao, Y.; Chan, P.A.; Halladay, C.W.; Rudolph, J.L.; Nugent, C.; Bosch, J.; et al. Durability of immunity and clinical protection in nursing home residents following bivalent SARS-CoV-2 vaccination. eBioMedicine 2024, 105, 105180. [Google Scholar] [CrossRef]
- Goodyear, C.S.; Patel, A.; Barnes, E.; Willicombe, M.; Siebert, S.; de Silva, T.I.; Snowden, J.A.; Lim, S.H.; Bowden, S.J.; Billingham, L.; et al. Immunogenicity of third dose COVID-19 vaccine strategies in patients who are immunocompromised with suboptimal immunity following two doses (OCTAVE-DUO): An open-label, multicentre, randomised, controlled, phase 3 trial. Lancet Rheumatol. 2024, 6, e339–e351. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.; Ohradanova-Repic, A.; Gebetsberger, L.; Tajti, G.; Kundi, M.; Stockinger, H.; Wiedermann, U.; Grabmeier-Pfistershammer, K. Full seroconversion in initial non-responders with higher antibody levels after heterologous COVID-19 vaccination schedule. Immunol. Lett. 2022, 250, 1–6. [Google Scholar] [CrossRef]
- Zhou, T.; Shi, T.; Li, A.; Zhu, L.; Zhao, X.; Mao, N.; Qin, W.; Bi, H.; Yang, M.; Dai, M.; et al. A third dose of inactivated SARS-CoV-2 vaccine induces robust antibody responses in people with inadequate response to two-dose vaccination. Natl. Sci. Rev. 2022, 9, nwac066. [Google Scholar] [CrossRef]
- Ferri, C.; Gragnani, L.; Raimondo, V.; Visentini, M.; Giuggioli, D.; Lorini, S.; Foti, R.; Cacciapaglia, F.; Caminiti, M.; Olivo, D.; et al. Absent or suboptimal response to booster dose of COVID-19 vaccine in patients with autoimmune systemic diseases. J. Autoimmun. 2022, 131, 102866. [Google Scholar] [CrossRef]
- Alejo, J.L.; Mitchell, J.; Chiang, T.P.; Abedon, A.T.; Boyarsky, B.J.; Avery, R.K.; Tobian, A.A.R.; Levan, M.L.; Massie, A.B.; Garonzik-Wang, J.M.; et al. Antibody Response to a Fourth Dose of a SARS-CoV-2 Vaccine in Solid Organ Transplant Recipients: A Case Series. Transplantation 2021, 105, e280–e281. [Google Scholar] [CrossRef]
- Martinelli, S.; Pascucci, D.; Laurenti, P. Humoral response after a fourth dose of SARS-CoV-2 vaccine in immunocompromised patients. Results of a systematic review. Front. Public Health 2023, 11, 1108546. [Google Scholar] [CrossRef]
- Nugent, C.; Abul, Y.; White, E.M.; Shehadeh, F.; Kaczynski, M.; Oscar Felix, L.; Ganesan, N.; Oyebanji, O.A.; Vishnepolskiy, I.; Didion, E.M.; et al. Second monovalent SARS-CoV-2 mRNA booster restores Omicron-specific neutralizing activity in both nursing home residents and health care workers. Vaccine 2023, 41, 3403–3409. [Google Scholar] [CrossRef] [PubMed]
- McConeghy, K.W.; White, E.M.; Blackman, C.; Santostefano, C.M.; Lee, Y.; Rudolph, J.L.; Canaday, D.; Zullo, A.R.; Jernigan, J.A.; Pilishvili, T.; et al. Effectiveness of a Second COVID-19 Vaccine Booster Dose Against Infection, Hospitalization, or Death Among Nursing Home Residents—19 States, March 29-July 25, 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1235–1238. [Google Scholar] [CrossRef] [PubMed]
- Martín Pérez, C.; Aguilar, R.; Jiménez, A.; Salmerón, G.; Canyelles, M.; Rubio, R.; Vidal, M.; Cuamba, I.; Barrios, D.; Díaz, N.; et al. Correlates of protection and determinants of SARS-CoV-2 breakthrough infections 1 year after third dose vaccination. BMC Med. 2024, 22, 103. [Google Scholar] [CrossRef]
- Regev-Yochay, G.; Lustig, Y.; Joseph, G.; Gilboa, M.; Barda, N.; Gens, I.; Indenbaum, V.; Halpern, O.; Katz-Likvornik, S.; Levin, T.; et al. Correlates of protection against COVID-19 infection and intensity of symptomatic disease in vaccinated individuals exposed to SARS-CoV-2 in households in Israel (ICoFS): A prospective cohort study. Lancet Microbe 2023, 4, e309–e318. [Google Scholar] [CrossRef] [PubMed]
- Vikström, L.; Fjällström, P.; Gwon, Y.-D.; Sheward, D.J.; Wigren-Byström, J.; Evander, M.; Bladh, O.; Widerström, M.; Molnar, C.; Rasmussen, G.; et al. Vaccine-induced correlate of protection against fatal COVID-19 in older and frail adults during waves of neutralization-resistant variants of concern: An observational study. Lancet Reg. Health–Eur. 2023, 30, 100646. [Google Scholar] [CrossRef]
- Kemlin, D.; Gemander, N.; Depickère, S.; Olislagers, V.; Georges, D.; Waegemans, A.; Pannus, P.; Lemy, A.; Goossens, M.E.; Desombere, I.; et al. Humoral and cellular immune correlates of protection against COVID-19 in kidney transplant recipients. Am. J. Transplant. 2023, 23, 649–658. [Google Scholar] [CrossRef]
- Sun, Z.; Wu, T.; Xie, H.; Li, Y.; Zhang, J.; Su, X.; Qi, H. The Role of Cellular Immunity in the Protective Efficacy of the SARS-CoV-2 Vaccines. Vaccines 2022, 10, 1103. [Google Scholar] [CrossRef]

| Subset I (n = 200) | Subset II (n = 121) | Subset III (n = 411) | ||
|---|---|---|---|---|
| Median age (IQR) | 86 (81–91) | 89 (84–92) | 88 (81–91) | |
| Female, n (%) | 159 (80%) | 96 (79%) | 307 (75%) | |
| Infection-primed, n (%) | 81 (41%) | 4 (3%) | 137 (33%) | |
| Care dependency level, n (%) | Low care dependency(category O, A, and B) | 130 (65%) | 79 (65%) | 281 (68%) |
| High care dependency(category C, Cd, and D) | 70 (35%) | 42 (35%) | 128 (31%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meyers, E.; De Rop, L.; Gioveni, C.; Engels, F.; Coen, A.; De Burghgraeve, T.; Digregorio, M.; Van Ngoc, P.; De Clercq, N.; Buret, L.; et al. Dynamics of SARS-CoV-2 IgG in Nursing Home Residents in Belgium Throughout Three BNT162b2 Vaccination Rounds: 19-Month Follow-Up. Vaccines 2025, 13, 409. https://doi.org/10.3390/vaccines13040409
Meyers E, De Rop L, Gioveni C, Engels F, Coen A, De Burghgraeve T, Digregorio M, Van Ngoc P, De Clercq N, Buret L, et al. Dynamics of SARS-CoV-2 IgG in Nursing Home Residents in Belgium Throughout Three BNT162b2 Vaccination Rounds: 19-Month Follow-Up. Vaccines. 2025; 13(4):409. https://doi.org/10.3390/vaccines13040409
Chicago/Turabian StyleMeyers, Eline, Liselore De Rop, Claudia Gioveni, Fien Engels, Anja Coen, Tine De Burghgraeve, Marina Digregorio, Pauline Van Ngoc, Nele De Clercq, Laëtitia Buret, and et al. 2025. "Dynamics of SARS-CoV-2 IgG in Nursing Home Residents in Belgium Throughout Three BNT162b2 Vaccination Rounds: 19-Month Follow-Up" Vaccines 13, no. 4: 409. https://doi.org/10.3390/vaccines13040409
APA StyleMeyers, E., De Rop, L., Gioveni, C., Engels, F., Coen, A., De Burghgraeve, T., Digregorio, M., Van Ngoc, P., De Clercq, N., Buret, L., Coenen, S., Padalko, E., Duysburgh, E., Scholtes, B., Verbakel, J. Y., Heytens, S., & Cools, P. (2025). Dynamics of SARS-CoV-2 IgG in Nursing Home Residents in Belgium Throughout Three BNT162b2 Vaccination Rounds: 19-Month Follow-Up. Vaccines, 13(4), 409. https://doi.org/10.3390/vaccines13040409









