Beyond the Pandemic Era: Recent Advances and Efficacy of SARS-CoV-2 Vaccines Against Emerging Variants of Concern
Abstract
:1. Introduction
2. SARS-CoV-2 Variants of Concern, Evolution, and Impact on Public Health
3. Major SARS-CoV-2 Variants of Concern (VOCs) Emerged During the Pandemic Era
3.1. Alpha Variant (B.1.1.7)
3.2. Beta Variant (B.1.351)
3.3. Gamma Variant (P.1)
3.4. Delta Variant (B.1.617.2)
3.5. Omicron Variant (B.1.1.529)
4. Newly Emerged SARS-CoV-2 Variants in the Post-Pandemic Era
4.1. Spring–Summer 2022: Emergence of BA.4 and BA.5
4.2. Summer 2022: Emergence of BA.2.75
4.3. Autumn–Winter 2022: Emergence of BQ.1.1 and XBB Lineages
4.4. Winter 2022–Spring 2023: Emergence of XBB.1.5
4.5. Spring–Summer 2023: Emergence of EG.5.1
4.6. Summer 2023–Winter 2024: Emergence of “FLip” Variants
4.7. Summer 2023–2024: Emergence of JN.1 and Other BA.2.86 Lineages
4.8. August–October 2024: Emergence of XEC and KP.3.1.1
5. SARS-CoV-2 Vaccines: Platforms, Efficacy, and Performance Against Variants
5.1. SARS-CoV-2 Vaccine Platforms: First-Generation and Beyond
5.2. Vaccine Efficacy Against SARS-CoV-2 VOCs and Emerging Sub-Variants
5.3. Booster Doses and the Dynamics of Adaptive Immunity
5.4. Determinants of Vaccine Performance: Host, Viral, and Environmental Factors
6. Recent Advancements in Vaccine Technology with Enhanced Breadth of Protection
6.1. Multivalent Vaccines: Targeting Multiple SARS-CoV-2 Variants
6.2. Pan-Coronavirus Strategies: Broad-Spectrum Immunity Approaches
7. Next-Generation SARS-CoV-2 Vaccines: Addressing the Challenges of Emerging Variants
7.1. Design and Platforms of Next-Generation SARS-CoV-2 Vaccines
7.1.1. Exosome-Based Vaccines
7.1.2. Virus-like Particle (VLP) Vaccines
7.1.3. Mucosal Vaccines
7.1.4. Nanomaterial-Based Vaccines
7.2. Innovations in Vaccine Delivery Systems
7.3. Next-Generation Vaccines: Advancing Broader Immunological Protection and Durability
7.4. Next-Generation Vaccine Efficacy Against Emerging Sub-Variants
7.4.1. BA.2-Derived Sub-Variants: BJ.1 and BM.1.1.1
7.4.2. XBB Lineages: XBB.1.9, XBB.1.5, and XBB.1.16
7.4.3. BA.2.86 (Pirola) Sub-Variants
7.5. Enhancing Cross-Variant Immunity and Immune Memory Through Next-Generation and Heterologous Vaccination Strategies
7.6. Immune Imprinting and Next-Generation Vaccines
8. Discussion: Challenges and Future Perspectives
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Roknuzzaman, A.; Sarker, R.; Nazmunnahar; Shahriar, M.; Mosharrafa, R.A.; Islam, M.R. The WHO has Declared COVID-19 is No Longer a Pandemic-Level Threat: A Perspective Evaluating Potential Public Health Impacts. Clin. Pathol. 2024, 17, 2632010X241228053. [Google Scholar] [CrossRef] [PubMed]
- Helmy, Y.A.; Fawzy, M.; Elaswad, A.; Sobieh, A.; Kenney, S.P.; Shehata, A.A. The COVID-19 Pandemic: A Comprehensive Review of Taxonomy, Genetics, Epidemiology, Diagnosis, Treatment, and Control. J. Clin. Med. 2020, 9, 1225. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.M.; Wang, W.; Song, Z.G.; Hu, Y.; Tao, Z.W.; Tian, J.H.; Pei, Y.Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef]
- Rastogi, M.; Pandey, N.; Shukla, A.; Singh, S.K. SARS coronavirus 2: From genome to infectome. Respir. Res. 2020, 21, 318. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Flores, D.; Zepeda-Cervantes, J.; Cruz-Resendiz, A.; Aguirre-Sampieri, S.; Sampieri, A.; Vaca, L. SARS-CoV-2 Vaccines Based on the Spike Glycoprotein and Implications of New Viral Variants. Front. Immunol. 2021, 12, 701501. [Google Scholar] [CrossRef]
- Sette, A.; Crotty, S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 2021, 184, 861–880. [Google Scholar] [CrossRef]
- Morales-Nunez, J.J.; Munoz-Valle, J.F.; Torres-Hernandez, P.C.; Hernandez-Bello, J. Overview of Neutralizing Antibodies and Their Potential in COVID-19. Vaccines 2021, 9, 1376. [Google Scholar] [CrossRef]
- Takada, K.; Ueda, M.T.; Shichinohe, S.; Kida, Y.; Ono, C.; Matsuura, Y.; Watanabe, T.; Nakagawa, S. Genomic diversity of SARS-CoV-2 can be accelerated by mutations in the nsp14 gene. iScience 2023, 26, 106210. [Google Scholar] [CrossRef]
- Eckerle, L.D.; Becker, M.M.; Halpin, R.A.; Li, K.; Venter, E.; Lu, X.; Scherbakova, S.; Graham, R.L.; Baric, R.S.; Stockwell, T.B.; et al. Infidelity of SARS-CoV Nsp14-exonuclease mutant virus replication is revealed by complete genome sequencing. PLoS Pathog. 2010, 6, e1000896. [Google Scholar] [CrossRef]
- Kumar, R.; Srivastava, Y.; Muthuramalingam, P.; Singh, S.K.; Verma, G.; Tiwari, S.; Tandel, N.; Beura, S.K.; Panigrahi, A.R.; Maji, S.; et al. Understanding Mutations in Human SARS-CoV-2 Spike Glycoprotein: A Systematic Review & Meta-Analysis. Viruses 2023, 15, 856. [Google Scholar] [CrossRef]
- Evans, J.P.; Zeng, C.; Qu, P.; Faraone, J.; Zheng, Y.M.; Carlin, C.; Bednash, J.S.; Zhou, T.; Lozanski, G.; Mallampalli, R.; et al. Neutralization of SARS-CoV-2 Omicron sub-lineages BA.1, BA.1.1, and BA.2. Cell Host Microbe 2022, 30, 1093–1102.e1093. [Google Scholar] [CrossRef] [PubMed]
- Lustig, Y.; Zuckerman, N.; Nemet, I.; Atari, N.; Kliker, L.; Regev-Yochay, G.; Sapir, E.; Mor, O.; Alroy-Preis, S.; Mendelson, E.; et al. Neutralising capacity against Delta (B.1.617.2) and other variants of concern following Comirnaty (BNT162b2, BioNTech/Pfizer) vaccination in health care workers, Israel. Euro Surveill. 2021, 26, 2100557. [Google Scholar] [CrossRef] [PubMed]
- Cantoni, D.; Mayora-Neto, M.; Nadesalingam, A.; Wells, D.A.; Carnell, G.W.; Ohlendorf, L.; Ferrari, M.; Palmer, P.; Chan, A.C.Y.; Smith, P.; et al. Neutralisation Hierarchy of SARS-CoV-2 Variants of Concern Using Standardised, Quantitative Neutralisation Assays Reveals a Correlation With Disease Severity; Towards Deciphering Protective Antibody Thresholds. Front. Immunol. 2022, 13, 773982. [Google Scholar] [CrossRef]
- Sanchez-Sendra, B.; Albert, E.; Zulaica, J.; Torres, I.; Gimenez, E.; Botija, P.; Beltran, M.J.; Rodado, C.; Geller, R.; Navarro, D. Neutralizing antibodies against SARS-CoV-2 variants of concern elicited by the comirnaty COVID-19 vaccine in nursing home residents. Sci. Rep. 2022, 12, 3788. [Google Scholar] [CrossRef]
- Kumar, A.; Tripathi, P.; Kumar, P.; Shekhar, R.; Pathak, R. From Detection to Protection: Antibodies and Their Crucial Role in Diagnosing and Combatting SARS-CoV-2. Vaccines 2024, 12, 459. [Google Scholar] [CrossRef]
- Miller, J.; Hachmann, N.P.; Collier, A.Y.; Lasrado, N.; Mazurek, C.R.; Patio, R.C.; Powers, O.; Surve, N.; Theiler, J.; Korber, B.; et al. Substantial Neutralization Escape by SARS-CoV-2 Omicron Variants BQ.1.1 and XBB.1. N. Engl. J. Med. 2023, 388, 662–664. [Google Scholar] [CrossRef]
- Wang, Q.; Iketani, S.; Li, Z.; Liu, L.; Guo, Y.; Huang, Y.; Bowen, A.D.; Liu, M.; Wang, M.; Yu, J.; et al. Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants. Cell 2023, 186, 279–286.e278. [Google Scholar] [CrossRef]
- Reeve, L.; Tessier, E.; Trindall, A.; Abdul Aziz, N.I.B.; Andrews, N.; Futschik, M.; Rayner, J.; Didier’Serre, A.; Hams, R.; Groves, N.; et al. High attack rate in a large care home outbreak of SARS-CoV-2 BA.2.86, East of England, August 2023. Euro Surveill. 2023, 28, 2300489. [Google Scholar] [CrossRef] [PubMed]
- Planas, D.; Staropoli, I.; Michel, V.; Lemoine, F.; Donati, F.; Prot, M.; Porrot, F.; Guivel-Benhassine, F.; Jeyarajah, B.; Brisebarre, A.; et al. Distinct evolution of SARS-CoV-2 Omicron XBB and BA.2.86/JN.1 lineages combining increased fitness and antibody evasion. Nat. Commun. 2024, 15, 2254. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, Y.; Liu, L.; Schwanz, L.T.; Li, Z.; Nair, M.S.; Ho, J.; Zhang, R.M.; Iketani, S.; Yu, J.; et al. Antigenicity and receptor affinity of SARS-CoV-2 BA.2.86 spike. Nature 2023, 624, 639–644. [Google Scholar] [CrossRef]
- Yang, S.; Yu, Y.; Jian, F.; Song, W.; Yisimayi, A.; Chen, X.; Xu, Y.; Wang, P.; Wang, J.; Yu, L.; et al. Antigenicity and infectivity characterisation of SARS-CoV-2 BA.2.86. Lancet Infect. Dis. 2023, 23, e457–e459. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Kempf, A.; Nehlmeier, I.; Cossmann, A.; Richter, A.; Bdeir, N.; Graichen, L.; Moldenhauer, A.S.; Dopfer-Jablonka, A.; Stankov, M.V.; et al. SARS-CoV-2 BA.2.86 enters lung cells and evades neutralizing antibodies with high efficiency. Cell 2024, 187, 596–608.e517. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.C.; Castro, J.; Lambrou, A.S.; Rose, E.B.; Cook, P.W.; Batra, D.; Cubenas, C.; Hughes, L.J.; MacCannell, D.R.; Mandal, P.; et al. Genomic Surveillance for SARS-CoV-2 Variants: Circulation of Omicron XBB and JN.1 Lineages—United States, May 2023–September 2024. MMWR Morb. Mortal. Wkly. Rep. 2024, 73, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Panagiotakopoulos, L.; Moulia, D.L.; Godfrey, M.; Link-Gelles, R.; Roper, L.; Havers, F.P.; Taylor, C.A.; Stokley, S.; Talbot, H.K.; Schechter, R.; et al. Use of COVID-19 Vaccines for Persons Aged >/=6 Months: Recommendations of the Advisory Committee on Immunization Practices—United States, 2024-2025. MMWR Morb. Mortal. Wkly. Rep. 2024, 73, 819–824. [Google Scholar] [CrossRef]
- Herwig, S.; Adler, J.M.; Vladimirova, D.; Trimpert, J.; Sehouli, J.; Cichon, G. Improving the Antigenicity of SARS-CoV-2 Vaccine Genes by Merging Mutations from Different Variants of Concern. Vaccines 2024, 12, 248. [Google Scholar] [CrossRef]
- Sheikh-Mohamed, S.; Isho, B.; Chao, G.Y.C.; Zuo, M.; Cohen, C.; Lustig, Y.; Nahass, G.R.; Salomon-Shulman, R.E.; Blacker, G.; Fazel-Zarandi, M.; et al. Systemic and mucosal IgA responses are variably induced in response to SARS-CoV-2 mRNA vaccination and are associated with protection against subsequent infection. Mucosal Immunol. 2022, 15, 799–808. [Google Scholar] [CrossRef]
- Sano, K.; Bhavsar, D.; Singh, G.; Floda, D.; Srivastava, K.; Gleason, C.; Group, P.S.; Carreno, J.M.; Simon, V.; Krammer, F. SARS-CoV-2 vaccination induces mucosal antibody responses in previously infected individuals. Nat. Commun. 2022, 13, 5135. [Google Scholar] [CrossRef]
- Fraser, R.; Orta-Resendiz, A.; Mazein, A.; Dockrell, D.H. Upper respiratory tract mucosal immunity for SARS-CoV-2 vaccines. Trends Mol. Med. 2023, 29, 255–267. [Google Scholar] [CrossRef]
- Hassan, A.O.; Shrihari, S.; Gorman, M.J.; Ying, B.; Yuan, D.; Raju, S.; Chen, R.E.; Dmitriev, I.P.; Kashentseva, E.; Adams, L.J.; et al. An intranasal vaccine durably protects against SARS-CoV-2 variants in mice. Cell Rep. 2021, 36, 109452. [Google Scholar] [CrossRef]
- Hassan, A.O.; Feldmann, F.; Zhao, H.; Curiel, D.T.; Okumura, A.; Tang-Huau, T.L.; Case, J.B.; Meade-White, K.; Callison, J.; Chen, R.E.; et al. A single intranasal dose of chimpanzee adenovirus-vectored vaccine protects against SARS-CoV-2 infection in rhesus macaques. Cell Rep. Med. 2021, 2, 100230. [Google Scholar] [CrossRef]
- Bricker, T.L.; Darling, T.L.; Hassan, A.O.; Harastani, H.H.; Soung, A.; Jiang, X.; Dai, Y.N.; Zhao, H.; Adams, L.J.; Holtzman, M.J.; et al. A single intranasal or intramuscular immunization with chimpanzee adenovirus-vectored SARS-CoV-2 vaccine protects against pneumonia in hamsters. Cell Rep. 2021, 36, 109400. [Google Scholar] [CrossRef] [PubMed]
- van Doremalen, N.; Purushotham, J.N.; Schulz, J.E.; Holbrook, M.G.; Bushmaker, T.; Carmody, A.; Port, J.R.; Yinda, C.K.; Okumura, A.; Saturday, G.; et al. Intranasal ChAdOx1 nCoV-19/AZD1222 vaccination reduces viral shedding after SARS-CoV-2 D614G challenge in preclinical models. Sci. Transl. Med. 2021, 13, eabh0755. [Google Scholar] [CrossRef]
- Mao, T.; Israelow, B.; Pena-Hernandez, M.A.; Suberi, A.; Zhou, L.; Luyten, S.; Reschke, M.; Dong, H.; Homer, R.J.; Saltzman, W.M.; et al. Unadjuvanted intranasal spike vaccine elicits protective mucosal immunity against sarbecoviruses. Science 2022, 378, eabo2523. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, L.; Liu, J.; Fang, E.; Liu, X.; Peng, Q.; Zhang, Z.; Li, M.; Liu, X.; Wu, X.; et al. Combining intramuscular and intranasal homologous prime-boost with a chimpanzee adenovirus-based COVID-19 vaccine elicits potent humoral and cellular immune responses in mice. Emerg. Microbes Infect. 2022, 11, 1890–1899. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Kar, S.; Chawla, B.; Hoang, T.; Yu, Y.; Wallace, S.M.; Andersen, H.; Berzofsky, J.A. Adjuvanted subunit intranasal vaccine prevents SARS-CoV-2 onward transmission in hamsters. bioRxiv 2024. [Google Scholar] [CrossRef]
- McMahan, K.; Wegmann, F.; Aid, M.; Sciacca, M.; Liu, J.; Hachmann, N.P.; Miller, J.; Jacob-Dolan, C.; Powers, O.; Hope, D.; et al. Mucosal boosting enhances vaccine protection against SARS-CoV-2 in macaques. Nature 2024, 626, 385–391. [Google Scholar] [CrossRef]
- Saraf, A.; Gurjar, R.; Kaviraj, S.; Kulkarni, A.; Kumar, D.; Kulkarni, R.; Virkar, R.; Krishnan, J.; Yadav, A.; Baranwal, E.; et al. An Omicron-specific, self-amplifying mRNA booster vaccine for COVID-19: A phase 2/3 randomized trial. Nat. Med. 2024, 30, 1363–1372. [Google Scholar] [CrossRef]
- Maruggi, G.; Mallett, C.P.; Westerbeck, J.W.; Chen, T.; Lofano, G.; Friedrich, K.; Qu, L.; Sun, J.T.; McAuliffe, J.; Kanitkar, A.; et al. A self-amplifying mRNA SARS-CoV-2 vaccine candidate induces safe and robust protective immunity in preclinical models. Mol. Ther. 2022, 30, 1897–1912. [Google Scholar] [CrossRef]
- Moore, K.A.; Leighton, T.; Ostrowsky, J.T.; Anderson, C.J.; Danila, R.N.; Ulrich, A.K.; Lackritz, E.M.; Mehr, A.J.; Baric, R.S.; Baylor, N.W.; et al. A research and development (R&D) roadmap for broadly protective coronavirus vaccines: A pandemic preparedness strategy. Vaccine 2023, 41, 2101–2112. [Google Scholar] [CrossRef]
- Chakraborty, S. E484K and N501Y SARS-CoV 2 spike mutants Increase ACE2 recognition but reduce affinity for neutralizing antibody. Int. Immunopharmacol. 2022, 102, 108424. [Google Scholar] [CrossRef]
- Jangra, S.; Ye, C.; Rathnasinghe, R.; Stadlbauer, D.; Personalized Virology Initiative Study Group; Krammer, F.; Simon, V.; Martinez-Sobrido, L.; Garcia-Sastre, A.; Schotsaert, M. SARS-CoV-2 spike E484K mutation reduces antibody neutralisation. Lancet Microbe 2021, 2, e283–e284. [Google Scholar] [CrossRef] [PubMed]
- Wrobel, A.G.; Benton, D.J.; Roustan, C.; Borg, A.; Hussain, S.; Martin, S.R.; Rosenthal, P.B.; Skehel, J.J.; Gamblin, S.J. Evolution of the SARS-CoV-2 spike protein in the human host. Nat. Commun. 2022, 13, 1178. [Google Scholar] [CrossRef]
- Raglow, Z.; Surie, D.; Chappell, J.D.; Zhu, Y.; Martin, E.T.; Kwon, J.H.; Frosch, A.E.; Mohamed, A.; Gilbert, J.; Bendall, E.E.; et al. SARS-CoV-2 shedding and evolution in patients who were immunocompromised during the omicron period: A multicentre, prospective analysis. Lancet Microbe 2024, 5, e235–e246. [Google Scholar] [CrossRef]
- Liu, W.; Huang, Z.; Xiao, J.; Wu, Y.; Xia, N.; Yuan, Q. Evolution of the SARS-CoV-2 Omicron Variants: Genetic Impact on Viral Fitness. Viruses 2024, 16, 184. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, P.; Mishra, S.K.; Behera, S.P.; Zaman, K.; Kant, R.; Singh, R. Genomic evolution of the SARS-CoV-2 Variants of Concern: COVID-19 pandemic waves in India. EXCLI J. 2023, 22, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.M.; et al. COVID-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef]
- Lopez Bernal, J.; Andrews, N.; Gower, C.; Gallagher, E.; Simmons, R.; Thelwall, S.; Stowe, J.; Tessier, E.; Groves, N.; Dabrera, G.; et al. Effectiveness of COVID-19 Vaccines against the B.1.617.2 (Delta) Variant. N. Engl. J. Med. 2021, 385, 585–594. [Google Scholar] [CrossRef]
- Emary, K.R.W.; Golubchik, T.; Aley, P.K.; Ariani, C.V.; Angus, B.; Bibi, S.; Blane, B.; Bonsall, D.; Cicconi, P.; Charlton, S.; et al. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): An exploratory analysis of a randomised controlled trial. Lancet 2021, 397, 1351–1362. [Google Scholar] [CrossRef]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cardenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against COVID-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef]
- Madhi, S.A.; Baillie, V.; Cutland, C.L.; Voysey, M.; Koen, A.L.; Fairlie, L.; Padayachee, S.D.; Dheda, K.; Barnabas, S.L.; Bhorat, Q.E.; et al. Efficacy of the ChAdOx1 nCoV-19 COVID-19 Vaccine against the B.1.351 Variant. N. Engl. J. Med. 2021, 384, 1885–1898. [Google Scholar] [CrossRef]
- Hitchings, M.D.T.; Ranzani, O.T.; Torres, M.S.S.; de Oliveira, S.B.; Almiron, M.; Said, R.; Borg, R.; Schulz, W.L.; de Oliveira, R.D.; da Silva, P.V.; et al. Effectiveness of CoronaVac among healthcare workers in the setting of high SARS-CoV-2 Gamma variant transmission in Manaus, Brazil: A test-negative case-control study. Lancet Reg. Health Am. 2021, 1, 100025. [Google Scholar] [CrossRef] [PubMed]
- Jara, A.; Undurraga, E.A.; Gonzalez, C.; Paredes, F.; Fontecilla, T.; Jara, G.; Pizarro, A.; Acevedo, J.; Leo, K.; Leon, F.; et al. Effectiveness of an Inactivated SARS-CoV-2 Vaccine in Chile. N. Engl. J. Med. 2021, 385, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R.; Sobieszczyk, M.E.; Hirsch, I.; Sproule, S.; Robb, M.L.; Corey, L.; Neuzil, K.M.; Hahn, W.; Hunt, J.; Mulligan, M.J.; et al. Phase 3 Safety and Efficacy of AZD1222 (ChAdOx1 nCoV-19) COVID-19 Vaccine. N. Engl. J. Med. 2021, 385, 2348–2360. [Google Scholar] [CrossRef] [PubMed]
- Mahadevaiah, A.; Doddamadaiah, C.; Sadananda, K.S.; Cholenahalli Nanjappa, M. Study of immunogenicity, safety and efficacy of covishield vaccine among health care workers in a tertiary cardiac care centre. Indian J. Med. Microbiol. 2022, 40, 200–203. [Google Scholar] [CrossRef]
- Collie, S.; Nayager, J.; Bamford, L.; Bekker, L.G.; Zylstra, M.; Gray, G. Effectiveness and Durability of the BNT162b2 Vaccine against Omicron Sublineages in South Africa. N. Engl. J. Med. 2022, 387, 1332–1333. [Google Scholar] [CrossRef]
- Filip, R.; Gheorghita Puscaselu, R.; Anchidin-Norocel, L.; Dimian, M.; Savage, W.K. Global Challenges to Public Health Care Systems during the COVID-19 Pandemic: A Review of Pandemic Measures and Problems. J. Pers. Med. 2022, 12, 1295. [Google Scholar] [CrossRef]
- Manathunga, S.S.; Abeyagunawardena, I.A.; Dharmaratne, S.D. A comparison of transmissibility of SARS-CoV-2 variants of concern. Virol. J. 2023, 20, 59. [Google Scholar] [CrossRef]
- Kherabi, Y.; Launay, O.; Luong Nguyen, L.B. COVID-19 Vaccines against Omicron Variant: Real-World Data on Effectiveness. Viruses 2022, 14, 2086. [Google Scholar] [CrossRef]
- Kumari, M.; Lu, R.M.; Li, M.C.; Huang, J.L.; Hsu, F.F.; Ko, S.H.; Ke, F.Y.; Su, S.C.; Liang, K.H.; Yuan, J.P.; et al. A critical overview of current progress for COVID-19: Development of vaccines, antiviral drugs, and therapeutic antibodies. J. Biomed. Sci. 2022, 29, 68. [Google Scholar] [CrossRef]
- Hedberg, P.; Parczewski, M.; Serwin, K.; Marchetti, G.; Bai, F.; Ole Jensen, B.E.; Pereira, J.P.V.; Drobniewski, F.; Reschreiter, H.; Naumovas, D.; et al. In-hospital mortality during the wild-type, alpha, delta, and omicron SARS-CoV-2 waves: A multinational cohort study in the EuCARE project. Lancet Reg. Health Eur. 2024, 38, 100855. [Google Scholar] [CrossRef]
- Zali, A.; Khodadoost, M.; Gholamzadeh, S.; Janbazi, S.; Piri, H.; Taraghikhah, N.; Hannani, K.; Looha, M.A.; Mohammadi, G. Mortality among hospitalized COVID-19 patients during surges of SARS-CoV-2 alpha (B.1.1.7) and delta (B.1.617.2) variants. Sci. Rep. 2022, 12, 18918. [Google Scholar] [CrossRef]
- Fairlie, R. The impact of COVID-19 on small business owners: Evidence from the first three months after widespread social-distancing restrictions. J. Econ. Manag. Strategy 2020, 29, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Naseer, S.; Khalid, S.; Parveen, S.; Abbass, K.; Song, H.; Achim, M.V. COVID-19 outbreak: Impact on global economy. Front. Public Health 2022, 10, 1009393. [Google Scholar] [CrossRef]
- Lambrou, A.S.; South, E.; Ballou, E.S.; Paden, C.R.; Fuller, J.A.; Bart, S.M.; Butryn, D.M.; Novak, R.T.; Browning, S.D.; Kirby, A.E.; et al. Early Detection and Surveillance of the SARS-CoV-2 Variant BA.2.86—Worldwide, July-October 2023. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 1162–1167. [Google Scholar] [CrossRef] [PubMed]
- Davidopoulou, C.; Kouvelas, D.; Ouranidis, A. COMPARING vaccine manufacturing technologies recombinant DNA vs in vitro transcribed (IVT) mRNA. Sci. Rep. 2024, 14, 21742. [Google Scholar] [CrossRef]
- Rohner, E.; Yang, R.; Foo, K.S.; Goedel, A.; Chien, K.R. Unlocking the promise of mRNA therapeutics. Nat. Biotechnol. 2022, 40, 1586–1600. [Google Scholar] [CrossRef]
- Chandler, R.E.; Balakrishnan, M.R.; Brasseur, D.; Bryan, P.; Espie, E.; Hartmann, K.; Jouquelet-Royer, C.; Milligan, J.; Nesbitt, L.; Pal, S.; et al. Collaboration within the global vaccine safety surveillance ecosystem during the COVID-19 pandemic: Lessons learnt and key recommendations from the COVAX Vaccine Safety Working Group. BMJ Glob. Health 2024, 9, e014544. [Google Scholar] [CrossRef]
- Singh, M.; de Wit, E. Antiviral agents for the treatment of COVID-19: Progress and challenges. Cell Rep. Med. 2022, 3, 100549. [Google Scholar] [CrossRef] [PubMed]
- Haddad, A.J.; Hachem, R.Y.; Moussa, M.; Jiang, Y.; Dagher, H.R.; Chaftari, P.; Chaftari, A.M.; Raad, I.I. Comparing Molnupiravir to Nirmatrelvir/Ritonavir (Paxlovid) in the Treatment of Mild-to-Moderate COVID-19 in Immunocompromised Cancer Patients. Cancers 2024, 16, 1055. [Google Scholar] [CrossRef]
- Alhamlan, F.S.; Al-Qahtani, A.A. SARS-CoV-2 Variants: Genetic Insights, Epidemiological Tracking, and Implications for Vaccine Strategies. Int. J. Mol. Sci. 2025, 26, 1263. [Google Scholar] [CrossRef]
- Tian, F.; Tong, B.; Sun, L.; Shi, S.; Zheng, B.; Wang, Z.; Dong, X.; Zheng, P. N501Y mutation of spike protein in SARS-CoV-2 strengthens its binding to receptor ACE2. Elife 2021, 10, e69091. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, J.; Plante, K.S.; Plante, J.A.; Xie, X.; Zhang, X.; Ku, Z.; An, Z.; Scharton, D.; Schindewolf, C.; et al. The N501Y spike substitution enhances SARS-CoV-2 transmission. bioRxiv 2021. [Google Scholar] [CrossRef]
- Bashir, A.; Li, S.; Ye, Y.; Zheng, Q.; Knanghat, R.; Bashir, F.; Shah, N.N.; Yang, D.; Xue, M.; Wang, H.; et al. SARS-CoV-2 S protein harbors furin cleavage site located in a short loop between antiparallel beta-strand. Int. J. Biol. Macromol. 2024, 281, 136020. [Google Scholar] [CrossRef]
- Walker, A.S.; Vihta, K.D.; Gethings, O.; Pritchard, E.; Jones, J.; House, T.; Bell, I.; Bell, J.I.; Newton, J.N.; Farrar, J.; et al. Tracking the Emergence of SARS-CoV-2 Alpha Variant in the United Kingdom. N. Engl. J. Med. 2021, 385, 2582–2585. [Google Scholar] [CrossRef] [PubMed]
- Earnest, R.; Uddin, R.; Matluk, N.; Renzette, N.; Siddle, K.J.; Loreth, C.; Adams, G.; Tomkins-Tinch, C.H.; Petrone, M.E.; Rothman, J.E.; et al. Comparative transmissibility of SARS-CoV-2 variants Delta and Alpha in New England, USA. medRxiv 2021. [Google Scholar] [CrossRef]
- Carabelli, A.M.; Peacock, T.P.; Thorne, L.G.; Harvey, W.T.; Hughes, J.; Consortium, C.-G.U.; Peacock, S.J.; Barclay, W.S.; de Silva, T.I.; Towers, G.J.; et al. SARS-CoV-2 variant biology: Immune escape, transmission and fitness. Nat. Rev. Microbiol. 2023, 21, 162–177. [Google Scholar] [CrossRef]
- Grint, D.J.; Wing, K.; Houlihan, C.; Gibbs, H.P.; Evans, S.J.W.; Williamson, E.; McDonald, H.I.; Bhaskaran, K.; Evans, D.; Walker, A.J.; et al. Severity of Severe Acute Respiratory System Coronavirus 2 (SARS-CoV-2) Alpha Variant (B.1.1.7) in England. Clin. Infect. Dis. 2022, 75, e1120–e1127. [Google Scholar] [CrossRef]
- Petrie, J.G.; Eisenberg, M.C.; Lauring, A.S.; Gilbert, J.; Harrison, S.M.; DeJonge, P.M.; Martin, E.T. The variant-specific burden of SARS-CoV-2 in Michigan: March 2020 through November 2021. J. Med. Virol. 2022, 94, 5251–5259. [Google Scholar] [CrossRef]
- Duong, D. Alpha, Beta, Delta, Gamma: What’s important to know about SARS-CoV-2 variants of concern? CMAJ 2021, 193, E1059–E1060. [Google Scholar] [CrossRef]
- Jin, P.; Zhu, F. Could Beta variant containing COVID-19 booster vaccines tackle Omicron variants? Lancet Reg. Health Eur. 2023, 28, 100623. [Google Scholar] [CrossRef]
- Pavot, V.; Berry, C.; Kishko, M.; Anosova, N.G.; Li, L.; Tibbitts, T.; Huang, D.; Raillard, A.; Gautheron, S.; Gutzeit, C.; et al. Beta variant COVID-19 protein booster vaccine elicits durable cross-neutralization against SARS-CoV-2 variants in non-human primates. Nat. Commun. 2023, 14, 1309. [Google Scholar] [CrossRef]
- Spira, B. The Impact of the Highly Virulent SARS-CoV-2 Gamma Variant on Young Adults in the State of Sao Paulo: Was It Inevitable? Cureus 2022, 14, e26486. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Herrera, N.; Araujo-Castillo, R.V.; Mestanza, O.; Galarza, M.; Rojas-Serrano, N.; Solari-Zerpa, L. SARS-CoV-2 Lambda and Gamma variants competition in Peru, a country with high seroprevalence. Lancet Reg. Health Am. 2022, 6, 100112. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.F.; Esteves, R.J.; Siza, C.; Soares, E.P.; Ramos, T.C.; Campelo, E.C.; da Costa, C.F.; de Alencar, L.C.; Cavalcante, R.P.; Florencio, C.R.; et al. Cluster of SARS-CoV-2 Gamma Variant Infections, Parintins, Brazil, March 2021. Emerg. Infect. Dis. 2022, 28, 262–264. [Google Scholar] [CrossRef] [PubMed]
- Banho, C.A.; Sacchetto, L.; Campos, G.R.F.; Bittar, C.; Possebon, F.S.; Ullmann, L.S.; Marques, B.C.; da Silva, G.C.D.; Moraes, M.M.; Parra, M.C.P.; et al. Impact of SARS-CoV-2 Gamma lineage introduction and COVID-19 vaccination on the epidemiological landscape of a Brazilian city. Commun. Med. 2022, 2, 41. [Google Scholar] [CrossRef]
- Dhawan, M.; Sharma, A.; Priyanka; Thakur, N.; Rajkhowa, T.K.; Choudhary, O.P. Delta variant (B.1.617.2) of SARS-CoV-2: Mutations, impact, challenges and possible solutions. Hum. Vaccines Immunother. 2022, 18, 2068883. [Google Scholar] [CrossRef]
- Zhang, J.; Fan, L.; Xu, H.; Fu, Y.; Peng, X.; Zheng, Y.; Yu, J.; He, J. Evolutionary Pattern Comparisons of the SARS-CoV-2 Delta Variant in Countries/Regions with High and Low Vaccine Coverage. Viruses 2022, 14, 2296. [Google Scholar] [CrossRef]
- Novelli, G.; Colona, V.L.; Pandolfi, P.P. A focus on the spread of the delta variant of SARS-CoV-2 in India. Indian J. Med. Res. 2021, 153, 537–541. [Google Scholar] [CrossRef]
- Yang, W.; Shaman, J. COVID-19 pandemic dynamics in India, the SARS-CoV-2 Delta variant, and implications for vaccination. medRxiv 2021. [Google Scholar] [CrossRef]
- Cella, E.; Ali, S.; Schmedes, S.E.; Rife Magalis, B.; Marini, S.; Salemi, M.; Blanton, J.; Azarian, T. Early Emergence Phase of SARS-CoV-2 Delta Variant in Florida, US. Viruses 2022, 14, 766. [Google Scholar] [CrossRef]
- Viana, R.; Moyo, S.; Amoako, D.G.; Tegally, H.; Scheepers, C.; Althaus, C.L.; Anyaneji, U.J.; Bester, P.A.; Boni, M.F.; Chand, M.; et al. Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa. Nature 2022, 603, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Al-Thani, H.; El-Menyar, A. The emergence of new SARS-CoV-2 variant (Omicron) and increasing calls for COVID-19 vaccine boosters-The debate continues. Travel. Med. Infect. Dis. 2022, 45, 102246. [Google Scholar] [CrossRef]
- Daria, S.; Islam, M.R. The SARS-CoV-2 omicron wave is indicating the end of the pandemic phase but the COVID-19 will continue. J. Med. Virol. 2022, 94, 2343–2345. [Google Scholar] [CrossRef]
- Lorenzo-Redondo, R.; de Sant’Anna Carvalho, A.M.; Hultquist, J.F.; Ozer, E.A. SARS-CoV-2 genomics and impact on clinical care for COVID-19. J. Antimicrob. Chemother. 2023, 78, ii25–ii36. [Google Scholar] [CrossRef] [PubMed]
- Tegally, H.; Moir, M.; Everatt, J.; Giovanetti, M.; Scheepers, C.; Wilkinson, E.; Subramoney, K.; Makatini, Z.; Moyo, S.; Amoako, D.G.; et al. Emergence of SARS-CoV-2 Omicron lineages BA.4 and BA.5 in South Africa. Nat. Med. 2022, 28, 1785–1790. [Google Scholar] [CrossRef]
- Kimura, I.; Yamasoba, D.; Tamura, T.; Nao, N.; Suzuki, T.; Oda, Y.; Mitoma, S.; Ito, J.; Nasser, H.; Zahradnik, J.; et al. Virological characteristics of the SARS-CoV-2 Omicron BA.2 subvariants, including BA.4 and BA.5. Cell 2022, 185, 3992–4007.e3916. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Tamura, T.; Zahradnik, J.; Deguchi, S.; Tabata, K.; Anraku, Y.; Kimura, I.; Ito, J.; Yamasoba, D.; Nasser, H.; et al. Virological characteristics of the SARS-CoV-2 Omicron BA.2.75 variant. Cell Host Microbe 2022, 30, 1540–1555.e1515. [Google Scholar] [CrossRef]
- Motozono, C.; Toyoda, M.; Zahradnik, J.; Saito, A.; Nasser, H.; Tan, T.S.; Ngare, I.; Kimura, I.; Uriu, K.; Kosugi, Y.; et al. SARS-CoV-2 spike L452R variant evades cellular immunity and increases infectivity. Cell Host Microbe 2021, 29, 1124–1136.e1111. [Google Scholar] [CrossRef]
- Focosi, D.; Quiroga, R.; McConnell, S.; Johnson, M.C.; Casadevall, A. Convergent Evolution in SARS-CoV-2 Spike Creates a Variant Soup from Which New COVID-19 Waves Emerge. Int. J. Mol. Sci. 2023, 24, 2264. [Google Scholar] [CrossRef]
- Ito, J.; Suzuki, R.; Uriu, K.; Itakura, Y.; Zahradnik, J.; Kimura, K.T.; Deguchi, S.; Wang, L.; Lytras, S.; Tamura, T.; et al. Convergent evolution of SARS-CoV-2 Omicron subvariants leading to the emergence of BQ.1.1 variant. Nat. Commun. 2023, 14, 2671. [Google Scholar] [CrossRef]
- Tamura, T.; Ito, J.; Uriu, K.; Zahradnik, J.; Kida, I.; Anraku, Y.; Nasser, H.; Shofa, M.; Oda, Y.; Lytras, S.; et al. Virological characteristics of the SARS-CoV-2 XBB variant derived from recombination of two Omicron subvariants. Nat. Commun. 2023, 14, 2800. [Google Scholar] [CrossRef] [PubMed]
- Uriu, K.; Ito, J.; Zahradnik, J.; Fujita, S.; Kosugi, Y.; Schreiber, G.; The Genotype to Phenotype Japan (G2P-Japan) Consortium; Sato, K. Enhanced transmissibility, infectivity, and immune resistance of the SARS-CoV-2 omicron XBB.1.5 variant. Lancet Infect. Dis. 2023, 23, 280–281. [Google Scholar] [CrossRef] [PubMed]
- Tsujino, S.; Deguchi, S.; Nomai, T.; Padilla-Blanco, M.; Plianchaisuk, A.; Wang, L.; Begum, M.M.; Uriu, K.; Mizuma, K.; Nao, N.; et al. Virological characteristics of the SARS-CoV-2 Omicron EG.5.1 variant. Microbiol. Immunol. 2024, 68, 305–330. [Google Scholar] [CrossRef]
- Focosi, D.; Spezia, P.G.; Gueli, F.; Maggi, F. The Era of the FLips: How Spike Mutations L455F and F456L (and A475V) Are Shaping SARS-CoV-2 Evolution. Viruses 2023, 16, 3. [Google Scholar] [CrossRef]
- Kosugi, Y.; Plianchaisuk, A.; Putri, O.; Uriu, K.; Kaku, Y.; Hinay, A.A., Jr.; Chen, L.; Kuramochi, J.; Sadamasu, K.; Yoshimura, K.; et al. Characteristics of the SARS-CoV-2 omicron HK.3 variant harbouring the FLip substitution. Lancet Microbe 2024, 5, e313. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, X.; Shi, J.; Wang, Y.; Liu, H.; Hu, Y.F.; Hu, B.; Shuai, H.; Yuen, T.T.; Chai, Y.; et al. Lineage-specific pathogenicity, immune evasion, and virological features of SARS-CoV-2 BA.2.86/JN.1 and EG.5.1/HK.3. Nat. Commun. 2024, 15, 8728. [Google Scholar] [CrossRef]
- Kaku, Y.; Okumura, K.; Padilla-Blanco, M.; Kosugi, Y.; Uriu, K.; Hinay, A.A., Jr.; Chen, L.; Plianchaisuk, A.; Kobiyama, K.; Ishii, K.J.; et al. Virological characteristics of the SARS-CoV-2 JN.1 variant. Lancet Infect. Dis. 2024, 24, e82. [Google Scholar] [CrossRef]
- He, Q.; An, Y.; Zhou, X.; Xie, H.; Tao, L.; Li, D.; Zheng, A.; Li, L.; Xu, Z.; Yu, S.; et al. Neutralization of EG.5, EG.5.1, BA.2.86, and JN.1 by antisera from dimeric receptor-binding domain subunit vaccines and 41 human monoclonal antibodies. Med 2024, 5, 401–413.e404. [Google Scholar] [CrossRef]
- Kaku, Y.; Okumura, K.; Kawakubo, S.; Uriu, K.; Chen, L.; Kosugi, Y.; Uwamino, Y.; Begum, M.M.; Leong, S.; Ikeda, T.; et al. Virological characteristics of the SARS-CoV-2 XEC variant. Lancet Infect. Dis. 2024, 24, e736. [Google Scholar] [CrossRef]
- Kaku, Y.; Uriu, K.; Okumura, K.; The Genotype to Phenotype Japan (G2P-Japan) Consortium; Ito, J.; Sato, K. Virological characteristics of the SARS-CoV-2 KP.3.1.1 variant. Lancet Infect. Dis. 2024, 24, e609. [Google Scholar] [CrossRef]
- Bagcchi, S. The world’s largest COVID-19 vaccination campaign. Lancet Infect. Dis. 2021, 21, 323. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.E.; Frenck, R.W., Jr.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and Immunogenicity of Two RNA-Based COVID-19 Vaccine Candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Hacisuleyman, E.; Hale, C.; Saito, Y.; Blachere, N.E.; Bergh, M.; Conlon, E.G.; Schaefer-Babajew, D.J.; DaSilva, J.; Muecksch, F.; Gaebler, C.; et al. Vaccine Breakthrough Infections with SARS-CoV-2 Variants. N. Engl. J. Med. 2021, 384, 2212–2218. [Google Scholar] [CrossRef]
- Rauch, S.; Roth, N.; Schwendt, K.; Fotin-Mleczek, M.; Mueller, S.O.; Petsch, B. mRNA-based SARS-CoV-2 vaccine candidate CVnCoV induces high levels of virus-neutralising antibodies and mediates protection in rodents. NPJ Vaccines 2021, 6, 57. [Google Scholar] [CrossRef]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An mRNA Vaccine against SARS-CoV-2—Preliminary Report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Hogan, M.J.; Naradikian, M.S.; Parkhouse, K.; Cain, D.W.; Jones, L.; Moody, M.A.; Verkerke, H.P.; Myles, A.; Willis, E.; et al. Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses. J. Exp. Med. 2018, 215, 1571–1588. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Frenck, R.W., Jr.; Klein, N.P.; Kitchin, N.; Gurtman, A.; Absalon, J.; Lockhart, S.; Perez, J.L.; Walter, E.B.; Senders, S.; Bailey, R.; et al. Safety, Immunogenicity, and Efficacy of the BNT162b2 COVID-19 Vaccine in Adolescents. N. Engl. J. Med. 2021, 385, 239–250. [Google Scholar] [CrossRef]
- CDC COVID-19 Response Team; Food and Drug Administration. Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Pfizer-BioNTech COVID-19 Vaccine—United States, December 14–23, 2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 46–51. [Google Scholar] [CrossRef]
- Shimabukuro, T.; Nair, N. Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Pfizer-BioNTech COVID-19 Vaccine. JAMA 2021, 325, 780–781. [Google Scholar] [CrossRef]
- Verma, A.K.; Lavine, K.J.; Lin, C.Y. Myocarditis after COVID-19 mRNA Vaccination. N. Engl. J. Med. 2021, 385, 1332–1334. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Knoll, M.D.; Wonodi, C. Oxford-AstraZeneca COVID-19 vaccine efficacy. Lancet 2021, 397, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.P.; Bezbaruah, R.; Valu, D.; Patel, B.; Kumar, A.; Prasad, S.; Kakoti, B.B.; Kaushik, A.; Jesawadawala, M. Adenoviral Vector-Based Vaccine Platform for COVID-19: Current Status. Vaccines 2023, 11, 432. [Google Scholar] [CrossRef] [PubMed]
- Hviid, A.; Hansen, J.V.; Thiesson, E.M.; Wohlfahrt, J. Association of AZD1222 and BNT162b2 COVID-19 Vaccination With Thromboembolic and Thrombocytopenic Events in Frontline Personnel: A Retrospective Cohort Study. Ann. Intern. Med. 2022, 175, 541–546. [Google Scholar] [CrossRef]
- Cines, D.B.; Bussel, J.B. SARS-CoV-2 Vaccine-Induced Immune Thrombotic Thrombocytopenia. N. Engl. J. Med. 2021, 384, 2254–2256. [Google Scholar] [CrossRef]
- Dotan, A.; Shoenfeld, Y. Perspectives on vaccine induced thrombotic thrombocytopenia. J. Autoimmun. 2021, 121, 102663. [Google Scholar] [CrossRef]
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, P.A.; Eichinger, S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N. Engl. J. Med. 2021, 384, 2092–2101. [Google Scholar] [CrossRef]
- Di Micco, P.; Camporese, G.; Cardillo, G.; Lodigiani, C.; Carannante, N.; Annunziata, A.; Fiorentino, G.; Russo, V.; Imbalzano, E. Pathophysiology of Vaccine-Induced Prothrombotic Immune Thrombocytopenia (VIPIT) and Vaccine-Induced Thrombocytopenic Thrombosis (VITT) and Their Diagnostic Approach in Emergency. Medicina 2021, 57, 997. [Google Scholar] [CrossRef]
- Scully, M.; Singh, D.; Lown, R.; Poles, A.; Solomon, T.; Levi, M.; Goldblatt, D.; Kotoucek, P.; Thomas, W.; Lester, W. Pathologic Antibodies to Platelet Factor 4 after ChAdOx1 nCoV-19 Vaccination. N. Engl. J. Med. 2021, 384, 2202–2211. [Google Scholar] [CrossRef]
- Nazy, I.; Jevtic, S.D.; Moore, J.C.; Huynh, A.; Smith, J.W.; Kelton, J.G.; Arnold, D.M. Platelet-activating immune complexes identified in critically ill COVID-19 patients suspected of heparin-induced thrombocytopenia. J. Thromb. Haemost. 2021, 19, 1342–1347. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, A. SARS-CoV-2 vaccine-induced cerebral venous thrombosis. Eur. J. Intern. Med. 2021, 89, 19–21. [Google Scholar] [CrossRef]
- Jeyanathan, M.; Afkhami, S.; Smaill, F.; Miller, M.S.; Lichty, B.D.; Xing, Z. Immunological considerations for COVID-19 vaccine strategies. Nat. Rev. Immunol. 2020, 20, 615–632. [Google Scholar] [CrossRef]
- Xia, S.; Duan, K.; Zhang, Y.; Zhao, D.; Zhang, H.; Xie, Z.; Li, X.; Peng, C.; Zhang, Y.; Zhang, W.; et al. Effect of an Inactivated Vaccine Against SARS-CoV-2 on Safety and Immunogenicity Outcomes: Interim Analysis of 2 Randomized Clinical Trials. JAMA 2020, 324, 951–960. [Google Scholar] [CrossRef]
- Fadlyana, E.; Rusmil, K.; Tarigan, R.; Rahmadi, A.R.; Prodjosoewojo, S.; Sofiatin, Y.; Khrisna, C.V.; Sari, R.M.; Setyaningsih, L.; Surachman, F.; et al. A phase III, observer-blind, randomized, placebo-controlled study of the efficacy, safety, and immunogenicity of SARS-CoV-2 inactivated vaccine in healthy adults aged 18-59 years: An interim analysis in Indonesia. Vaccine 2021, 39, 6520–6528. [Google Scholar] [CrossRef] [PubMed]
- Tanriover, M.D.; Doganay, H.L.; Akova, M.; Guner, H.R.; Azap, A.; Akhan, S.; Kose, S.; Erdinc, F.S.; Akalin, E.H.; Tabak, O.F.; et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): Interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet 2021, 398, 213–222. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, G.; Pan, H.; Li, C.; Hu, Y.; Chu, K.; Han, W.; Chen, Z.; Tang, R.; Yin, W.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021, 21, 181–192. [Google Scholar] [CrossRef]
- Wu, Z.; Hu, Y.; Xu, M.; Chen, Z.; Yang, W.; Jiang, Z.; Li, M.; Jin, H.; Cui, G.; Chen, P.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021, 21, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Bueno, S.M.; Abarca, K.; Gonzalez, P.A.; Galvez, N.M.S.; Soto, J.A.; Duarte, L.F.; Schultz, B.M.; Pacheco, G.A.; Gonzalez, L.A.; Vazquez, Y.; et al. Safety and Immunogenicity of an Inactivated Severe Acute Respiratory Syndrome Coronavirus 2 Vaccine in a Subgroup of Healthy Adults in Chile. Clin. Infect. Dis. 2022, 75, e792–e804. [Google Scholar] [CrossRef]
- Li, Y.; Tenchov, R.; Smoot, J.; Liu, C.; Watkins, S.; Zhou, Q. A Comprehensive Review of the Global Efforts on COVID-19 Vaccine Development. ACS Cent. Sci. 2021, 7, 512–533. [Google Scholar] [CrossRef]
- Pulendran, B.; Ahmed, R. Immunological mechanisms of vaccination. Nat. Immunol. 2011, 12, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Wheatley, A.K.; Kent, S.J.; DeKosky, B.J. Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies. Nat. Microbiol. 2020, 5, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.F.; Tseng, S.P.; Yen, C.H.; Yang, J.Y.; Tsao, C.H.; Shen, C.W.; Chen, K.H.; Liu, F.T.; Liu, W.T.; Chen, Y.M.; et al. Antibody-dependent SARS coronavirus infection is mediated by antibodies against spike proteins. Biochem. Biophys. Res. Commun. 2014, 451, 208–214. [Google Scholar] [CrossRef]
- Xu, L.; Ma, Z.; Li, Y.; Pang, Z.; Xiao, S. Antibody dependent enhancement: Unavoidable problems in vaccine development. Adv. Immunol. 2021, 151, 99–133. [Google Scholar] [CrossRef] [PubMed]
- Halstead, S.B. Vaccine-Associated Enhanced Viral Disease: Implications for Viral Vaccine Development. BioDrugs 2021, 35, 505–515. [Google Scholar] [CrossRef]
- Tian, J.H.; Patel, N.; Haupt, R.; Zhou, H.; Weston, S.; Hammond, H.; Logue, J.; Portnoff, A.D.; Norton, J.; Guebre-Xabier, M.; et al. SARS-CoV-2 spike glycoprotein vaccine candidate NVX-CoV2373 immunogenicity in baboons and protection in mice. Nat. Commun. 2021, 12, 372. [Google Scholar] [CrossRef]
- Stertman, L.; Palm, A.E.; Zarnegar, B.; Carow, B.; Lunderius Andersson, C.; Magnusson, S.E.; Carnrot, C.; Shinde, V.; Smith, G.; Glenn, G.; et al. The Matrix-M adjuvant: A critical component of vaccines for the 21(st) century. Hum. Vaccines Immunother. 2023, 19, 2189885. [Google Scholar] [CrossRef]
- Boni, M.F. Vaccination and antigenic drift in influenza. Vaccine 2008, 26 (Suppl. 3), C8–C14. [Google Scholar] [CrossRef]
- Yewdell, J.W. Antigenic drift: Understanding COVID-19. Immunity 2021, 54, 2681–2687. [Google Scholar] [CrossRef]
- Mykytyn, A.Z.; Fouchier, R.A.; Haagmans, B.L. Antigenic evolution of SARS coronavirus 2. Curr. Opin. Virol. 2023, 62, 101349. [Google Scholar] [CrossRef]
- Vousden, N.; Knight, M. Lessons learned from the A (H1N1) influenza pandemic. Best Pract. Res. Clin. Obstet. Gynaecol. 2021, 76, 41–52. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, C.Y.; Wan, X.F. Antigenic characterization of influenza and SARS-CoV-2 viruses. Anal. Bioanal. Chem. 2022, 414, 2841–2881. [Google Scholar] [CrossRef] [PubMed]
- Belongia, E.A.; Skowronski, D.M.; McLean, H.Q.; Chambers, C.; Sundaram, M.E.; De Serres, G. Repeated annual influenza vaccination and vaccine effectiveness: Review of evidence. Expert Rev. Vaccines 2017, 16, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, C.M.; Kistner, O.; Montomoli, E.; Viviani, S.; Marchi, S. Influenza Viruses and Vaccines: The Role of Vaccine Effectiveness Studies for Evaluation of the Benefits of Influenza Vaccines. Vaccines 2022, 10, 714. [Google Scholar] [CrossRef]
- Law, J.L.M.; Logan, M.; Joyce, M.A.; Landi, A.; Hockman, D.; Crawford, K.; Johnson, J.; LaChance, G.; Saffran, H.A.; Shields, J.; et al. SARS-COV-2 recombinant Receptor-Binding-Domain (RBD) induces neutralizing antibodies against variant strains of SARS-CoV-2 and SARS-CoV-1. Vaccine 2021, 39, 5769–5779. [Google Scholar] [CrossRef] [PubMed]
- Changrob, S.; Fu, Y.; Guthmiller, J.J.; Halfmann, P.J.; Li, L.; Stamper, C.T.; Dugan, H.L.; Accola, M.; Rehrauer, W.; Zheng, N.Y.; et al. Cross-Neutralization of Emerging SARS-CoV-2 Variants of Concern by Antibodies Targeting Distinct Epitopes on Spike. mBio 2021, 12, e0297521. [Google Scholar] [CrossRef]
- Mahumud, R.A.; Ali, M.A.; Kundu, S.; Rahman, M.A.; Kamara, J.K.; Renzaho, A.M.N. Effectiveness of COVID-19 Vaccines against Delta Variant (B.1.617.2): A Meta-Analysis. Vaccines 2022, 10, 277. [Google Scholar] [CrossRef]
- Andre, M.; Lau, L.S.; Pokharel, M.D.; Ramelow, J.; Owens, F.; Souchak, J.; Akkaoui, J.; Ales, E.; Brown, H.; Shil, R.; et al. From Alpha to Omicron: How Different Variants of Concern of the SARS-Coronavirus-2 Impacted the World. Biology 2023, 12, 1267. [Google Scholar] [CrossRef]
- Hill, V.; Du Plessis, L.; Peacock, T.P.; Aggarwal, D.; Colquhoun, R.; Carabelli, A.M.; Ellaby, N.; Gallagher, E.; Groves, N.; Jackson, B.; et al. The origins and molecular evolution of SARS-CoV-2 lineage B.1.1.7 in the UK. Virus Evol. 2022, 8, veac080. [Google Scholar] [CrossRef]
- Cevik, M.; Grubaugh, N.D.; Iwasaki, A.; Openshaw, P. COVID-19 vaccines: Keeping pace with SARS-CoV-2 variants. Cell 2021, 184, 5077–5081. [Google Scholar] [CrossRef]
- Dhawan, M.; Saied, A.A.; Mitra, S.; Alhumaydhi, F.A.; Emran, T.B.; Wilairatana, P. Omicron variant (B.1.1.529) and its sublineages: What do we know so far amid the emergence of recombinant variants of SARS-CoV-2? Biomed. Pharmacother. 2022, 154, 113522. [Google Scholar] [CrossRef]
- Haykal, N.M.; Fadilah, F.; Dewi, B.E.; Erlina, L.; Prawiningrum, A.F.; Hegar, B. Dynamics of SARS-CoV-2 Spike RBD Protein Mutation and Pathogenicity Consequences in Indonesian Circulating Variants in 2020-2022. Genes 2024, 15, 1468. [Google Scholar] [CrossRef]
- Singh, P.; Negi, S.S.; Bhargava, A.; Kolla, V.P.; Arora, R.D. A Preliminary Genomic Analysis of the Omicron Variants of SARS-CoV-2 in Central India During the third wave of the COVID-19 Pandemic. Arch. Med. Res. 2022, 53, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Tallei, T.E.; Alhumaid, S.; AlMusa, Z.; Fatimawali; Kusumawaty, D.; Alynbiawi, A.; Alshukairi, A.N.; Rabaan, A.A. Update on the omicron sub-variants BA.4 and BA.5. Rev. Med. Virol. 2023, 33, e2391. [Google Scholar] [CrossRef] [PubMed]
- Chaguza, C.; Coppi, A.; Earnest, R.; Ferguson, D.; Kerantzas, N.; Warner, F.; Young, H.P.; Breban, M.I.; Billig, K.; Koch, R.T.; et al. Rapid emergence of SARS-CoV-2 Omicron variant is associated with an infection advantage over Delta in vaccinated persons. Med 2022, 3, 325–334.e324. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; He, J.; Wang, C.; Bao, J.; Leng, T.; Chen, F. The importance of booster vaccination in the context of Omicron wave. Front. Immunol. 2022, 13, 977972. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Goldberg, Y.; Mandel, M.; Bodenheimer, O.; Amir, O.; Freedman, L.; Alroy-Preis, S.; Ash, N.; Huppert, A.; Milo, R. Protection by a Fourth Dose of BNT162b2 against Omicron in Israel. N. Engl. J. Med. 2022, 386, 1712–1720. [Google Scholar] [CrossRef]
- Scheaffer, S.M.; Lee, D.; Whitener, B.; Ying, B.; Wu, K.; Liang, C.Y.; Jani, H.; Martin, P.; Amato, N.J.; Avena, L.E.; et al. Bivalent SARS-CoV-2 mRNA vaccines increase breadth of neutralization and protect against the BA.5 Omicron variant in mice. Nat. Med. 2023, 29, 247–257. [Google Scholar] [CrossRef]
- Chenchula, S.; Karunakaran, P.; Sharma, S.; Chavan, M. Current evidence on efficacy of COVID-19 booster dose vaccination against the Omicron variant: A systematic review. J. Med. Virol. 2022, 94, 2969–2976. [Google Scholar] [CrossRef]
- Black, B.; Atanasov, V.; Glatman-Freedman, A.; Keinan-Boker, L.; Reichman, A.; Franchi, L.; Meurer, J.; Luo, Q.; Thaw, D.B.; Moghtaderi, A. COVID-19 Boosters: If The US Had Matched Israel’s Speed And Take-Up, An Estimated 29,000 US Lives Would Have Been Saved. Health Aff. 2023, 42, 1747–1757. [Google Scholar] [CrossRef]
- Krause, P.R.; Fleming, T.R.; Peto, R.; Longini, I.M.; Figueroa, J.P.; Sterne, J.A.C.; Cravioto, A.; Rees, H.; Higgins, J.P.T.; Boutron, I.; et al. Considerations in boosting COVID-19 vaccine immune responses. Lancet 2021, 398, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.Y.; Chiang, B.L.; Chi, W.K.; Chang, M.H.; Ni, Y.H.; Hsu, H.M.; Twu, S.J.; Su, I.J.; Huang, L.M.; Lee, C.Y. Waning immunity to plasma-derived hepatitis B vaccine and the need for boosters 15 years after neonatal vaccination. Hepatology 2004, 40, 1415–1420. [Google Scholar] [CrossRef]
- Doyon-Plourde, P.; Przepiorkowski, J.; Young, K.; Zhao, L.; Sinilaite, A. Intraseasonal waning immunity of seasonal influenza vaccine—A systematic review and meta-analysis. Vaccine 2023, 41, 4462–4471. [Google Scholar] [CrossRef] [PubMed]
- Perez-Alos, L.; Hansen, C.B.; Almagro Armenteros, J.J.; Madsen, J.R.; Heftdal, L.D.; Hasselbalch, R.B.; Pries-Heje, M.M.; Bayarri-Olmos, R.; Jarlhelt, I.; Hamm, S.R.; et al. Previous immunity shapes immune responses to SARS-CoV-2 booster vaccination and Omicron breakthrough infection risk. Nat. Commun. 2023, 14, 5624. [Google Scholar] [CrossRef] [PubMed]
- Goel, R.R.; Apostolidis, S.A.; Painter, M.M.; Mathew, D.; Pattekar, A.; Kuthuru, O.; Gouma, S.; Hicks, P.; Meng, W.; Rosenfeld, A.M.; et al. Distinct antibody and memory B cell responses in SARS-CoV-2 naive and recovered individuals following mRNA vaccination. Sci. Immunol. 2021, 6, eabi6950. [Google Scholar] [CrossRef]
- Gavish, N.; Yaari, R.; Huppert, A.; Katriel, G. Population-level implications of the Israeli booster campaign to curtail COVID-19 resurgence. Sci. Transl. Med. 2022, 14, eabn9836. [Google Scholar] [CrossRef]
- Haas, E.J.; Angulo, F.J.; McLaughlin, J.M.; Anis, E.; Singer, S.R.; Khan, F.; Brooks, N.; Smaja, M.; Mircus, G.; Pan, K.; et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: An observational study using national surveillance data. Lancet 2021, 397, 1819–1829. [Google Scholar] [CrossRef]
- Tamandjou Tchuem, C.R.; Auvigne, V.; Vaux, S.; Montagnat, C.; Paireau, J.; Monnier Besnard, S.; Gabet, A.; Benhajkassen, N.; Le Strat, Y.; Parent Du Chatelet, I.; et al. Vaccine effectiveness and duration of protection of COVID-19 mRNA vaccines against Delta and Omicron BA.1 symptomatic and severe COVID-19 outcomes in adults aged 50 years and over in France. Vaccine 2023, 41, 2280–2288. [Google Scholar] [CrossRef]
- Reinholm, A.; Maljanen, S.; Jalkanen, P.; Altan, E.; Tauriainen, S.; Belik, M.; Skon, M.; Haveri, A.; Osterlund, P.; Iakubovskaia, A.; et al. Neutralizing antibodies after the third COVID-19 vaccination in healthcare workers with or without breakthrough infection. Commun Med. 2024, 4, 28. [Google Scholar] [CrossRef]
- Bayart, J.L.; Douxfils, J.; Gillot, C.; David, C.; Mullier, F.; Elsen, M.; Eucher, C.; Van Eeckhoudt, S.; Roy, T.; Gerin, V.; et al. Waning of IgG, Total and Neutralizing Antibodies 6 Months Post-Vaccination with BNT162b2 in Healthcare Workers. Vaccines 2021, 9, 1092. [Google Scholar] [CrossRef]
- Alhabbab, R.Y.; Algaissi, A.; Mahmoud, A.B.; Alkayyal, A.A.; Al-Amri, S.; Alfaleh, M.A.; Basabrain, M.; Alsubki, R.A.; Almarshad, I.S.; Alhudaithi, A.M.; et al. Middle East Respiratory Syndrome Coronavirus Infection Elicits Long-lasting Specific Antibody, T and B Cell Immune Responses in Recovered Individuals. Clin. Infect. Dis. 2023, 76, e308–e318. [Google Scholar] [CrossRef]
- Lapuente, D.; Winkler, T.H.; Tenbusch, M. B-cell and antibody responses to SARS-CoV-2: Infection, vaccination, and hybrid immunity. Cell Mol. Immunol. 2024, 21, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, M.; Rabaan, A.A.; Fawarah, M.M.A.; Almuthree, S.A.; Alsubki, R.A.; Alfaraj, A.H.; Mashraqi, M.M.; Alshamrani, S.A.; Abduljabbar, W.A.; Alwashmi, A.S.S.; et al. Updated Insights into the T Cell-Mediated Immune Response against SARS-CoV-2: A Step towards Efficient and Reliable Vaccines. Vaccines 2023, 11, 101. [Google Scholar] [CrossRef]
- Moss, P. The T cell immune response against SARS-CoV-2. Nat. Immunol. 2022, 23, 186–193. [Google Scholar] [CrossRef]
- Costa Clemens, S.A.; Weckx, L.; Clemens, R.; Almeida Mendes, A.V.; Ramos Souza, A.; Silveira, M.B.V.; da Guarda, S.N.F.; de Nobrega, M.M.; de Moraes Pinto, M.I.; Gonzalez, I.G.S.; et al. Heterologous versus homologous COVID-19 booster vaccination in previous recipients of two doses of CoronaVac COVID-19 vaccine in Brazil (RHH-001): A phase 4, non-inferiority, single blind, randomised study. Lancet 2022, 399, 521–529. [Google Scholar] [CrossRef]
- Lambert, N.D.; Ovsyannikova, I.G.; Pankratz, V.S.; Jacobson, R.M.; Poland, G.A. Understanding the immune response to seasonal influenza vaccination in older adults: A systems biology approach. Expert. Rev. Vaccines 2012, 11, 985–994. [Google Scholar] [CrossRef] [PubMed]
- McElhaney, J.E. Influenza vaccine responses in older adults. Ageing Res. Rev. 2011, 10, 379–388. [Google Scholar] [CrossRef]
- Suarez Castillo, M.; Khaoua, H.; Courtejoie, N. Vaccine effectiveness and duration of protection against symptomatic infections and severe COVID-19 outcomes in adults aged 50 years and over, France, January to mid-December 2021. Glob. Epidemiol. 2022, 4, 100076. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Pouwels, K.B.; Stoesser, N.; Matthews, P.C.; Diamond, I.; Studley, R.; Rourke, E.; Cook, D.; Bell, J.I.; Newton, J.N.; et al. Antibody responses and correlates of protection in the general population after two doses of the ChAdOx1 or BNT162b2 vaccines. Nat. Med. 2022, 28, 1072–1082. [Google Scholar] [CrossRef]
- Bigdelou, B.; Sepand, M.R.; Najafikhoshnoo, S.; Negrete, J.A.T.; Sharaf, M.; Ho, J.Q.; Sullivan, I.; Chauhan, P.; Etter, M.; Shekarian, T.; et al. COVID-19 and Preexisting Comorbidities: Risks, Synergies, and Clinical Outcomes. Front. Immunol. 2022, 13, 890517. [Google Scholar] [CrossRef]
- Nam, S.Y.; Jeon, S.W.; Jung, D.K.; Heo, S.J. Body Weight is Inversely Associated with Anti-SARS-CoV-2 Antibody Levels after BNT162b2 mRNA Vaccination in Young and Middle Aged Adults. Infect. Chemother. 2022, 54, 504–516. [Google Scholar] [CrossRef]
- Nasr, M.C.; Geerling, E.; Pinto, A.K. Impact of Obesity on Vaccination to SARS-CoV-2. Front. Endocrinol. 2022, 13, 898810. [Google Scholar] [CrossRef]
- DeWolf, S.; Laracy, J.C.; Perales, M.A.; Kamboj, M.; van den Brink, M.R.M.; Vardhana, S. SARS-CoV-2 in immunocompromised individuals. Immunity 2022, 55, 1779–1798. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, K.; Carreno, J.M.; Gleason, C.; Monahan, B.; Singh, G.; Abbad, A.; Tcheou, J.; Raskin, A.; Kleiner, G.; van Bakel, H.; et al. SARS-CoV-2-infection- and vaccine-induced antibody responses are long lasting with an initial waning phase followed by a stabilization phase. Immunity 2024, 57, 587–599.e584. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Nicols, A.; Turtle, L.; Richter, A.; Duncan, C.J.; Dunachie, S.J.; Klenerman, P.; Payne, R.P. T cell immune memory after COVID-19 and vaccination. BMJ Med. 2023, 2, e000468. [Google Scholar] [CrossRef] [PubMed]
- Hachmann, N.P.; Miller, J.; Collier, A.Y.; Ventura, J.D.; Yu, J.; Rowe, M.; Bondzie, E.A.; Powers, O.; Surve, N.; Hall, K.; et al. Neutralization Escape by SARS-CoV-2 Omicron Subvariants BA.2.12.1, BA.4, and BA.5. N. Engl. J. Med. 2022, 387, 86–88. [Google Scholar] [CrossRef]
- Altarawneh, H.N.; Chemaitelly, H.; Ayoub, H.H.; Hasan, M.R.; Coyle, P.; Yassine, H.M.; Al-Khatib, H.A.; Smatti, M.K.; Al-Kanaani, Z.; Al-Kuwari, E.; et al. Protective Effect of Previous SARS-CoV-2 Infection against Omicron BA.4 and BA.5 Subvariants. N. Engl. J. Med. 2022, 387, 1620–1622. [Google Scholar] [CrossRef]
- Le Bert, N.; Tan, A.T.; Kunasegaran, K.; Tham, C.Y.L.; Hafezi, M.; Chia, A.; Chng, M.H.Y.; Lin, M.; Tan, N.; Linster, M.; et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 2020, 584, 457–462. [Google Scholar] [CrossRef]
- Haralambieva, I.H.; Ovsyannikova, I.G.; Pankratz, V.S.; Kennedy, R.B.; Jacobson, R.M.; Poland, G.A. The genetic basis for interindividual immune response variation to measles vaccine: New understanding and new vaccine approaches. Expert Rev. Vaccines 2013, 12, 57–70. [Google Scholar] [CrossRef]
- Lunt, R.; Quinot, C.; Kirsebom, F.; Andrews, N.; Skarnes, C.; Letley, L.; Haskins, D.; Angel, C.; Firminger, S.; Ratcliffe, K.; et al. The impact of vaccination and SARS-CoV-2 variants on the virological response to SARS-CoV-2 infections during the Alpha, Delta, and Omicron waves in England. J. Infect. 2024, 88, 21–29. [Google Scholar] [CrossRef]
- Chang, S.; Shin, K.S.; Park, B.; Park, S.; Shin, J.; Park, H.; Jung, I.K.; Kim, J.H.; Bae, S.E.; Kim, J.O.; et al. Strategy to develop broadly effective multivalent COVID-19 vaccines against emerging variants based on Ad5/35 platform. Proc. Natl. Acad. Sci. USA 2024, 121, e2313681121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhao, J.; Zhu, X.; Guan, Q.; Liu, S.; Li, M.; Gao, J.; Tan, J.; Cao, F.; Gan, B.; et al. Efficacy of the tetravalent protein COVID-19 vaccine, SCTV01E: A phase 3 double-blind, randomized, placebo-controlled trial. Nat. Commun. 2024, 15, 6255. [Google Scholar] [CrossRef] [PubMed]
- Hannawi, S.; Abuquta, A.; Eldin, L.S.; Hassan, A.; Alamadi, A.; Gao, C.; Baidoo, A.A.H.; Yang, X.; Su, H.; Zhang, J.; et al. Immunogenicity and Safety of Omicron-Containing Multivalent COVID-19 Vaccines in Unvaccinated and Previously Vaccinated Adults. Vaccines 2024, 12, 1109. [Google Scholar] [CrossRef]
- Qian, G.; Gao, C.; Zhang, M.; Chen, Y.; Xie, L. A Review of Protein-Based COVID-19 Vaccines: From Monovalent to Multivalent Formulations. Vaccines 2024, 12, 579. [Google Scholar] [CrossRef]
- Tang, J.; Xu, Q.; Zhu, C.; Xuan, K.; Li, T.; Li, Q.; Pang, X.; Zha, Z.; Li, J.; Qiao, L.; et al. Immunogenicity of Tetravalent Protein Vaccine SCTV01E-2 against SARS-CoV-2 EG.5 Subvaraint: A Phase 2 Trial. Vaccines 2024, 12, 175. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, L.; Fang, Z.; Li, J.; Li, C.; Song, W.; Huang, Z.; Chen, R.; Zhang, Y.; Li, J. Analysis of the protective efficacy of approved COVID-19 vaccines against Omicron variants and the prospects for universal vaccines. Front. Immunol. 2023, 14, 1294288. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; He, X.; Shi, H.; He, C.; Lei, H.; He, H.; Yang, L.; Wang, W.; Shen, G.; Yang, J.; et al. Recombinant XBB.1.5 boosters induce robust neutralization against KP.2- and KP.3-included JN.1 sublineages. Signal Transduct. Target. Ther. 2025, 10, 47. [Google Scholar] [CrossRef]
- Cankat, S.; Demael, M.U.; Swadling, L. In search of a pan-coronavirus vaccine: Next-generation vaccine design and immune mechanisms. Cell Mol. Immunol. 2024, 21, 103–118. [Google Scholar] [CrossRef]
- Bartsch, S.M.; O’Shea, K.J.; John, D.C.; Strych, U.; Bottazzi, M.E.; Martinez, M.F.; Ciciriello, A.; Chin, K.L.; Weatherwax, C.; Velmurugan, K.; et al. The potential epidemiologic, clinical, and economic value of a universal coronavirus vaccine: A modelling study. EClinicalMedicine 2024, 68, 102369. [Google Scholar] [CrossRef]
- Dolgin, E. Pan-coronavirus vaccine pipeline takes form. Nat. Rev. Drug Discov. 2022, 21, 324–326. [Google Scholar] [CrossRef]
- Patel, R.S.; Agrawal, B. Heterologous immunity induced by 1(st) generation COVID-19 vaccines and its role in developing a pan-coronavirus vaccine. Front. Immunol. 2022, 13, 952229. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, M.O.; Raftery, M.J.; Weihs, J.; Bielawski, O.; Edel, R.; Koppke, J.; Vladimirova, D.; Adler, J.M.; Firsching, T.; Voss, A.; et al. Early protective effect of a (“pan”) coronavirus vaccine (PanCoVac) in Roborovski dwarf hamsters after single-low dose intranasal administration. Front. Immunol. 2023, 14, 1166765. [Google Scholar] [CrossRef]
- Brunner, T.; Mueller, C. Cytotoxic T cells: Double-barreled shot guns. Nat. Med. 1999, 5, 20. [Google Scholar] [CrossRef]
- Koup, R.A.; Douek, D.C. Vaccine design for CD8 T lymphocyte responses. Cold Spring Harb. Perspect. Med. 2011, 1, a007252. [Google Scholar] [CrossRef] [PubMed]
- Tobery, T.W.; Siliciano, R.F. Targeting of HIV-1 antigens for rapid intracellular degradation enhances cytotoxic T lymphocyte (CTL) recognition and the induction of de novo CTL responses in vivo after immunization. J. Exp. Med. 1997, 185, 909–920. [Google Scholar] [CrossRef]
- Gaucher, D.; Therrien, R.; Kettaf, N.; Angermann, B.R.; Boucher, G.; Filali-Mouhim, A.; Moser, J.M.; Mehta, R.S.; Drake, D.R., 3rd; Castro, E.; et al. Yellow fever vaccine induces integrated multilineage and polyfunctional immune responses. J. Exp. Med. 2008, 205, 3119–3131. [Google Scholar] [CrossRef]
- Zhang, B.; Upadhyay, R.; Hao, Y.; Samanovic, M.I.; Herati, R.S.; Blair, J.D.; Axelrad, J.; Mulligan, M.J.; Littman, D.R.; Satija, R. Multimodal single-cell datasets characterize antigen-specific CD8(+) T cells across SARS-CoV-2 vaccination and infection. Nat. Immunol. 2023, 24, 1725–1734. [Google Scholar] [CrossRef]
- Li, M.; Zeng, J.; Li, R.; Wen, Z.; Cai, Y.; Wallin, J.; Shu, Y.; Du, X.; Sun, C. Rational Design of a Pan-Coronavirus Vaccine Based on Conserved CTL Epitopes. Viruses 2021, 13, 333. [Google Scholar] [CrossRef]
- Kumar, M.; Al Khodor, S. “Armed for the future Coronavirus pandemic”: A promising use of the multimeric SARS-CoV-2 receptor binding domain nanoparticle as a new Pan-Coronavirus vaccine. Signal Transduct. Target. Ther. 2021, 6, 305. [Google Scholar] [CrossRef]
- Hao, X.; Yuan, F.; Yao, X. Advances in virus-like particle-based SARS-CoV-2 vaccines. Front. Cell Infect. Microbiol. 2024, 14, 1406091. [Google Scholar] [CrossRef]
- Hsieh, C.L.; Leist, S.R.; Miller, E.H.; Zhou, L.; Powers, J.M.; Tse, A.L.; Wang, A.; West, A.; Zweigart, M.R.; Schisler, J.C.; et al. Prefusion-stabilized SARS-CoV-2 S2-only antigen provides protection against SARS-CoV-2 challenge. Nat. Commun. 2024, 15, 1553. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Ramirez, N.J.; Garcia-Cordero, J.; Shrivastava, G.; Cedillo-Barron, L. The Key to Increase Immunogenicity of Next-Generation COVID-19 Vaccines Lies in the Inclusion of the SARS-CoV-2 Nucleocapsid Protein. J. Immunol. Res. 2024, 2024, 9313267. [Google Scholar] [CrossRef]
- Miyamoto, S.; Suzuki, T. Infection-mediated immune response in SARS-CoV-2 breakthrough infection and implications for next-generation COVID-19 vaccine development. Vaccine 2024, 42, 1401–1406. [Google Scholar] [CrossRef] [PubMed]
- Ross, K.A.; Kelly, S.; Phadke, K.S.; Peroutka-Bigus, N.; Fasina, O.; Siddoway, A.; Mallapragada, S.K.; Wannemuehler, M.J.; Bellaire, B.H.; Narasimhan, B. Next-generation nanovaccine induces durable immunity and protects against SARS-CoV-2. Acta Biomater. 2024, 183, 318–329. [Google Scholar] [CrossRef]
- Tedjakusuma, S.N.; Lester, C.A.; Neuhaus, E.D.; Dora, E.G.; Gutierrez, S.; Braun, M.R.; Tucker, S.N.; Flitter, B.A. A Next-Generation Adenoviral Vaccine Elicits Mucosal and Systemic Immunogenicity and Reduces Viral Shedding after SARS-CoV-2 Challenge in Nonhuman Primates. Vaccines 2024, 12, 132. [Google Scholar] [CrossRef] [PubMed]
- Warner, B.M.; Yates, J.G.E.; Vendramelli, R.; Truong, T.; Meilleur, C.; Chan, L.; Leacy, A.; Pham, P.H.; Pei, Y.; Susta, L.; et al. Intranasal vaccination with an NDV-vectored SARS-CoV-2 vaccine protects against Delta and Omicron challenges. NPJ Vaccines 2024, 9, 90. [Google Scholar] [CrossRef]
- Zhang, Y.; Chamblee, M.; Xu, J.; Qu, P.; Shamseldin, M.M.; Yoo, S.J.; Misny, J.; Thongpan, I.; Kc, M.; Hall, J.M.; et al. Three SARS-CoV-2 spike protein variants delivered intranasally by measles and mumps vaccines are broadly protective. Nat. Commun. 2024, 15, 5589. [Google Scholar] [CrossRef]
- Bloom, K.; Ely, A.; Maepa, M.B.; Arbuthnot, P. Bridging gene therapy and next-generation vaccine technologies. Gene Ther. 2025, 32, 4–7. [Google Scholar] [CrossRef]
- Chalkias, S.; Pragalos, A.; Akinsola, A.; Berman, G.; Ampajwala, M.; Meyer, J.; Schoch, L.; Zhou, W.; Paila, Y.D.; Deng, W.; et al. Safety and Immunogenicity of SARS-CoV-2 Spike Receptor-Binding Domain andN-Terminal Domain mRNA Vaccine. J. Infect. Dis. 2025, jiaf022. [Google Scholar] [CrossRef]
- Yassini, P.; Hutchens, M.; Paila, Y.D.; Schoch, L.; Aunins, A.; Siangphoe, U.; Paris, R. Interim analysis of a phase 1 randomized clinical trial on the safety and immunogenicity of the mRNA-1283 SARS-CoV-2 vaccine in adults. Hum. Vaccines Immunother. 2023, 19, 2190690. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.; Song, Y.; Coleman, J.R.; Stawowczyk, M.; Tafrova, J.; Tasker, S.; Boltz, D.; Baker, R.; Garcia, L.; et al. Scalable live-attenuated SARS-CoV-2 vaccine candidate demonstrates preclinical safety and efficacy. Proc. Natl. Acad. Sci. USA 2021, 118. [Google Scholar] [CrossRef]
- Suzuki Okutani, M.; Okamura, S.; Gis, T.; Sasaki, H.; Lee, S.; Kashiwabara, A.; Goto, S.; Matsumoto, M.; Yamawaki, M.; Miyazaki, T.; et al. Immunogenicity and safety of a live-attenuated SARS-CoV-2 vaccine candidate based on multiple attenuation mechanisms. Elife 2025, 13, RP97532. [Google Scholar] [CrossRef] [PubMed]
- Ho, N.T.; Hughes, S.G.; Ta, V.T.; Phan, L.T.; Do, Q.; Nguyen, T.V.; Pham, A.T.V.; Thi Ngoc Dang, M.; Nguyen, L.V.; Trinh, Q.V.; et al. Safety, immunogenicity and efficacy of the self-amplifying mRNA ARCT-154 COVID-19 vaccine: Pooled phase 1, 2, 3a and 3b randomized, controlled trials. Nat. Commun. 2024, 15, 4081. [Google Scholar] [CrossRef]
- Oda, Y.; Kumagai, Y.; Kanai, M.; Iwama, Y.; Okura, I.; Minamida, T.; Yagi, Y.; Kurosawa, T.; Chivukula, P.; Zhang, Y.; et al. Persistence of immune responses of a self-amplifying RNA COVID-19 vaccine (ARCT-154) versus BNT162b2. Lancet Infect. Dis. 2024, 24, 341–343. [Google Scholar] [CrossRef]
- Singh, C.; Verma, S.; Reddy, P.; Diamond, M.S.; Curiel, D.T.; Patel, C.; Jain, M.K.; Redkar, S.V.; Bhate, A.S.; Gundappa, V.; et al. Phase III Pivotal comparative clinical trial of intranasal (iNCOVACC) and intramuscular COVID 19 vaccine (Covaxin((R))). NPJ Vaccines 2023, 8, 125. [Google Scholar] [CrossRef] [PubMed]
- Sunagar, R.; Prasad, S.D.; Ella, R.; Vadrevu, K.M. Preclinical evaluation of safety and immunogenicity of a primary series intranasal COVID-19 vaccine candidate (BBV154) and humoral immunogenicity evaluation of a heterologous prime-boost strategy with COVAXIN (BBV152). Front. Immunol. 2022, 13, 1063679. [Google Scholar] [CrossRef]
- Cacciottolo, M.; Nice, J.B.; Li, Y.; LeClaire, M.J.; Twaddle, R.; Mora, C.L.; Adachi, S.Y.; Chin, E.R.; Young, M.; Angeles, J.; et al. Exosome-Based Multivalent Vaccine: Achieving Potent Immunization, Broadened Reactivity, and Strong T-Cell Responses with Nanograms of Proteins. Microbiol. Spectr. 2023, 11, e0050323. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Popowski, K.D.; Zhu, D.; de Juan Abad, B.L.; Wang, X.; Liu, M.; Lutz, H.; De Naeyer, N.; DeMarco, C.T.; Denny, T.N.; et al. Exosomes decorated with a recombinant SARS-CoV-2 receptor-binding domain as an inhalable COVID-19 vaccine. Nat. Biomed. Eng. 2022, 6, 791–805. [Google Scholar] [CrossRef]
- Charland, N.; Gobeil, P.; Pillet, S.; Boulay, I.; Seguin, A.; Makarkov, A.; Heizer, G.; Bhutada, K.; Mahmood, A.; Trepanier, S.; et al. Safety and immunogenicity of an AS03-adjuvanted plant-based SARS-CoV-2 vaccine in Adults with and without Comorbidities. NPJ Vaccines 2022, 7, 142. [Google Scholar] [CrossRef]
- Hager, K.J.; Perez Marc, G.; Gobeil, P.; Diaz, R.S.; Heizer, G.; Llapur, C.; Makarkov, A.I.; Vasconcellos, E.; Pillet, S.; Riera, F.; et al. Efficacy and Safety of a Recombinant Plant-Based Adjuvanted COVID-19 Vaccine. N. Engl. J. Med. 2022, 386, 2084–2096. [Google Scholar] [CrossRef]
- Lu, B.; Lim, J.M.; Yu, B.; Song, S.; Neeli, P.; Sobhani, N.; K, P.; Bonam, S.R.; Kurapati, R.; Zheng, J.; et al. The next-generation DNA vaccine platforms and delivery systems: Advances, challenges and prospects. Front. Immunol. 2024, 15, 1332939. [Google Scholar] [CrossRef]
- Momin, T.; Kansagra, K.; Patel, H.; Sharma, S.; Sharma, B.; Patel, J.; Mittal, R.; Sanmukhani, J.; Maithal, K.; Dey, A.; et al. Safety and Immunogenicity of a DNA SARS-CoV-2 vaccine (ZyCoV-D): Results of an open-label, non-randomized phase I part of phase I/II clinical study by intradermal route in healthy subjects in India. EClinicalMedicine 2021, 38, 101020. [Google Scholar] [CrossRef]
- Khobragade, A.; Bhate, S.; Ramaiah, V.; Deshpande, S.; Giri, K.; Phophle, H.; Supe, P.; Godara, I.; Revanna, R.; Nagarkar, R.; et al. Efficacy, safety, and immunogenicity of the DNA SARS-CoV-2 vaccine (ZyCoV-D): The interim efficacy results of a phase 3, randomised, double-blind, placebo-controlled study in India. Lancet 2022, 399, 1313–1321. [Google Scholar] [CrossRef]
- Heath, P.T.; Galiza, E.P.; Baxter, D.N.; Boffito, M.; Browne, D.; Burns, F.; Chadwick, D.R.; Clark, R.; Cosgrove, C.A.; Galloway, J.; et al. Safety and Efficacy of the NVX-CoV2373 Coronavirus Disease 2019 Vaccine at Completion of the Placebo-Controlled Phase of a Randomized Controlled Trial. Clin. Infect. Dis. 2023, 76, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Chiba, S.; Halfmann, P.J.; Iida, S.; Hirata, Y.; Sato, Y.; Kuroda, M.; Armbrust, T.; Spyra, S.; Suzuki, T.; Kawaoka, Y. Recombinant spike protein vaccines coupled with adjuvants that have different modes of action induce protective immunity against SARS-CoV-2. Vaccine 2023, 41, 6025–6035. [Google Scholar] [CrossRef] [PubMed]
- Meo, S.A.; ElToukhy, R.A.; Meo, A.S.; Klonoff, D.C. Comparison of Biological, Pharmacological Characteristics, Indications, Contraindications, Efficacy, and Adverse Effects of Inactivated Whole-Virus COVID-19 Vaccines Sinopharm, CoronaVac, and Covaxin: An Observational Study. Vaccines 2023, 11, 826. [Google Scholar] [CrossRef] [PubMed]
- Ella, R.; Reddy, S.; Blackwelder, W.; Potdar, V.; Yadav, P.; Sarangi, V.; Aileni, V.K.; Kanungo, S.; Rai, S.; Reddy, P.; et al. Efficacy, safety, and lot-to-lot immunogenicity of an inactivated SARS-CoV-2 vaccine (BBV152): Interim results of a randomised, double-blind, controlled, phase 3 trial. Lancet 2021, 398, 2173–2184. [Google Scholar] [CrossRef]
- Liu, C.; Xu, S.; Zheng, Y.; Xie, Y.; Xu, K.; Chai, Y.; Luo, T.; Dai, L.; Gao, G.F. Mosaic RBD nanoparticle elicits immunodominant antibody responses across sarbecoviruses. Cell Rep. 2024, 43, 114235. [Google Scholar] [CrossRef]
- Cohen, A.A.; van Doremalen, N.; Greaney, A.J.; Andersen, H.; Sharma, A.; Starr, T.N.; Keeffe, J.R.; Fan, C.; Schulz, J.E.; Gnanapragasam, P.N.P.; et al. Mosaic RBD nanoparticles protect against challenge by diverse sarbecoviruses in animal models. Science 2022, 377, eabq0839. [Google Scholar] [CrossRef]
- Tan, D.; Li, G.; Fu, W.; Lei, C. Exosomes: The next frontier in vaccine development and delivery. Front. Immunol. 2024, 15, 1435426. [Google Scholar] [CrossRef]
- Hong, S.; Ruan, S.; Greenberg, Z.; He, M.; McGill, J.L. Development of surface engineered antigenic exosomes as vaccines for respiratory syncytial virus. Sci. Rep. 2021, 11, 21358. [Google Scholar] [CrossRef]
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Liejendekker, R.; Harding, C.V.; Melief, C.J.; Geuze, H.J. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996, 183, 1161–1172. [Google Scholar] [CrossRef]
- Li, Q.; Wang, H.; Peng, H.; Huyan, T.; Cacalano, N.A. Exosomes: Versatile Nano Mediators of Immune Regulation. Cancers 2019, 11, 1557. [Google Scholar] [CrossRef] [PubMed]
- El Safadi, D.; Mokhtari, A.; Krejbich, M.; Lagrave, A.; Hirigoyen, U.; Lebeau, G.; Viranaicken, W.; Krejbich-Trotot, P. Exosome-Mediated Antigen Delivery: Unveiling Novel Strategies in Viral Infection Control and Vaccine Design. Vaccines 2024, 12, 280. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Thapa, N. Exosome-Based COVID-19 Vaccine. Methods Mol. Biol. 2023, 2668, 301–311. [Google Scholar] [CrossRef]
- Joe, C.C.D.; Chatterjee, S.; Lovrecz, G.; Adams, T.E.; Thaysen-Andersen, M.; Walsh, R.; Locarnini, S.A.; Smooker, P.; Netter, H.J. Glycoengineered hepatitis B virus-like particles with enhanced immunogenicity. Vaccine 2020, 38, 3892–3901. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Yadav, R.; Kunda, N.K.; Anderson, D.; Bruckner, E.; Miller, E.K.; Basu, R.; Muttil, P.; Tumban, E. Oral immunization with bacteriophage MS2-L2 VLPs protects against oral and genital infection with multiple HPV types associated with head & neck cancers and cervical cancer. Antiviral Res. 2019, 166, 56–65. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, Q.; Li, Y.; Garner, L.V.; Watkins, S.P.; Carter, L.J.; Smoot, J.; Gregg, A.C.; Daniels, A.D.; Jervey, S.; et al. Research and Development on Therapeutic Agents and Vaccines for COVID-19 and Related Human Coronavirus Diseases. ACS Cent. Sci. 2020, 6, 315–331. [Google Scholar] [CrossRef]
- Rosales-Mendoza, S.; Marquez-Escobar, V.A.; Gonzalez-Ortega, O.; Nieto-Gomez, R.; Arevalo-Villalobos, J.I. What Does Plant-Based Vaccine Technology Offer to the Fight against COVID-19? Vaccines 2020, 8, 183. [Google Scholar] [CrossRef]
- Tariq, H.; Batool, S.; Asif, S.; Ali, M.; Abbasi, B.H. Virus-Like Particles: Revolutionary Platforms for Developing Vaccines Against Emerging Infectious Diseases. Front. Microbiol. 2021, 12, 790121. [Google Scholar] [CrossRef]
- Neutra, M.R.; Kozlowski, P.A. Mucosal vaccines: The promise and the challenge. Nat. Rev. Immunol. 2006, 6, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Lavelle, E.C.; Ward, R.W. Mucosal vaccines—Fortifying the frontiers. Nat. Rev. Immunol. 2022, 22, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Xiao, H.; Yang, X.; Cheng, T.; Yuan, L.; Xia, N. Novel vaccine strategies to induce respiratory mucosal immunity: Advances and implications. MedComm 2025, 6, e70056. [Google Scholar] [CrossRef]
- Hemmi, T.; Ainai, A.; Hashiguchi, T.; Tobiume, M.; Kanno, T.; Iwata-Yoshikawa, N.; Iida, S.; Sato, Y.; Miyamoto, S.; Ueno, A.; et al. Intranasal vaccination induced cross-protective secretory IgA antibodies against SARS-CoV-2 variants with reducing the potential risk of lung eosinophilic immunopathology. Vaccine 2022, 40, 5892–5903. [Google Scholar] [CrossRef] [PubMed]
- Pavot, V.; Rochereau, N.; Genin, C.; Verrier, B.; Paul, S. New insights in mucosal vaccine development. Vaccine 2012, 30, 142–154. [Google Scholar] [CrossRef]
- Dotiwala, F.; Upadhyay, A.K. Next Generation Mucosal Vaccine Strategy for Respiratory Pathogens. Vaccines 2023, 11, 1585. [Google Scholar] [CrossRef]
- Ackerson, B.K.; Bruxvoort, K.J.; Qian, L.; Sy, L.S.; Qiu, S.; Tubert, J.E.; Lee, G.S.; Ku, J.H.; Florea, A.; Luo, Y.; et al. Effectiveness and durability of mRNA-1273 BA.4/BA.5 bivalent vaccine (mRNA-1273.222) against SARS-CoV-2 BA.4/BA.5 and XBB sublineages. Hum. Vaccines Immunother. 2024, 20, 2335052. [Google Scholar] [CrossRef]
- Wibowo, D.; Jorritsma, S.H.T.; Gonzaga, Z.J.; Evert, B.; Chen, S.; Rehm, B.H.A. Polymeric nanoparticle vaccines to combat emerging and pandemic threats. Biomaterials 2021, 268, 120597. [Google Scholar] [CrossRef]
- Prabhakar, P.K.; Khurana, N.; Vyas, M.; Sharma, V.; Batiha, G.E.; Kaur, H.; Singh, J.; Kumar, D.; Sharma, N.; Kaushik, A.; et al. Aspects of Nanotechnology for COVID-19 Vaccine Development and Its Delivery Applications. Pharmaceutics 2023, 15, 451. [Google Scholar] [CrossRef]
- Albaz, A.A.; Rafeeq, M.M.; Sain, Z.M.; Almutairi, W.A.; Alamri, A.S.; Aloufi, A.H.; Almalki, W.H.; Tarique, M. Nanotechnology-based approaches in the fight against SARS-CoV-2. AIMS Microbiol. 2021, 7, 368–398. [Google Scholar] [CrossRef]
- Filipic, B.; Pantelic, I.; Nikolic, I.; Majhen, D.; Stojic-Vukanic, Z.; Savic, S.; Krajisnik, D. Nanoparticle-Based Adjuvants and Delivery Systems for Modern Vaccines. Vaccines 2023, 11, 1172. [Google Scholar] [CrossRef] [PubMed]
- Bezbaruah, R.; Chavda, V.P.; Nongrang, L.; Alom, S.; Deka, K.; Kalita, T.; Ali, F.; Bhattacharjee, B.; Vora, L. Nanoparticle-Based Delivery Systems for Vaccines. Vaccines 2022, 10, 1946. [Google Scholar] [CrossRef] [PubMed]
- Reichmuth, A.M.; Oberli, M.A.; Jaklenec, A.; Langer, R.; Blankschtein, D. mRNA vaccine delivery using lipid nanoparticles. Ther. Deliv. 2016, 7, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, L.C.; Costa, P.A.C.; Scalzo Junior, S.R.A.; Ferreira, H.A.S.; Braga, A.C.S.; de Oliveira, L.C.; Figueiredo, M.M.; Shepherd, S.; Hamilton, A.; Queiroz-Junior, C.M.; et al. Nanoparticle-based DNA vaccine protects against SARS-CoV-2 variants in female preclinical models. Nat. Commun. 2024, 15, 590. [Google Scholar] [CrossRef]
- Rauf, A.; Abu-Izneid, T.; Khalil, A.A.; Hafeez, N.; Olatunde, A.; Rahman, M.; Semwal, P.; Al-Awthan, Y.S.; Bahattab, O.S.; Khan, I.N.; et al. Nanoparticles in clinical trials of COVID-19: An update. Int. J. Surg. 2022, 104, 106818. [Google Scholar] [CrossRef]
- Kim, Y.C.; Park, J.H.; Prausnitz, M.R. Microneedles for drug and vaccine delivery. Adv. Drug Deliv. Rev. 2012, 64, 1547–1568. [Google Scholar] [CrossRef]
- Kisakov, D.N.; Belyakov, I.M.; Kisakova, L.A.; Yakovlev, V.A.; Tigeeva, E.V.; Karpenko, L.I. The use of electroporation to deliver DNA-based vaccines. Expert. Rev. Vaccines 2024, 23, 102–123. [Google Scholar] [CrossRef]
- Kennedy, R.B.; Ovsyannikova, I.G.; Palese, P.; Poland, G.A. Current Challenges in Vaccinology. Front. Immunol. 2020, 11, 1181. [Google Scholar] [CrossRef]
- Demongeot, J.; Fougere, C. mRNA COVID-19 Vaccines-Facts and Hypotheses on Fragmentation and Encapsulation. Vaccines 2022, 11, 40. [Google Scholar] [CrossRef]
- Magazine, N.; Zhang, T.; Bungwon, A.D.; McGee, M.C.; Wu, Y.; Veggiani, G.; Huang, W. Immune Epitopes of SARS-CoV-2 Spike Protein and Considerations for Universal Vaccine Development. bioRxiv 2023. [Google Scholar] [CrossRef]
- Jiang, N.; Malone, M.; Chizari, S. Antigen-specific and cross-reactive T cells in protection and disease. Immunol. Rev. 2023, 316, 120–135. [Google Scholar] [CrossRef]
- Ou, B.S.; Baillet, J.; Filsinger Interrante, M.V.; Adamska, J.Z.; Zhou, X.; Saouaf, O.M.; Yan, J.; Klich, J.H.; Jons, C.K.; Meany, E.L.; et al. Saponin nanoparticle adjuvants incorporating Toll-like receptor agonists drive distinct immune signatures and potent vaccine responses. Sci. Adv. 2024, 10, eadn7187. [Google Scholar] [CrossRef] [PubMed]
- Pulendran, B.; Arunachalam, P.S.; O’Hagan, D.T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 2021, 20, 454–475. [Google Scholar] [CrossRef] [PubMed]
- Pooley, N.; Abdool Karim, S.S.; Combadiere, B.; Ooi, E.E.; Harris, R.C.; El Guerche Seblain, C.; Kisomi, M.; Shaikh, N. Durability of Vaccine-Induced and Natural Immunity Against COVID-19: A Narrative Review. Infect. Dis. Ther. 2023, 12, 367–387. [Google Scholar] [CrossRef]
- Thompson, M.G.; Clippard, J.; Petrie, J.G.; Jackson, M.L.; McLean, H.Q.; Gaglani, M.; Reis, E.C.; Flannery, B.; Monto, A.S.; Jackson, L.; et al. Influenza Vaccine Effectiveness for Fully and Partially Vaccinated Children 6 Months to 8 Years Old During 2011–2012 and 2012–2013: The Importance of Two Priming Doses. Pediatr. Infect. Dis. J. 2016, 35, 299–308. [Google Scholar] [CrossRef]
- Yin, Q.; Wang, Y.; Xiang, Y.; Xu, F. Nanovaccines: Merits, and diverse roles in boosting antitumor immune responses. Hum. Vaccines Immunother. 2022, 18, 2119020. [Google Scholar] [CrossRef]
- Ou, B.S.; Saouaf, O.M.; Baillet, J.; Appel, E.A. Sustained delivery approaches to improving adaptive immune responses. Adv. Drug Deliv. Rev. 2022, 187, 114401. [Google Scholar] [CrossRef] [PubMed]
- Kerfoot, S.M.; Yaari, G.; Patel, J.R.; Johnson, K.L.; Gonzalez, D.G.; Kleinstein, S.H.; Haberman, A.M. Germinal center B cell and T follicular helper cell development initiates in the interfollicular zone. Immunity 2011, 34, 947–960. [Google Scholar] [CrossRef]
- Yaugel-Novoa, M.; Bourlet, T.; Paul, S. Role of the humoral immune response during COVID-19: Guilty or not guilty? Mucosal Immunol. 2022, 15, 1170–1180. [Google Scholar] [CrossRef]
- Moga, E.; Lynton-Pons, E.; Domingo, P. The Robustness of Cellular Immunity Determines the Fate of SARS-CoV-2 Infection. Front. Immunol. 2022, 13, 904686. [Google Scholar] [CrossRef]
- Hermens, J.M.; Kesmir, C. Role of T cells in severe COVID-19 disease, protection, and long term immunity. Immunogenetics 2023, 75, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Niessl, J.; Sekine, T.; Buggert, M. T cell immunity to SARS-CoV-2. Semin. Immunol. 2021, 55, 101505. [Google Scholar] [CrossRef]
- Rijkers, G.T.; Weterings, N.; Obregon-Henao, A.; Lepolder, M.; Dutt, T.S.; van Overveld, F.J.; Henao-Tamayo, M. Antigen Presentation of mRNA-Based and Virus-Vectored SARS-CoV-2 Vaccines. Vaccines 2021, 9, 848. [Google Scholar] [CrossRef]
- Nguyen, J.L.; Mitratza, M.; Volkman, H.R.; de Munter, L.; Tran, T.M.P.; Marques, C.; Mustapha, M.; Valluri, S.; Yang, J.; Anton, A.; et al. Effectiveness of the BNT162b2 XBB.1.5-adapted vaccine against COVID-19 hospitalization related to the JN.1 variant in Europe: A test-negative case-control study using the id.DRIVE platform. EClinicalMedicine 2025, 79, 102995. [Google Scholar] [CrossRef]
- Parums, D.V. Editorial: The XBB.1.5 (‘Kraken’) Subvariant of Omicron SARS-CoV-2 and its Rapid Global Spread. Med. Sci. Monit. 2023, 29, e939580. [Google Scholar] [CrossRef]
- Giancotti, R.; Lomoio, U.; Puccio, B.; Tradigo, G.; Vizza, P.; Torti, C.; Veltri, P.; Guzzi, P.H. The Omicron XBB.1 Variant and Its Descendants: Genomic Mutations, Rapid Dissemination and Notable Characteristics. Biology 2024, 13, 90. [Google Scholar] [CrossRef]
- Patel, N.; Trost, J.F.; Guebre-Xabier, M.; Zhou, H.; Norton, J.; Jiang, D.; Cai, Z.; Zhu, M.; Marchese, A.M.; Greene, A.M.; et al. XBB.1.5 spike protein COVID-19 vaccine induces broadly neutralizing and cellular immune responses against EG.5.1 and emerging XBB variants. Sci. Rep. 2023, 13, 19176. [Google Scholar] [CrossRef] [PubMed]
- Curlin, M.E.; Bates, T.A.; Guzman, G.; Schoen, D.; McBride, S.K.; Carpenter, S.D.; Tafesse, F.G. Omicron neutralizing antibody response following booster vaccination compared with breakthrough infection. Med 2022, 3, 827–837.e823. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.; Rahai, N.; Beck, E.; Beebe, E.; Conroy, B.; Esposito, D.; Govil, P.; Kopel, H.; Lu, T.; Mansi, J.; et al. Evaluating the Effectiveness of mRNA-1273.815 Against COVID-19 Hospitalization Among Adults Aged >/= 18 Years in the United States. Infect. Dis. Ther. 2025, 14, 199–216. [Google Scholar] [CrossRef]
- Focosi, D.; Spezia, P.G.; Maggi, F. SARS-CoV-2 BA.2.86 (“Pirola”): Is it Pi or Just Another Omicron Sublineage? Vaccines 2023, 11, 1634. [Google Scholar] [CrossRef]
- Satapathy, P.; Kumar, P.; Gupta, J.K.; Rabaan, A.A.; Al Kaabi, N.A.; Mohanty, D.; Naveen, P.; Khatib, M.N.; Gaidhane, S.; Zahiruddin, Q.S.; et al. The emergence and implications of SARS-CoV-2 omicron subvariant BA.2.86 on global health. Int. J. Surg. 2024, 110, 2498–2501. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jiang, S.; Ma, W.; Li, X.; Wei, K.; Xie, F.; Zhao, C.; Zhao, X.; Wang, S.; Li, C.; et al. Enhanced neutralization of SARS-CoV-2 variant BA.2.86 and XBB sub-lineages by a tetravalent COVID-19 vaccine booster. Cell Host Microbe 2024, 32, 25–34.e25. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Guo, Y.; Bowen, A.; Mellis, I.A.; Valdez, R.; Gherasim, C.; Gordon, A.; Liu, L.; Ho, D.D. XBB.1.5 monovalent mRNA vaccine booster elicits robust neutralizing antibodies against XBB subvariants and JN.1. Cell Host Microbe 2024, 32, 315–321.e313. [Google Scholar] [CrossRef]
- Krammer, F.; Ellebedy, A.H. Variant-adapted COVID-19 booster vaccines. Science 2023, 382, 157–159. [Google Scholar] [CrossRef]
- Goel, R.R.; Painter, M.M.; Lundgreen, K.A.; Apostolidis, S.A.; Baxter, A.E.; Giles, J.R.; Mathew, D.; Pattekar, A.; Reynaldi, A.; Khoury, D.S.; et al. Efficient recall of Omicron-reactive B cell memory after a third dose of SARS-CoV-2 mRNA vaccine. Cell 2022, 185, 1875–1887.e1878. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, Y.C.; Chen, J.W.; Kang, J.; Burns, M.D.; St Denis, K.J.; Sheehan, M.L.; Davis, J.P.; Edlow, A.G.; Balazs, A.B.; Yonker, L.M.; et al. BNT162b2 induces robust cross-variant SARS-CoV-2 immunity in children. NPJ Vaccines 2022, 7, 158. [Google Scholar] [CrossRef]
- Yang, J.; Hong, W.; Shi, H.; He, C.; Lei, H.; Zhou, Y.; Yang, H.; Alu, A.; Chen, Z.; Yang, Y.; et al. Trivalent recombinant protein vaccine induces cross-neutralization against XBB lineage and JN.1 subvariants: Preclinical and phase 1 clinical trials. Nat. Commun. 2024, 15, 10778. [Google Scholar] [CrossRef]
- Wang, C.Y.; Peng, W.J.; Kuo, B.S.; Ho, Y.H.; Wang, M.S.; Yang, Y.T.; Chang, P.Y.; Shen, Y.H.; Hwang, K.P. Toward a pan-SARS-CoV-2 vaccine targeting conserved epitopes on spike and non-spike proteins for potent, broad and durable immune responses. PLoS Pathog. 2023, 19, e1010870. [Google Scholar] [CrossRef]
- O’Hagan, D.T.; van der Most, R.; Lodaya, R.N.; Coccia, M.; Lofano, G. “World in motion”—Emulsion adjuvants rising to meet the pandemic challenges. NPJ Vaccines 2021, 6, 158. [Google Scholar] [CrossRef]
- Sohail, M.S.; Ahmed, S.F.; Quadeer, A.A.; McKay, M.R. Cross-Reactivity Assessment of Vaccine-Derived SARS-CoV-2 T Cell Responses against BA.2.86 and JN.1. Viruses 2024, 16, 473. [Google Scholar] [CrossRef]
- Siddiqui, A.; Adnan, A.; Abbas, M.; Taseen, S.; Ochani, S.; Essar, M.Y. Revival of the heterologous prime-boost technique in COVID-19: An outlook from the history of outbreaks. Health Sci. Rep. 2022, 5, e531. [Google Scholar] [CrossRef]
- Suphanchaimat, R.; Nittayasoot, N.; Jiraphongsa, C.; Thammawijaya, P.; Bumrungwong, P.; Tulyathan, A.; Cheewaruangroj, N.; Pittayawonganon, C.; Tharmaphornpilas, P. Real-World Effectiveness of Mix-and-Match Vaccine Regimens against SARS-CoV-2 Delta Variant in Thailand: A Nationwide Test-Negative Matched Case-Control Study. Vaccines 2022, 10, 1080. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, I.R.; Sebastian, S. Novel viral vectors in infectious diseases. Immunology 2018, 153, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pollet, J.; Chen, W.H.; Strych, U. Recombinant protein vaccines, a proven approach against coronavirus pandemics. Adv. Drug Deliv. Rev. 2021, 170, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Atmar, R.L.; Lyke, K.E.; Deming, M.E.; Jackson, L.A.; Branche, A.R.; El Sahly, H.M.; Rostad, C.A.; Martin, J.M.; Johnston, C.; Rupp, R.E.; et al. Homologous and Heterologous COVID-19 Booster Vaccinations. N. Engl. J. Med. 2022, 386, 1046–1057. [Google Scholar] [CrossRef]
- Song, Y.; Wang, J.; Yang, Z.; He, Q.; Bao, C.; Xie, Y.; Sun, Y.; Li, S.; Quan, Y.; Yang, H.; et al. Heterologous booster vaccination enhances antibody responses to SARS-CoV-2 by improving Tfh function and increasing B-cell clonotype SHM frequency. Front. Immunol. 2024, 15, 1406138. [Google Scholar] [CrossRef]
- Ozbay Kurt, F.G.; Lepper, A.; Gerhards, C.; Roemer, M.; Lasser, S.; Arkhypov, I.; Bitsch, R.; Bugert, P.; Altevogt, P.; Gouttefangeas, C.; et al. Booster dose of mRNA vaccine augments waning T cell and antibody responses against SARS-CoV-2. Front. Immunol. 2022, 13, 1012526. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, Y.; Liu, X.; Hou, F.; Cai, R.; Yu, Z.; Liu, F.; Yang, G.; Ding, J.; Xu, J.; et al. Heterologous boost with mRNA vaccines against SARS-CoV-2 Delta/Omicron variants following an inactivated whole-virus vaccine. Antiviral Res. 2023, 212, 105556. [Google Scholar] [CrossRef]
- Sritipsukho, P.; Khawcharoenporn, T.; Siribumrungwong, B.; Damronglerd, P.; Suwantarat, N.; Satdhabudha, A.; Chaiyakulsil, C.; Sinlapamongkolkul, P.; Tangsathapornpong, A.; Bunjoungmanee, P.; et al. Real-life effectiveness of COVID-19 vaccine during the Omicron variant-dominant pandemic: How many booster doses do we need? Emerg. Microbes Infect. 2023, 12, 2174779. [Google Scholar] [CrossRef]
- Sapkota, B.; Saud, B.; Shrestha, R.; Al-Fahad, D.; Sah, R.; Shrestha, S.; Rodriguez-Morales, A.J. Heterologous prime-boost strategies for COVID-19 vaccines. J. Travel. Med. 2022, 29, taab191. [Google Scholar] [CrossRef]
- Kim, D.; Pekgun, P.; Yildirim, I.; Keskinocak, P. Resource allocation for different types of vaccines against COVID-19: Tradeoffs and synergies between efficacy and reach. Vaccine 2021, 39, 6876–6882. [Google Scholar] [CrossRef] [PubMed]
- Sekulovski, M.; Mileva, N.; Vasilev, G.V.; Miteva, D.; Gulinac, M.; Peshevska-Sekulovska, M.; Chervenkov, L.; Batselova, H.; Vasilev, G.H.; Tomov, L.; et al. Blood Coagulation and Thrombotic Disorders following SARS-CoV-2 Infection and COVID-19 Vaccination. Biomedicines 2023, 11, 2813. [Google Scholar] [CrossRef]
- Al Fayez, N.; Nassar, M.S.; Alshehri, A.A.; Alnefaie, M.K.; Almughem, F.A.; Alshehri, B.Y.; Alawad, A.O.; Tawfik, E.A. Recent Advancement in mRNA Vaccine Development and Applications. Pharmaceutics 2023, 15, 1972. [Google Scholar] [CrossRef] [PubMed]
- Aguas, R.; Bharath, A.; White, L.J.; Gao, B.; Pollard, A.J.; Voysey, M.; Shretta, R. Potential global impacts of alternative dosing regimen and rollout options for the ChAdOx1 nCoV-19 vaccine. Nat. Commun. 2021, 12, 6370. [Google Scholar] [CrossRef]
- Bollaerts, K.; Wyndham-Thomas, C.; Miller, E.; Izurieta, H.S.; Black, S.; Andrews, N.; Rubbrecht, M.; Van Heuverswyn, F.; Neels, P. The role of real-world evidence for regulatory and public health decision-making for Accelerated Vaccine Deployment—A meeting report. Biologicals 2024, 85, 101750. [Google Scholar] [CrossRef]
- Matrajt, L.; Eaton, J.; Leung, T.; Dimitrov, D.; Schiffer, J.T.; Swan, D.A.; Janes, H. Optimizing vaccine allocation for COVID-19 vaccines: Potential role of single-dose vaccination. medRxiv 2021. [Google Scholar] [CrossRef]
- Maltseva, M.; Keeshan, A.; Cooper, C.; Langlois, M.A. Immune imprinting: The persisting influence of the first antigenic encounter with rapidly evolving viruses. Hum. Vaccines Immunother. 2024, 20, 2384192. [Google Scholar] [CrossRef] [PubMed]
- Goel, R.R.; Painter, M.M.; Apostolidis, S.A.; Mathew, D.; Meng, W.; Rosenfeld, A.M.; Lundgreen, K.A.; Reynaldi, A.; Khoury, D.S.; Pattekar, A.; et al. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science 2021, 374, abm0829. [Google Scholar] [CrossRef]
- Johnston, T.S.; Li, S.H.; Painter, M.M.; Atkinson, R.K.; Douek, N.R.; Reeg, D.B.; Douek, D.C.; Wherry, E.J.; Hensley, S.E. Immunological imprinting shapes the specificity of human antibody responses against SARS-CoV-2 variants. Immunity 2024, 57, 912–925. [Google Scholar] [CrossRef]
- Silva, M.; Kato, Y.; Melo, M.B.; Phung, I.; Freeman, B.L.; Li, Z.; Roh, K.; Van Wijnbergen, J.W.; Watkins, H.; Enemuo, C.A.; et al. A particulate saponin/TLR agonist vaccine adjuvant alters lymph flow and modulates adaptive immunity. Sci. Immunol. 2021, 6, eabf1152. [Google Scholar] [CrossRef]
- Deng, S.; Liang, H.; Chen, P.; Li, Y.; Li, Z.; Fan, S.; Wu, K.; Li, X.; Chen, W.; Qin, Y.; et al. Viral Vector Vaccine Development and Application during the COVID-19 Pandemic. Microorganisms 2022, 10, 1450. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Sun, J.; Wang, Y.; Tan, D.; Cao, Z.; Gao, L.; Guan, Y.; Jia, X.; Mao, J. A Review of Inactivated COVID-19 Vaccine Development in China: Focusing on Safety and Efficacy in Special Populations. Vaccines 2023, 11, 1045. [Google Scholar] [CrossRef] [PubMed]
- Pourrazavi, S.; Fathifar, Z.; Sharma, M.; Allahverdipour, H. COVID-19 vaccine hesitancy: A Systematic review of cognitive determinants. Health Promot. Perspect. 2023, 13, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Asundi, A.; O’Leary, C.; Bhadelia, N. Global COVID-19 vaccine inequity: The scope, the impact, and the challenges. Cell Host Microbe 2021, 29, 1036–1039. [Google Scholar] [CrossRef]
- Saad-Roy, C.M.; Morris, S.E.; Boots, M.; Baker, R.E.; Lewis, B.L.; Farrar, J.; Marathe, M.V.; Graham, A.L.; Levin, S.A.; Wagner, C.E.; et al. Impact of waning immunity against SARS-CoV-2 severity exacerbated by vaccine hesitancy. PLoS Comput. Biol. 2024, 20, e1012211. [Google Scholar] [CrossRef]
- Tuckerman, J.; Kaufman, J.; Danchin, M. Effective Approaches to Combat Vaccine Hesitancy. Pediatr. Infect. Dis. J. 2022, 41, e243–e245. [Google Scholar] [CrossRef]
- Majekodunmi, P.; Tulli-Shah, M.; Kemei, J.; Kayode, I.; Maduforo, A.N.; Salami, B. Interventions employed to address vaccine hesitancy among Black populations outside of African and Caribbean countries: A scoping review. BMC Public Health 2024, 24, 3147. [Google Scholar] [CrossRef]
- Yoo, K.J.; Mehta, A.; Mak, J.; Bishai, D.; Chansa, C.; Patenaude, B. COVAX and equitable access to COVID-19 vaccines. Bull. World Health Organ. 2022, 100, 315–328. [Google Scholar] [CrossRef]
- Moore, S.; Hill, E.M.; Dyson, L.; Tildesley, M.J.; Keeling, M.J. Retrospectively modeling the effects of increased global vaccine sharing on the COVID-19 pandemic. Nat. Med. 2022, 28, 2416–2423. [Google Scholar] [CrossRef]
- Clemente-Suarez, V.J.; Hormeno-Holgado, A.; Jimenez, M.; Benitez-Agudelo, J.C.; Navarro-Jimenez, E.; Perez-Palencia, N.; Maestre-Serrano, R.; Laborde-Cardenas, C.C.; Tornero-Aguilera, J.F. Dynamics of Population Immunity Due to the Herd Effect in the COVID-19 Pandemic. Vaccines 2020, 8, 236. [Google Scholar] [CrossRef]
- Chirico, F.; Teixeira da Silva, J.A. Evidence-based policies in public health to address COVID-19 vaccine hesitancy. Future Virol. 2023, 18, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Mistry, P.; Barmania, F.; Mellet, J.; Peta, K.; Strydom, A.; Viljoen, I.M.; James, W.; Gordon, S.; Pepper, M.S. SARS-CoV-2 Variants, Vaccines, and Host Immunity. Front. Immunol. 2021, 12, 809244. [Google Scholar] [CrossRef]
- Keyel, A.C.; Russell, A.; Plitnick, J.; Rowlands, J.V.; Lamson, D.M.; Rosenberg, E.; St George, K. SARS-CoV-2 Vaccine Breakthrough by Omicron and Delta Variants, New York, USA. Emerg. Infect. Dis. 2022, 28, 1990–1998. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.P.; Bezbaruah, R.; Deka, K.; Nongrang, L.; Kalita, T. The Delta and Omicron Variants of SARS-CoV-2: What We Know So Far. Vaccines 2022, 10, 1926. [Google Scholar] [CrossRef]
- Verbeke, R.; Hogan, M.J.; Lore, K.; Pardi, N. Innate immune mechanisms of mRNA vaccines. Immunity 2022, 55, 1993–2005. [Google Scholar] [CrossRef] [PubMed]
- Verheul, M.K.; Nijhof, K.H.; de Zeeuw-Brouwer, M.L.; Duijm, G.; Ten Hulscher, H.; de Rond, L.; Beckers, L.; Eggink, D.; van Tol, S.; Reimerink, J.; et al. Booster Immunization Improves Memory B Cell Responses in Older Adults Unresponsive to Primary SARS-CoV-2 Immunization. Vaccines 2023, 11, 1196. [Google Scholar] [CrossRef]
- Yaamika, H.; Muralidas, D.; Elumalai, K. Review of adverse events associated with COVID-19 vaccines, highlighting their frequencies and reported cases. J. Taibah Univ. Med. Sci. 2023, 18, 1646–1661. [Google Scholar] [CrossRef]
- Ferrara, P.; Ponticelli, D.; Losa, L.; Romeo, C.; Magliuolo, R.; Vitale, A.; Zampella, A.; Alleanza, L.; Borrelli, M.; Schiavone, B.; et al. Risk of Repeated Adverse Effects following Booster Dose of mRNA COVID-19 Vaccine: Results from the MOSAICO Study. Vaccines 2023, 11, 247. [Google Scholar] [CrossRef]
- Shah, A.; Coiado, O.C. COVID-19 vaccine and booster hesitation around the world: A literature review. Front Med. 2022, 9, 1054557. [Google Scholar] [CrossRef]
- Lazarus, J.V.; Wyka, K.; White, T.M.; Picchio, C.A.; Gostin, L.O.; Larson, H.J.; Rabin, K.; Ratzan, S.C.; Kamarulzaman, A.; El-Mohandes, A. A survey of COVID-19 vaccine acceptance across 23 countries in 2022. Nat. Med. 2023, 29, 366–375. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kruger, N.; Schulz, S.; Cossmann, A.; Rocha, C.; Kempf, A.; Nehlmeier, I.; Graichen, L.; Moldenhauer, A.S.; Winkler, M.S.; et al. The Omicron variant is highly resistant against antibody-mediated neutralization: Implications for control of the COVID-19 pandemic. Cell 2022, 185, 447–456.e411. [Google Scholar] [CrossRef]
- San Filippo, S.; Crovetto, B.; Bucek, J.; Nahass, R.G.; Milano, M.; Brunetti, L. Comparative Efficacy of Early COVID-19 Monoclonal Antibody Therapies: A Retrospective Analysis. Open Forum Infect. Dis. 2022, 9, ofac080. [Google Scholar] [CrossRef] [PubMed]
- Corwin, D.S.; Ender, P.T.; Sahu, N.; Durgham, R.A.; McGorry, D.M., Jr.; Rahman, A.; Stoltzfus, J.; Jahre, J.A. The Efficacy of Bamlanivimab in Reducing Emergency Department Visits and Hospitalizations in a Real-world Setting. Open Forum Infect. Dis. 2021, 8, ofab305. [Google Scholar] [CrossRef]
- Pinto, P.B.A.; Timis, J.; Chuensirikulchai, K.; Li, Q.H.; Lu, H.H.; Maule, E.; Nguyen, M.; Alves, R.; Verma, S.K.; Ana-Sosa-Batiz, F.; et al. Co-immunization with spike and nucleocapsid based DNA vaccines for long-term protective immunity against SARS-CoV-2 Omicron. NPJ Vaccines 2024, 9, 252. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Verma, A.K.; Shi, J.; Guan, X.; Meyerholz, D.K.; Bu, F.; Wen, W.; Liu, B.; Li, F.; Perlman, S.; et al. Universal subunit vaccine protects against multiple SARS-CoV-2 variants and SARS-CoV. NPJ Vaccines 2024, 9, 133. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Ganguly, A.; Kumar, A.; Srivastava, N.; Pathak, R. Harnessing Epigenetics: Innovative Approaches in Diagnosing and Combating Viral Acute Respiratory Infections. Pathogens 2025, 14, 129. [Google Scholar] [CrossRef]
- Majewska, M.; Mazdziarz, M.; Krawczyk, K.; Paukszto, L.; Makowczenko, K.G.; Lepiarczyk, E.; Lipka, A.; Wiszpolska, M.; Gorska, A.; Moczulska, B.; et al. SARS-CoV-2 disrupts host gene networks: Unveiling key hub genes as potential therapeutic targets for COVID-19 management. Comput. Biol. Med. 2024, 183, 109343. [Google Scholar] [CrossRef]
- Kee, J.; Thudium, S.; Renner, D.M.; Glastad, K.; Palozola, K.; Zhang, Z.; Li, Y.; Lan, Y.; Cesare, J.; Poleshko, A.; et al. SARS-CoV-2 disrupts host epigenetic regulation via histone mimicry. Nature 2022, 610, 381–388. [Google Scholar] [CrossRef]
- Zhai, L.Y.; Su, A.M.; Liu, J.F.; Zhao, J.J.; Xi, X.G.; Hou, X.M. Recent advances in applying G-quadruplex for SARS-CoV-2 targeting and diagnosis: A review. Int. J. Biol. Macromol. 2022, 221, 1476–1490. [Google Scholar] [CrossRef]
- Panera, N.; Tozzi, A.E.; Alisi, A. The G-Quadruplex/Helicase World as a Potential Antiviral Approach Against COVID-19. Drugs 2020, 80, 941–946. [Google Scholar] [CrossRef]
- Zhao, C.; Qin, G.; Niu, J.; Wang, Z.; Wang, C.; Ren, J.; Qu, X. Targeting RNA G-Quadruplex in SARS-CoV-2: A Promising Therapeutic Target for COVID-19? Angew. Chem. Int. Ed. Engl. 2021, 60, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Pathak, R. G-Quadruplexes in the Viral Genome: Unlocking Targets for Therapeutic Interventions and Antiviral Strategies. Viruses 2023, 15, 2216. [Google Scholar] [CrossRef]
- Qin, G.; Zhao, C.; Liu, Y.; Zhang, C.; Yang, G.; Yang, J.; Wang, Z.; Wang, C.; Tu, C.; Guo, Z.; et al. RNA G-quadruplex formed in SARS-CoV-2 used for COVID-19 treatment in animal models. Cell Discov. 2022, 8, 86. [Google Scholar] [CrossRef]
- Agampodi, S.; Mogeni, O.D.; Chandler, R.; Pansuriya, M.; Kim, J.H.; Excler, J.L. Global pandemic preparedness: Learning from the COVID-19 vaccine development and distribution. Expert. Rev. Vaccines 2024, 23, 761–772. [Google Scholar] [CrossRef] [PubMed]
- McLean, G.; Kamil, J.; Lee, B.; Moore, P.; Schulz, T.F.; Muik, A.; Sahin, U.; Tureci, O.; Pather, S. The Impact of Evolving SARS-CoV-2 Mutations and Variants on COVID-19 Vaccines. mBio 2022, 13, e0297921. [Google Scholar] [CrossRef]
- Rahman, M.M.; Masum, M.H.U.; Wajed, S.; Talukder, A. A comprehensive review on COVID-19 vaccines: Development, effectiveness, adverse effects, distribution and challenges. Virusdisease 2022, 33, 1–22. [Google Scholar] [CrossRef]
- Arashkia, A.; Jalilvand, S.; Mohajel, N.; Afchangi, A.; Azadmanesh, K.; Salehi-Vaziri, M.; Fazlalipour, M.; Pouriayevali, M.H.; Jalali, T.; Mousavi Nasab, S.D.; et al. Severe acute respiratory syndrome-coronavirus-2 spike (S) protein based vaccine candidates: State of the art and future prospects. Rev. Med. Virol. 2021, 31, e2183. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N.; Redwan, E.M.; Makis, W.; Rubio-Casillas, A. IgG4 Antibodies Induced by Repeated Vaccination May Generate Immune Tolerance to the SARS-CoV-2 Spike Protein. Vaccines 2023, 11, 991. [Google Scholar] [CrossRef]
- Haq, M.A.; Roy, A.K.; Ahmed, R.; Kuddusi, R.U.; Sinha, M.; Hossain, M.S.; Vandenent, M.; Islam, M.Z.; Zaman, R.U.; Kibria, M.G.; et al. Antibody longevity and waning following COVID-19 vaccination in a 1-year longitudinal cohort in Bangladesh. Sci. Rep. 2024, 14, 11467. [Google Scholar] [CrossRef]
- Baker, J.R., Jr.; Farazuddin, M.; Wong, P.T.; O’Konek, J.J. The unfulfilled potential of mucosal immunization. J. Allergy Clin. Immunol. 2022, 150, 1–11. [Google Scholar] [CrossRef]
- Stolovich-Rain, M.; Kumari, S.; Friedman, A.; Kirillov, S.; Socol, Y.; Billan, M.; Pal, R.R.; Das, K.; Golding, P.; Oiknine-Djian, E.; et al. Intramuscular mRNA BNT162b2 vaccine against SARS-CoV-2 induces neutralizing salivary IgA. Front. Immunol. 2022, 13, 933347. [Google Scholar] [CrossRef] [PubMed]
- Harvey, W.T.; Carabelli, A.M.; Jackson, B.; Gupta, R.K.; Thomson, E.C.; Harrison, E.M.; Ludden, C.; Reeve, R.; Rambaut, A.; Consortium, C.-G.U.; et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021, 19, 409–424. [Google Scholar] [CrossRef]
- Cele, S.; Jackson, L.; Khoury, D.S.; Khan, K.; Moyo-Gwete, T.; Tegally, H.; San, J.E.; Cromer, D.; Scheepers, C.; Amoako, D.G.; et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature 2022, 602, 654–656. [Google Scholar] [CrossRef]
- Tang, J.; Zeng, C.; Cox, T.M.; Li, C.; Son, Y.M.; Cheon, I.S.; Wu, Y.; Behl, S.; Taylor, J.J.; Chakaraborty, R.; et al. Respiratory mucosal immunity against SARS-CoV-2 after mRNA vaccination. Sci. Immunol. 2022, 7, eadd4853. [Google Scholar] [CrossRef] [PubMed]
- Kundu, R.; Narean, J.S.; Wang, L.; Fenn, J.; Pillay, T.; Fernandez, N.D.; Conibear, E.; Koycheva, A.; Davies, M.; Tolosa-Wright, M.; et al. Cross-reactive memory T cells associate with protection against SARS-CoV-2 infection in COVID-19 contacts. Nat. Commun. 2022, 13, 80. [Google Scholar] [CrossRef] [PubMed]
- Tarke, A.; Sidney, J.; Methot, N.; Yu, E.D.; Zhang, Y.; Dan, J.M.; Goodwin, B.; Rubiro, P.; Sutherland, A.; Wang, E.; et al. Impact of SARS-CoV-2 variants on the total CD4(+) and CD8(+) T cell reactivity in infected or vaccinated individuals. Cell Rep. Med. 2021, 2, 100355. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, J.; Jian, F.; Xiao, T.; Song, W.; Yisimayi, A.; Huang, W.; Li, Q.; Wang, P.; An, R.; et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature 2022, 602, 657–663. [Google Scholar] [CrossRef]
- Kurhade, C.; Zou, J.; Xia, H.; Liu, M.; Chang, H.C.; Ren, P.; Xie, X.; Shi, P.Y. Low neutralization of SARS-CoV-2 Omicron BA.2.75.2, BQ.1.1 and XBB.1 by parental mRNA vaccine or a BA.5 bivalent booster. Nat. Med. 2023, 29, 344–347. [Google Scholar] [CrossRef]
- Gagne, M.; Moliva, J.I.; Foulds, K.E.; Andrew, S.F.; Flynn, B.J.; Werner, A.P.; Wagner, D.A.; Teng, I.T.; Lin, B.C.; Moore, C.; et al. mRNA-1273 or mRNA-Omicron boost in vaccinated macaques elicits similar B cell expansion, neutralizing responses, and protection from Omicron. Cell 2022, 185, 1556–1571.e1518. [Google Scholar] [CrossRef]
- Cohen, A.A.; Gnanapragasam, P.N.P.; Lee, Y.E.; Hoffman, P.R.; Ou, S.; Kakutani, L.M.; Keeffe, J.R.; Wu, H.J.; Howarth, M.; West, A.P.; et al. Mosaic nanoparticles elicit cross-reactive immune responses to zoonotic coronaviruses in mice. Science 2021, 371, 735–741. [Google Scholar] [CrossRef]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 2020, 181, 1489–1501.e1415. [Google Scholar] [CrossRef] [PubMed]
- Nouailles, G.; Adler, J.M.; Pennitz, P.; Peidli, S.; Teixeira Alves, L.G.; Baumgardt, M.; Bushe, J.; Voss, A.; Langenhagen, A.; Langner, C.; et al. Live-attenuated vaccine sCPD9 elicits superior mucosal and systemic immunity to SARS-CoV-2 variants in hamsters. Nat. Microbiol. 2023, 8, 860–874. [Google Scholar] [CrossRef] [PubMed]
- Dhama, K.; Dhawan, M.; Tiwari, R.; Emran, T.B.; Mitra, S.; Rabaan, A.A.; Alhumaid, S.; Alawi, Z.A.; Al Mutair, A. COVID-19 intranasal vaccines: Current progress, advantages, prospects, and challenges. Hum. Vaccines Immunother. 2022, 18, 2045853. [Google Scholar] [CrossRef]
- Li, L.; Yang, Z.; Li, J. Exosomes and SARS-CoV-2 infection. Front. Immunol. 2024, 15, 1467109. [Google Scholar] [CrossRef] [PubMed]
- Minor, P.D. Live attenuated vaccines: Historical successes and current challenges. Virology 2015, 479–480, 379–392. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Goldberg, Y.; Mandel, M.; Bar-On, Y.M.; Bodenheimer, O.; Freedman, L.S.; Ash, N.; Alroy-Preis, S.; Huppert, A.; Milo, R. Protection and Waning of Natural and Hybrid Immunity to SARS-CoV-2. N. Engl. J. Med. 2022, 386, 2201–2212. [Google Scholar] [CrossRef]
- Russell, M.W.; Moldoveanu, Z.; Ogra, P.L.; Mestecky, J. Mucosal Immunity in COVID-19: A Neglected but Critical Aspect of SARS-CoV-2 Infection. Front. Immunol. 2020, 11, 611337. [Google Scholar] [CrossRef]
- Poon, M.M.L.; Rybkina, K.; Kato, Y.; Kubota, M.; Matsumoto, R.; Bloom, N.I.; Zhang, Z.; Hastie, K.M.; Grifoni, A.; Weiskopf, D.; et al. SARS-CoV-2 infection generates tissue-localized immunological memory in humans. Sci. Immunol. 2021, 6, eabl9105. [Google Scholar] [CrossRef]
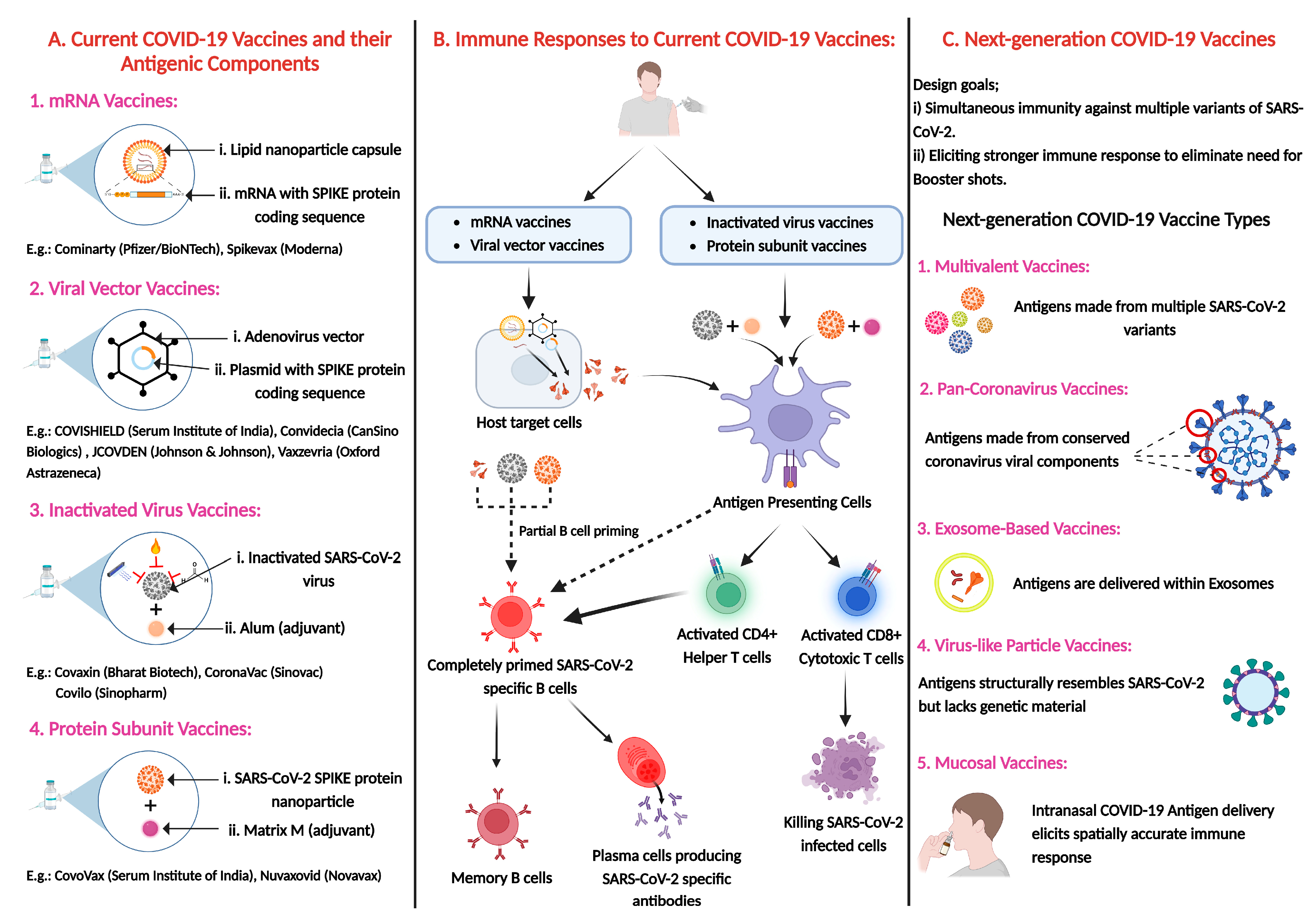
| Variants of Concern (VOCs) | Country/First Appearance | Key Mutations | Virulence | Immune Evasion | Global Impact | Most Effective Vaccines | References |
|---|---|---|---|---|---|---|---|
| Alpha (B.1.1.7) | UK, September 2020 | N501Y, P681H, Deletion 69–70 | Increased transmissibility, similar severity | Minimal, reduced neutralization by some antibodies | Dominated Europe and North America; increased case numbers | Pfizer-BioNTech, Moderna (effective with high neutralization) | [47,48] |
| Beta (B.1.351) | South Africa, May 2020 | E484K, K417N, N501Y | Moderate, immune escape raises concerns | Moderate, reduced vaccine efficacy | Severe second wave in South Africa; vaccine efficacy challenges | Johnson & Johnson, mRNA vaccines (reduced efficacy for mild cases, good for severe disease) | [49,50] |
| Gamma (P.1) | Brazil, November 2020 | E484K, K417T, N501Y | Moderate, severe outbreaks in seroprevalent regions | Moderate, reinfections in high seroprevalence areas | Regional crises in Brazil and Japan; limited global spread | CoronaVac (moderate efficacy), mRNA vaccines (boosters improve protection) | [51,52] |
| Delta (B.1.617.2) | India, October 2020 | L452R, P681R, D614G | High, increased severity and hospitalization | Minimal to moderate, partially vaccine-resistant | Global dominance; severe outbreaks in India and US | mRNA vaccines with booster doses, AstraZeneca (good for severe disease) | [47,53,54] |
| Omicron (B.1.1.529) | South Africa, November 2021 | E484A, G446S, Deletion 69–70 | Reduced intrinsic virulence, high transmissibility | High, reinfections and breakthrough cases common | Massive surges globally; ongoing evolution with sub-lineages | Bivalent mRNA vaccines (targeting Omicron sub-lineages), Booster doses critical | [46,55] |
| Variants of Concern (VOCs) | Vaccines | Neutralizing Antibodies (nAbs) | Monoclonal Antibodies (mAbs) | References |
|---|---|---|---|---|
| Alpha(B.1.1.7) | High efficacy with mRNA vaccines (Pfizer-BioNTech, Andover, MA, USA;Moderna, Norwood, MA, USA); AstraZeneca effective | Minimal reduction; effective neutralization by vaccine and natural infection antibodies | Retained efficacy; mutations did not affect primary binding sites | [47,48] |
| Beta(B.1.351) | Moderate decrease in efficacy; mRNA vaccines better than vector-based vaccines | Significant reduction; immune escape via E484K mutation | Reduced efficacy for some monoclonal therapies; mutations altered key epitopes | [49,50] |
| Gamma(P.1) | Reduction similar to Beta; mRNA vaccines effective for severe disease | Moderate to significant reduction; reinfections observed | Reduced neutralizing activity; some therapies less effective | [51,52] |
| Delta(B.1.617.2) | Efficacy against infection reduced, strong protection against severe disease with boosters | Modest reduction, protection against severe outcomes maintained | Most treatments effective, though some showed reduced potency | [47,157] |
| Omicron(B.1.1.529) | Significant reduction in protection, strong efficacy against severe disease after boosters or updated vaccines | Substantial decrease in neutralization; reinfections and breakthrough cases common | Significant reduction or loss of efficacy for many therapies; updates needed | [46,55] |
| Vaccine Type | Examples | Antigen Target | Mechanism of Action | Formulation Type | Efficacy (Preclinical/Clinical) | Notable Secondary Effects | Limitations | References |
|---|---|---|---|---|---|---|---|---|
| mRNA-Based | mRNA-1283 | Full-length Spike or RBD | Encodes viral protein; translated in host cells to induce humoral and cellular immunity | Lipid nanoparticle | High antibody titers; improved thermal stability (preclinical) | Injection site pain, fatigue, mild fever | Data largely preclinical; cold-chain still needed | [229,230] |
| Live Attenuated | FluMist, COVI-VAC, BK2102 (experimental) | Whole virus | Uses replication-competent but weakened virus to induce robust immunity | Intranasal spray or oral drops | Strong mucosal and systemic immunity; effective in animal models (preclinical studies) | Nasal irritation, mild symptoms; caution in immunocompromised | Reversion risk; cold chain and safety in immunocompromised | [231,232] |
| Self-Amplifying RNA (saRNA) | ARCT-154, VLPCOV-01 | Spike variants | Intracellular RNA amplification enhances antigen expression and immune response | Lipid nanoparticle | Promising immunogenicity; dose-sparing (Phase I/II trials) | Tolerable; less reactogenic than standard mRNA | Durability and dose optimization under evaluation | [233,234] |
| Intranasal/Inhaled | BBV154, AdCOVID, VXA-CoV2-1, iNCOVACC | Spike | Induces mucosal IgA and tissue-resident T cell responses | Nasal spray, aerosol, or drops | Shown to reduce nasal viral load; blocks transmission (animals) | Nasal congestion, mild irritation (early trials) | Variable immunogenicity; delivery complexity | [235,236] |
| Exosome-Based | STX-S + N | Spike + Nucleocapsid | Delivers antigens via engineered exosomes mimicking natural presentation | Engineered extracellular vesicles | Strong CD8+ T cell and antibody responses in preclinical models | Not fully reported | Clinical efficacy and scalability yet unknown | [237,238] |
| Virus-Like Particles (VLPs) | CoVLP + AS03 | Spike | Presents spike protein in repetitive arrays to stimulate B cells | Protein-based with adjuvant | ~70–80% efficacy against ancestral strains (Phase III) | Fatigue, injection site pain; mild systemic symptoms | Cold-chain requirements and manufacturing cost | [239,240] |
| DNA-Based | ZyCoV-D | Spike | DNA plasmid is transcribed in host cells to produce viral antigen | Needle-free intradermal jet | ~67% efficacy; moderate antibody response (India trials) | Injection site swelling, fatigue | Lower immunogenicity; boosters often needed | [241,242,243] |
| Recombinant Protein-Based | Novavax NVX-CoV2373 | Spike | Protein subunit with adjuvant elicits targeted immune response | Protein subunit with Matrix-M adjuvant | High efficacy against symptomatic COVID-19 in trials | Fatigue, headache, local/injection site pain | Multiple dose requirement; logistical hurdles | [244,245] |
| Inactivated Virus | Covaxin, Sinopharm, Sinovac, BBV152 | Whole virus | Chemically inactivated virus triggers broad immune response | Inactivated virus with adjuvant | Moderate protection against severe disease | Fever, fatigue, injection site pain | Requires multiple doses; lower immunogenicity | [246,247] |
| Universal Coronavirus Vaccines | Mosaic nanoparticles, pan-sarbecovirus platforms | Conserved epitopes from multiple strains | Designed to induce broad immunity against variants and future coronaviruses | mRNA, protein nanoparticle, viral vector | Broad cross-reactive immunity in animal studies | Mild local/systemic effects in preclinical studies | No licensed product yet; efficacy under investigation | [248,249] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saha, A.; Ghosh Roy, S.; Dwivedi, R.; Tripathi, P.; Kumar, K.; Nambiar, S.M.; Pathak, R. Beyond the Pandemic Era: Recent Advances and Efficacy of SARS-CoV-2 Vaccines Against Emerging Variants of Concern. Vaccines 2025, 13, 424. https://doi.org/10.3390/vaccines13040424
Saha A, Ghosh Roy S, Dwivedi R, Tripathi P, Kumar K, Nambiar SM, Pathak R. Beyond the Pandemic Era: Recent Advances and Efficacy of SARS-CoV-2 Vaccines Against Emerging Variants of Concern. Vaccines. 2025; 13(4):424. https://doi.org/10.3390/vaccines13040424
Chicago/Turabian StyleSaha, Ankita, Sounak Ghosh Roy, Richa Dwivedi, Prajna Tripathi, Kamal Kumar, Shashank Manohar Nambiar, and Rajiv Pathak. 2025. "Beyond the Pandemic Era: Recent Advances and Efficacy of SARS-CoV-2 Vaccines Against Emerging Variants of Concern" Vaccines 13, no. 4: 424. https://doi.org/10.3390/vaccines13040424
APA StyleSaha, A., Ghosh Roy, S., Dwivedi, R., Tripathi, P., Kumar, K., Nambiar, S. M., & Pathak, R. (2025). Beyond the Pandemic Era: Recent Advances and Efficacy of SARS-CoV-2 Vaccines Against Emerging Variants of Concern. Vaccines, 13(4), 424. https://doi.org/10.3390/vaccines13040424







