Intratumoral Immunotherapy in Breast Cancer
Abstract
1. Introduction
2. Oncolytic Viruses
2.1. Clinical Data
2.2. Safety Profile and Limitations
2.3. Summary
3. Nucleic Acids
3.1. Clinical Data
3.1.1. DNA-Based Therapy
3.1.2. mRNA-Based Therapy
3.2. Safety Profile and Limitations
- •
- The safety profile of nucleic acid-based therapy seems to be reasonable; however, there are relatively few studies. No severe AEs were attributable to plasmid IL-12 [27,28]. However, this study only evaluated a single dose of the therapy and included a limited number of patients. mRNA-2752 had a single dose-limiting toxicity (DLT) at 8 mg, characterized as cytokine-release syndrome [29].
- •
- Studies of nucleic acid-based therapy have generally focused on advanced TNBC, and their utility is unknown in patients with early-stage cancers. Moreover, advancements in delivery systems for DNA-based therapies are required to enhance their immunogenicity and potency. Continued research in tumor antigen selection will improve efficacy and personalization of these therapies.
3.3. Summary
- •
- Nucleic acids therapies are not yet well-studied in the BC population. Early studies do show some evidence of an immune response; however, this is not well clinically correlated with objective tumor regression. These therapies seem to have a generally acceptable safety profile and may work better in combination with pembrolizumab. Ultimately, more research is necessary to further explore their use in BC.
4. Innate Immune Agonists
4.1. Clinical Data
4.1.1. Toll-like Receptors
4.1.2. STING Pathway
4.2. Safety Profile and Limitations
4.3. Summary
5. Bacteria
5.1. Clinical Data
5.2. Safety Profile and Limitations
5.3. Summary
6. CAR T Cells
6.1. Clinical Data
6.2. Safety Profile and Limitations
6.3. Summary
7. Dendritic Cells
7.1. Clinical Data
7.2. Safety Profile and Limitations
7.3. Summary
8. The Lymph Node’s Role in Promoting Response to Immunotherapy
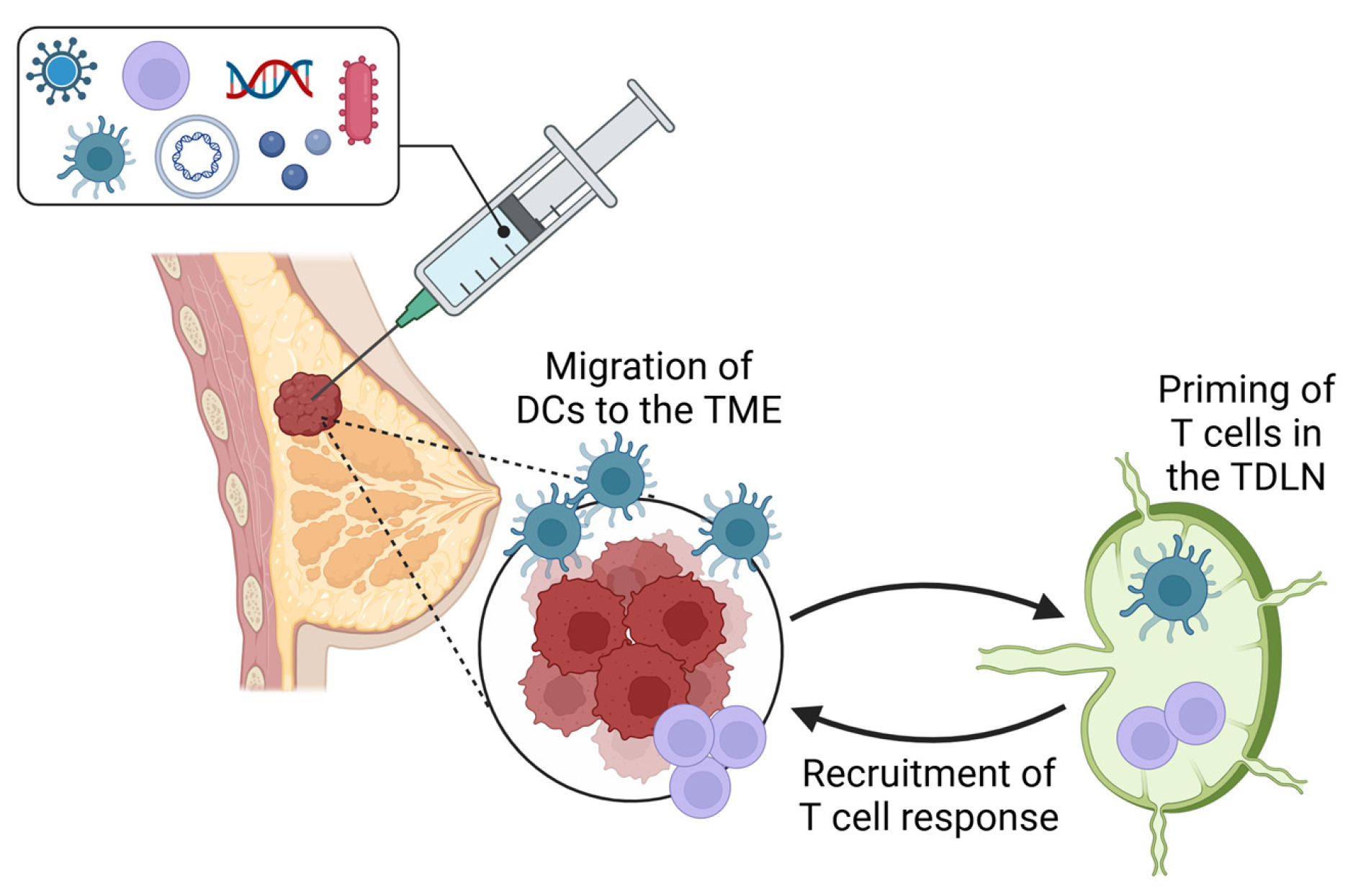
9. Future Perspectives
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AE | adverse event |
| APC | antigen-presenting cell |
| BC | breast cancer |
| CAR | chimeric antigen receptor |
| cDC1 | conventional type 1 dendritic cell |
| DC | dendritic cell |
| DCIS | ductal carcinoma in situ |
| DNA | deoxyribonucleic acid |
| EGFR | epidermal growth factor receptor |
| GM-CSF | granulocyte–macrophage colony-stimulating factor |
| HER | human epidermal growth factor |
| HSV | herpes simplex virus |
| ICI | immune checkpoint inhibitor |
| IFN | interferon |
| MDSC | myeloid-derived suppressor cells |
| MHC | major histocompatibility complex |
| mRNA | messenger ribonucleic acid |
| NK | natural killer |
| NKT | natural killer T cell |
| OV | oncolytic virus |
| PAMP | pathogen-associated molecular pattern |
| pCR | pathologic complete response |
| PD | programmed death protein |
| PDL | programmed death ligand |
| PMN | polymorphonuclear leukocyte |
| RCB | residual cancer burden |
| RNA | ribonucleic acid |
| STING | stimulator of interferon gamma |
| TAA | tumor-associated antigen |
| TAM | tumor-associated macrophage |
| TDLN | tumor-draining lymph node |
| TIL | tumor-infiltrating lymphocytes |
| TLR | toll-like receptor |
| TLS | tertiary lymphoid structure |
| TME | tumor microenvironment |
| TNBC | triple-negative breast cancer |
| TVEC | talimogene laherparepvec |
References
- World Health Organization. Breast Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 2 February 2025).
- Riggio, A.I.; Varley, K.E.; Welm, A.L. The lingering mysteries of metastatic recurrence in breast cancer. Br. J. Cancer 2021, 124, 13–26. [Google Scholar] [CrossRef]
- Mantooth, S.M.; Abdou, Y.; Saez-Ibanez, A.R.; Upadhaya, S.; Zaharoff, D.A. Intratumoral delivery of immunotherapy to treat breast cancer: Current development in clinical and preclinical studies. Front. Immunol. 2024, 15, 1385484. [Google Scholar] [CrossRef]
- Abdou, Y.; Goudarzi, A.; Yu, J.X.; Upadhaya, S.; Vincent, B.; Carey, L.A. Immunotherapy in triple negative breast cancer: Beyond checkpoint inhibitors. NPJ Breast Cancer 2022, 8, 121. [Google Scholar] [CrossRef]
- Shiravand, Y.; Khodadadi, F.; Kashani, S.M.A.; Hosseini-Fard, S.R.; Hosseini, S.; Sadeghirad, H.; Ladwa, R.; O’Byrne, K.; Kulasinghe, A. Immune checkpoint inhibitors in cancer therapy. Curr. Oncol. 2022, 29, 3044–3060. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Dvir, K.; Giordano, S.; Leone, J.P. Immunotherapy in breast cancer. Int. J. Mol. Sci. 2024, 25, 7517. [Google Scholar] [CrossRef] [PubMed]
- Balibegloo, M.; Nejadghaderi, S.A.; Sadeghalvad, M.; Soleymanitabar, A.; Salehi Nezamabadi, S.; Saghazadeh, A.; Rezaei, N. Adverse events associated with immune checkpoint inhibitors in patients with breast cancer: A systematic review and meta-analysis. Int. Immunopharmacol. 2021, 96, 107796. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z. The history and advances in cancer immunotherapy: Understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell. Mol. Immunol. 2020, 17, 807–821. [Google Scholar] [CrossRef]
- Li, J.J.; Tsang, J.Y.; Tse, G.M. Tumor microenvironment in breast cancer—Updates on therapeutic implications and pathologic assessment. Cancers 2021, 13, 4233. [Google Scholar] [CrossRef]
- Planes-Laine, G.; Rochigneux, P.; Bertucci, F.; Chrétien, A.; Viens, P.; Sabatier, R.; Gonçalves, A. PD-1/PD-L1 targeting in breast cancer: The first clinical evidences are Emerging—A literature review. Cancers 2019, 11, 1033. [Google Scholar] [CrossRef]
- Dominguez-Cejudo, M.A.; Gil-Torralvo, A.; Cejuela, M.; Molina-Pinelo, S.; Salvador Bofill, J. Targeting the tumor microenvironment in breast cancer: Prognostic and predictive significance and therapeutic opportunities. Int. J. Mol. Sci. 2023, 24, 16771. [Google Scholar] [CrossRef]
- Mehraj, U.; Dar, A.H.; Wani, N.A.; Mir, M.A. Tumor microenvironment promotes breast cancer chemoresistance. Cancer Chemother. Pharmacol. 2021, 87, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Cejalvo, J.M.; Falato, C.; Villanueva, L.; Tolosa, P.; Gonzalez, X.; Pascal, M.; Canes, J.; Gavila, J.; Manso, L.; Pascual, T.; et al. Oncolytic viruses: A new immunotherapeutic approach for breast cancer treatment? Cancer Treat. Rev. 2022, 106, 102392. [Google Scholar] [CrossRef] [PubMed]
- Soliman, H.; Hogue, D.; Han, H.; Mooney, B.; Costa, R.; Lee, M.C.; Niell, B.; Williams, A.; Chau, A.; Falcon, S.; et al. Oncolytic T-VEC virotherapy plus neoadjuvant chemotherapy in nonmetastatic triple-negative breast cancer: A phase 2 trial. Nat. Med. 2023, 29, 450–457. [Google Scholar] [CrossRef]
- Martini, V.; D’Avanzo, F.; Maggiora, P.M.; Varughese, F.M.; Sica, A.; Gennari, A. Oncolytic virotherapy: New weapon for breast cancer treatment. Ecancermedicalscience 2020, 14, 1149. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, H.L.; Kohlhapp, F.J.; Zloza, A. Oncolytic viruses: A new class of immunotherapy drugs. Nat. Rev. Drug Discov. 2015, 14, 642–662. [Google Scholar] [CrossRef]
- Wang, L.; Chard Dunmall, L.S.; Cheng, Z.; Wang, Y. Remodeling the tumor microenvironment by oncolytic viruses: Beyond oncolysis of tumor cells for cancer treatment. J. Immunother. Cancer 2022, 10, e004167. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, X.; Cheng, P. Remodeling of tumor immune microenvironment by oncolytic viruses. Front. Oncol. 2021, 10, 561372. [Google Scholar] [CrossRef]
- Ferrucci, P.F.; Pala, L.; Conforti, F.; Cocorocchio, E. Talimogene laherparepvec (T-VEC): An intralesional cancer immunotherapy for advanced melanoma. Cancers 2021, 13, 1383. [Google Scholar] [CrossRef]
- Nguyen, V.P.; Campbell, K.M.; Nowicki, T.S.; Elumalai, N.; Medina, E.; Baselga-Carretero, I.; DiNome, M.L.; Chang, H.R.; Oseguera, D.K.; Ribas, A.; et al. A pilot study of neoadjuvant nivolumab, ipilimumab, and intralesional oncolytic virotherapy for HER2-negative breast cancer. Cancer Res. Commun. 2023, 3, 1628–1637. [Google Scholar] [CrossRef]
- Malvehy, J.; Samoylenko, I.; Schadendorf, D.; Gutzmer, R.; Grob, J.; Sacco, J.J.; Gorski, K.S.; Anderson, A.; Pickett, C.A.; Liu, K.; et al. Talimogene laherparepvec upregulates immune-cell populations in non-injected lesions: Findings from a phase II, multicenter, open-label study in patients with stage IIIB–IVM1c melanoma. J. Immunother. Cancer 2021, 9, e001621. [Google Scholar] [CrossRef] [PubMed]
- Kai, M.; Marx, A.N.; Liu, D.D.; Shen, Y.; Gao, H.; Reuben, J.M.; Whitman, G.; Krishnamurthy, S.; Ross, M.I.; Litton, J.K.; et al. A phase II study of talimogene laherparepvec for patients with inoperable locoregional recurrence of breast cancer. Sci. Rep. 2021, 11, 22242. [Google Scholar] [CrossRef]
- Hecht, J.R.; Chan, A.; Martin, M.; Mach, N.; Hurvitz, S.A.; Rottey, S.; Pelzer, U.; Liu, C.; Chan, E. Phase ib study of talimogene laherparepvec (T-VEC) injection into liver metastases (LMs) in combination with intravenous (IV) atezolizumab in patients (pts) with metastatic triple-negative breast cancer (TNBC) or colorectal cancer (CRC). J. Clin. Oncol. 2019, 37, TPS725. [Google Scholar] [CrossRef]
- Saleh, R.O.; Ibrahim, F.M.; Pallathadka, H.; Kaur, I.; Ahmad, I.; Ali, S.H.J.; Redhee, A.H.; Ghildiyal, P.; Jawad, M.A.; Alsaadi, S.B. Nucleic acid vaccines-based therapy for triple-negative breast cancer: A new paradigm in tumor immunotherapy arena. Cell Biochem. Funct. 2024, 42, e3992. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Chen, W.; Cole, J.; Zhu, G. Delivery of nucleic acid therapeutics for cancer immunotherapy. Med. Drug Discov. 2020, 6, 100023. [Google Scholar] [CrossRef]
- Telli, M.L.; Nagata, H.; Wapnir, I.; Acharya, C.R.; Zablotsky, K.; Fox, B.A.; Bifulco, C.B.; Jensen, S.M.; Ballesteros-Merino, C.; Le, M.H.; et al. Intratumoral plasmid IL12 expands CD8(+) T cells and induces a CXCR3 gene signature in triple-negative breast tumors that sensitizes patients to anti-PD-1 therapy. Clin. Cancer Res. 2021, 27, 2481–2493. [Google Scholar] [CrossRef]
- Telli, M.L.; Wapnir, I.; Devitt, B.; Cuff, K.; Soliman, H.; Vinayak, S.; Canton, D.A.; Twitty, C.; Foerter, K.M.; Joshi, R. Abstract P3-09-04: Phase 2, open-label study of intratumoral tavokinogene telseplasmid (tavo) plus electroporation in combination with intravenous pembrolizumab therapy in patients with inoperable locally advanced or metastatic triple-negative breast cancer (mTNBC) (KEYNOTE-890/OMS-I141). Cancer Res. 2020, 80, P3–P4. [Google Scholar] [CrossRef]
- Patel, M.; Jimeno, A.; Wang, D.; Stemmer, S.; Bauer, T.; Sweis, R.; Geva, R.; Kummar, S.; Reagan, P.; Perets, R.; et al. 539 phase 1 study of mRNA-2752, a lipid nanoparticle encapsulating mRNAs encoding human OX40L/IL-23/IL-36γ, for intratumoral (ITu) injection +/− durvalumab in advanced solid tumors and lymphoma. J. Immunother. Cancer 2021, 9, A569. [Google Scholar] [CrossRef]
- Cossu, C.; Di Lorenzo, A.; Fiorilla, I.; Todesco, A.M.; Audrito, V.; Conti, L. The role of the toll-like receptor 2 and the cGAS-STING pathways in breast cancer: Friends or foes? Int. J. Mol. Sci. 2023, 25, 456. [Google Scholar] [CrossRef]
- Toroghian, Y.; Khayyami, R.; Hassanian, S.M.; Nassiri, M.; Ferns, G.A.; Khazaei, M.; Avan, A. The therapeutic potential of targeting the toll-like receptor pathway in breast cancer. Curr. Pharm. Des. 2022, 28, 2203–2210. [Google Scholar] [CrossRef]
- Babiker, H.; Borazanci, E.; Subbiah, V.; Agarwala, S.; Algazi, A.; Schachter, J.; Lotem, M.; Maurice-Dror, C.; Hendler, D.; Rahimian, S.; et al. Tilsotolimod exploits the TLR9 pathway to promote antigen presentation and type 1 IFN signaling in solid tumors: A multicenter international phase I/II trial (ILLUMINATE-101). Clin. Cancer Res. 2022, 28, 5079–5087. [Google Scholar] [CrossRef]
- Frank, M.J.; Reagan, P.M.; Bartlett, N.L.; Gordon, L.I.; Friedberg, J.W.; Czerwinski, D.K.; Long, S.R.; Hoppe, R.T.; Janssen, R.; Candia, A.F.; et al. In situ vaccination with a TLR9 agonist and local low-dose radiation induces systemic responses in untreated indolent lymphoma. Cancer Discov. 2018, 8, 1258–1269. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, J. Role of STING protein in breast cancer: Mechanisms and therapeutic implications. Med. Oncol. 2022, 40, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Le Naour, J.; Zitvogel, L.; Galluzzi, L.; Vacchelli, E.; Kroemer, G. Trial watch: STING agonists in cancer therapy. OncoImmunology 2020, 9, 1777624. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.J.; Delgado, K.; Sawant, K.; Roy, J.; Gupta, U.; Song, C.S.; Poojary, R.; de Figueiredo, P.; Song, J. Harnessing bacterial agents to modulate the tumor microenvironment and enhance cancer immunotherapy. Cancers 2024, 16, 3810. [Google Scholar] [CrossRef]
- Janku, F.; Zhang, H.H.; Pezeshki, A.; Goel, S.; Murthy, R.; Wang-Gillam, A.; Shepard, D.R.; Helgason, T.; Masters, T.; Hong, D.S.; et al. Intratumoral injection of clostridium novyi-NT spores in patients with treatment-refractory advanced solid tumors. Clin. Cancer Res. 2021, 27, 96–106. [Google Scholar] [CrossRef]
- Nelson, B.E.; Janku, F.; Fu, S.; Dumbrava, E.I.; Hong, D.S.; Karp, D.; Naing, A.; Rodon, J.; Tsimberidou, A.; Amaria, R.N.; et al. Abstract, CT107: Phase ib study of pembrolizumab in combination with intratumoral injection of clostridium novyi-NT in patients with advanced solid tumors. Cancer Res. 2023, 83, CT107. [Google Scholar] [CrossRef]
- Kwon, S.; Thi-Thu Ngo, H.; Son, J.; Hong, Y.; Min, J. Exploiting bacteria for cancer immunotherapy. Nat. Rev. Clin. Oncol. 2024, 21, 569–589. [Google Scholar] [CrossRef]
- Huang, X.; Pan, J.; Xu, F.; Shao, B.; Wang, Y.; Guo, X.; Zhou, S. Bacteria-based cancer immunotherapy. Adv. Sci. 2021, 8, 2003572. [Google Scholar] [CrossRef]
- Tchou, J.; Zhao, Y.; Levine, B.L.; Zhang, P.J.; Davis, M.M.; Melenhorst, J.J.; Kulikovskaya, I.; Brennan, A.L.; Liu, X.; Lacey, S.F.; et al. Safety and efficacy of intratumoral injections of chimeric antigen receptor (CAR) T cells in metastatic breast cancer. Cancer Immunol. Res. 2017, 5, 1152–1161. [Google Scholar] [CrossRef]
- Ye, F.; Dewanjee, S.; Li, Y.; Jha, N.K.; Chen, Z.; Kumar, A.; Vishakha; Behl, T.; Jha, S.K.; Tang, H. Advancements in clinical aspects of targeted therapy and immunotherapy in breast cancer. Mol. Cancer 2023, 22, 105. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.D.; Huang, A.C.; Xu, X.; Orlowski, R.; Amaravadi, R.K.; Schuchter, L.M.; Zhang, P.; Tchou, J.; Matlawski, T.; Cervini, A.; et al. Phase I trial of autologous RNA-electroporated cMET-directed CAR T cells administered intravenously in patients with melanoma and breast carcinoma. Cancer Res. Commun. 2023, 3, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.A.; Savas, P.; Virassamy, B.; O’Malley, M.M.R.; Kay, J.; Mueller, S.N.; Mackay, L.K.; Salgado, R.; Loi, S. Towards targeting the breast cancer immune microenvironment. Nat. Rev. Cancer 2024, 24, 554–577. [Google Scholar] [CrossRef] [PubMed]
- Grosser, R.; Cherkassky, L.; Chintala, N.; Adusumilli, P.S. Combination immunotherapy with CAR T cells and checkpoint blockade for the treatment of solid tumors. Cancer Cell 2019, 36, 471–482. [Google Scholar] [CrossRef]
- Sterner, R.C.; Sterner, R.M. CAR-T cell therapy: Current limitations and potential strategies. Blood Cancer J. 2021, 11, 69. [Google Scholar] [CrossRef]
- Gautam, N.; Ramamoorthi, G.; Champion, N.; Han, H.S.; Czerniecki, B.J. Reviewing the significance of dendritic cell vaccines in interrupting breast cancer development. Mol. Aspects Med. 2024, 95, 101239. [Google Scholar] [CrossRef]
- Basu, A.; Albert, G.K.; Awshah, S.; Datta, J.; Kodumudi, K.N.; Gallen, C.; Beyer, A.; Smalley, K.S.M.; Rodriguez, P.C.; Duckett, D.R.; et al. Identification of immunogenic MHC class II human HER3 peptides that mediate anti-HER3 CD4+ Th1 responses and potential use as a cancer vaccine. Cancer Immunol. Res. 2022, 10, 108–125. [Google Scholar] [CrossRef]
- Lowenfeld, L.; Mick, R.; Datta, J.; Xu, S.; Fitzpatrick, E.; Fisher, C.S.; Fox, K.R.; DeMichele, A.; Zhang, P.J.; Weinstein, S.P.; et al. Dendritic cell vaccination enhances immune responses and induces regression of HER2(pos) DCIS independent of route: Results of randomized selection design trial. Clin. Cancer Res. 2017, 23, 2961–2971. [Google Scholar] [CrossRef]
- Han, H.S.; Aldrich, A.L.; Garg, S.K.; Weinfurtner, R.J.; Nguyen, J.V.; Mo, Q.; Whiting, J.; Childress, J.; Soliman, H.; Costa, R.; et al. Alteration of the tumor microenvironment with intratumoral dendritic cells before chemotherapy in ERBB2 breast cancer: A nonrandomized clinical trial. JAMA Oncol. 2025, 11, 119–127. [Google Scholar] [CrossRef]
- Gardner, A.; de Mingo Pulido, Á.; Ruffell, B. Dendritic cells and their role in immunotherapy. Front. Immunol. 2020, 11, 924. [Google Scholar] [CrossRef]
- Xu, Z.; Li, Z.; Cao, L.; Zhong, N.; Liu, X.; Wang, G.; Xiao, Y.; Liu, B.; Bu, L. Seizing the fate of lymph nodes in immunotherapy: To preserve or not? Cancer Lett. 2024, 588, 216740. [Google Scholar] [CrossRef]
- Van Pul, K.M.; Fransen, M.F.; van de Ven, R.; de Gruijl, T.D. Immunotherapy goes local: The central role of lymph nodes in driving tumor infiltration and efficacy. Front. Immunol. 2021, 12, 643291. [Google Scholar] [CrossRef] [PubMed]
- Dotta, E.; Maciola, A.K.; Baccega, T.; Pasqual, G. Dendritic cells steering antigen and leukocyte traffic in lymph nodes. FEBS Lett. 2024, in press.
- Ramamoorthi, G.; Kodumudi, K.; Snyder, C.; Grover, P.; Zhang, H.; Greene, M.I.; Basu, A.; Gallen, C.; Wiener, D.; Costa, R.L.B.; et al. Intratumoral delivery of dendritic cells plus anti-HER2 therapy triggers both robust systemic antitumor immunity and complete regression in HER2 mammary carcinoma. J. Immunother. Cancer 2022, 10, e004841. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthi, G.; Lee, M.C.; Farrell, C.M.; Snyder, C.; Garg, S.K.; Aldrich, A.L.; Lok, V.; Dominguez-Viqueira, W.; Olson-Mcpeek, S.K.; Rosa, M.; et al. Antitumor CD4+ T helper 1 cells target and control the outgrowth of disseminated cancer cells. Cancer Immunol. Res. 2025. [Google Scholar] [CrossRef]
- Andtbacka, R.H.I.; Ross, M.; Puzanov, I.; Milhem, M.; Collichio, F.; Delman, K.A.; Amatruda, T.; Zager, J.S.; Cranmer, L.; Hsueh, E.; et al. Patterns of clinical response with talimogene laherparepvec (T-VEC) in patients with melanoma treated in the OPTiM phase III clinical trial. Ann. Surg. Oncol. 2016, 23, 4169–4177. [Google Scholar] [CrossRef]
- Kaufman, H.L.; Amatruda, T.; Reid, T.; Gonzalez, R.; Glaspy, J.; Whitman, E.; Harrington, K.; Nemunaitis, J.; Zloza, A.; Wolf, M.; et al. Systemic versus local responses in melanoma patients treated with talimogene laherparepvec from a multi-institutional phase II study. J. Immunother. Cancer 2016, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Van den Hout, M.F.; Sluijter, B.J.R.; Santegoets, S.J.A.M.; van Leeuwen, P.A.M.; van den Tol, M.P.; van den Eertwegh, A.J.M.; Scheper, R.J.; de Gruijl, T.D. Local delivery of CpG-B and GM-CSF induces concerted activation of effector and regulatory T cells in the human melanoma sentinel lymph node. Cancer Immunol. Immunother. 2016, 65, 405–415. [Google Scholar] [CrossRef][Green Version]
- Sluijter, B.J.R.; van den Hout, M.F.; Koster, B.D.; van Leeuwen, P.A.M.; Schneiders, F.L.; van de Ven, R.; Molenkamp, B.G.; Vosslamber, S.; Verweij, C.L.; van den Tol, M.P.; et al. Arming the melanoma sentinel lymph node through local administration of CpG-B and GM-CSF: Recruitment and activation of BDCA3/CD141(+) dendritic cells and enhanced cross-presentation. Cancer Immunol. Res. 2015, 3, 495–505. [Google Scholar] [CrossRef]
- Chesney, J.A.; Puzanov, I.; Collichio, F.A.; Singh, P.; Milhem, M.M.; Glaspy, J.; Hamid, O.; Ross, M.; Friedlander, P.; Garbe, C.; et al. Talimogene laherparepvec in combination with ipilimumab versus ipilimumab alone for advanced melanoma: 5-year final analysis of a multicenter, randomized, open-label, phase II trial. J. Immunother. Cancer 2023, 11, e006270. [Google Scholar] [CrossRef]
- Weber, J.S.; Carlino, M.S.; Khattak, A.; Meniawy, T.; Ansstas, G.; Taylor, M.H.; Kim, K.B.; McKean, M.; Long, G.V.; Sullivan, R.J.; et al. Individualised neoantigen therapy mRNA-4157 (V940) plus pembrolizumab versus pembrolizumab monotherapy in resected melanoma (KEYNOTE-942): A randomised, phase 2b study. Lancet 2024, 403, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Koeneman, B.J.; Schreibelt, G.; Gorris, M.A.J.; Hins-de Bree, S.; Westdorp, H.; Ottevanger, P.B.; de Vries, I.J.M. Dendritic cell vaccination combined with carboplatin/paclitaxel for metastatic endometrial cancer patients: Results of a phase I/II trial. Front. Immunol. 2024, 15, 1368103. [Google Scholar] [CrossRef] [PubMed]
| Trial | Status | Phase | Treatment | Adjunct Treatment | Indication | Patient Enrollment | Immune Response | Clinical Response |
|---|---|---|---|---|---|---|---|---|
| NCT04185311 | Terminated | 1 | T-VEC | Ipilimumab, nivolumab | TNBC or ER+/HER2− localized BC | 6 | ↑ CD8+ T cells, cytotoxic lymphocytes, monocytes, NK cells | 16.7% pCR |
| NCT03554044 | Active, not recruiting | 1b | T-VEC | Gemcitabine/carboplatin, nab-paclitaxel, paclitaxel, or endocrine therapy | HER2− (HR+/−) metastatic, unresectable, or locoregionally recurrent | 20 | ↓ circulating lymphocytes and TIM3 expression | 0% pCR, 61% partial response |
| NCT02779855 | Active, not recruiting | 1/2 | Talimogene laherparepvec | Paclitaxel, doxorubicin, cyclophosphamide | Nonmetastatic TNBC | 37 | ↑ CD3+CD8+ effector T cells, CD3+CD45RO+ memory T cells | 45.9% RCB-0 |
| NCT02658812 | Terminated | 2 | T-VEC | None | Inoperable recurrent | 11 | Not available | 0% response |
| NCT03256344 | Completed | 1 | T-VEC | Atezolizumab | TNBC with liver metastases | 36 | Not available | 0% complete response; 10% overall response rate |
| NCT02531425 | Completed | 1 | IT-pIL-12 EP (Tavo) | Electroporation | Locally advanced or metastatic TNBC | 10 | ↑ CD8+ TILs, ↑ expression of PD-1/PD-L1, CXCL9/10/11/CXCR3 pathways | Not available |
| NCT03567720 | Active, not recruiting | 2 | Tavo | Electroporation; pembrolizumab OR pembrolizumab + nab-pacitaxel or gemcitabine plus carboplatin | Inoperable recurrent or metastatic TNBC | 65 | Not available | 27.3% ORR |
| NCT03739931 | Active, not recruiting | 1 | mRNA-2752 | Durvalumab | Solid tumors including TNBC | 134 | ↑ IL-12 and IL-36 gamma expression; ↑ IFN-gamma, TNF-alpha, PD-L1, T-cell infiltration | Not available in TNBC |
| NCT03052205 | Completed | 1b | IMO-2125 (TLR9 agonist) | None | Refractory solid tumors | 54 | ↑ MHC I and II expression, IFN-gamma expression | Not available |
| NCT04144140 | Terminated | 1 | E7766 (STING agonist) | None | Advanced solid tumors | 24 | Not available | Not available |
| NCT01924689 | Completed | 1 | Clostridium noyvi-NT | None | Advanced solid tumors | 24 | Overall, 3 patients with increased T-cell infiltration | Of BC pts, 1 pt with stable disease, 1 unevaluable |
| NCT03435952 | Active, not recruiting | 1 | Clostridium noyvi-NT | Pembrolizumab, doxycycline | Advanced solid tumors | 16 | Not available | 25% ORR |
| NCT01837602 | Completed | 1 | c-Met-CAR T-cells | None | Metastatic | 6 | ↑ CD4+ T cell and mononuclear immune cells | Not available |
| NCT05325632 | Completed | 1 | cDC1 | Trastuzumab, pertuzumab | ERBB2+ breast cancer | 12 | ↑ CD3+, CD4+, CD8+ T cells, B-cells, NKT cells | 58% pCR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baumrucker, C.C.; Harris, N.; Hoover, S.; Czerniecki, B.J. Intratumoral Immunotherapy in Breast Cancer. Vaccines 2025, 13, 429. https://doi.org/10.3390/vaccines13040429
Baumrucker CC, Harris N, Hoover S, Czerniecki BJ. Intratumoral Immunotherapy in Breast Cancer. Vaccines. 2025; 13(4):429. https://doi.org/10.3390/vaccines13040429
Chicago/Turabian StyleBaumrucker, Camille C., Nicole Harris, Susan Hoover, and Brian J. Czerniecki. 2025. "Intratumoral Immunotherapy in Breast Cancer" Vaccines 13, no. 4: 429. https://doi.org/10.3390/vaccines13040429
APA StyleBaumrucker, C. C., Harris, N., Hoover, S., & Czerniecki, B. J. (2025). Intratumoral Immunotherapy in Breast Cancer. Vaccines, 13(4), 429. https://doi.org/10.3390/vaccines13040429







