Evaluation of Immunogenicity of Mycobacterium tuberculosis ag85ab DNA Vaccine Delivered by Pulmonary Administration
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Experimental Animals
2.3. M. tb ag85a/b Chimeric DNA Vaccine and Recombinant Ag85AB Chimeric Protein
2.4. Immunization Regimen of ag85ab DNA Vaccine
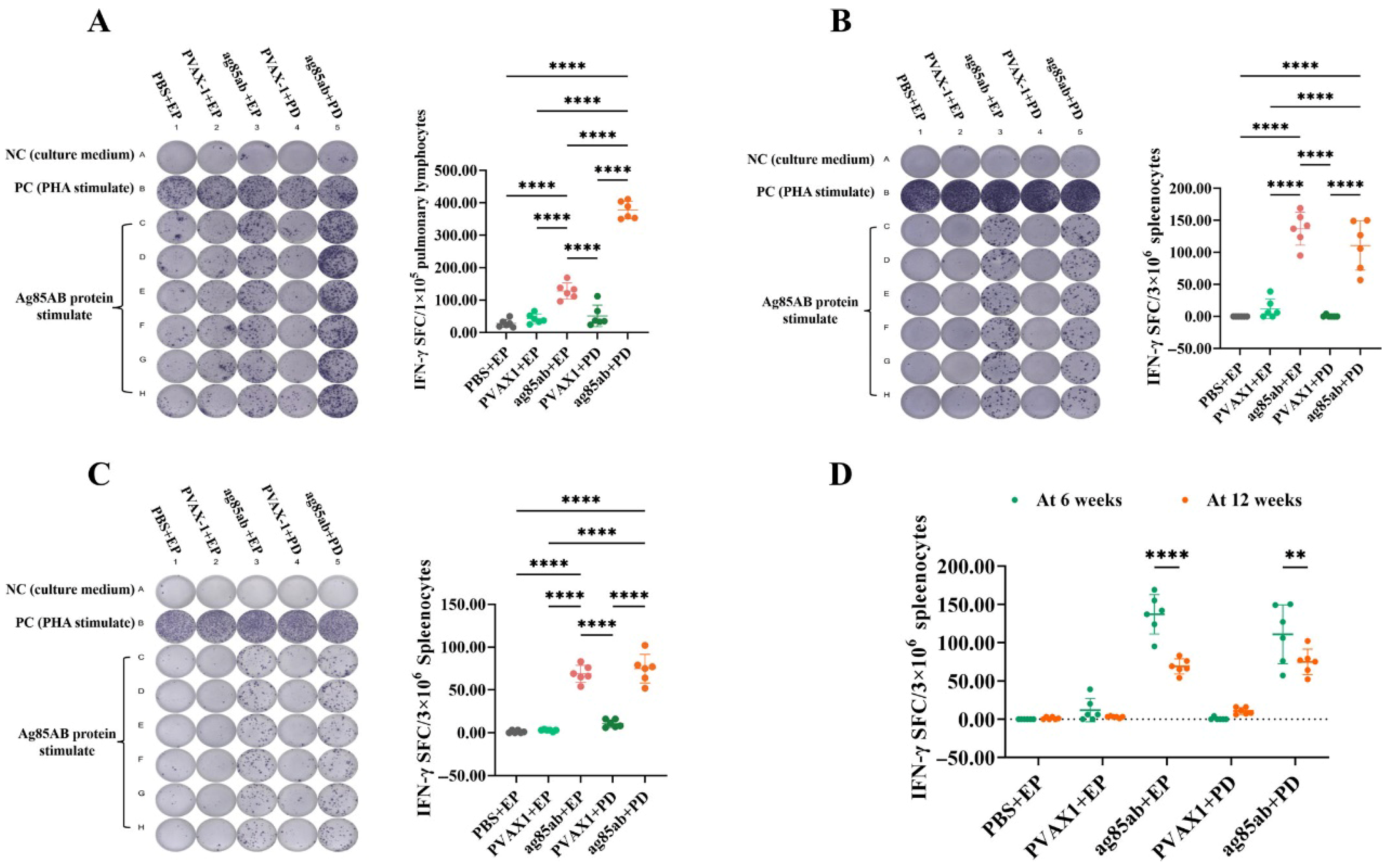
2.5. Determination of the Number of IFN-γ-Secreting Effector T-Lymphocyte Spots in Lung or Spleen Lymphocytes by ELISPOT Assay
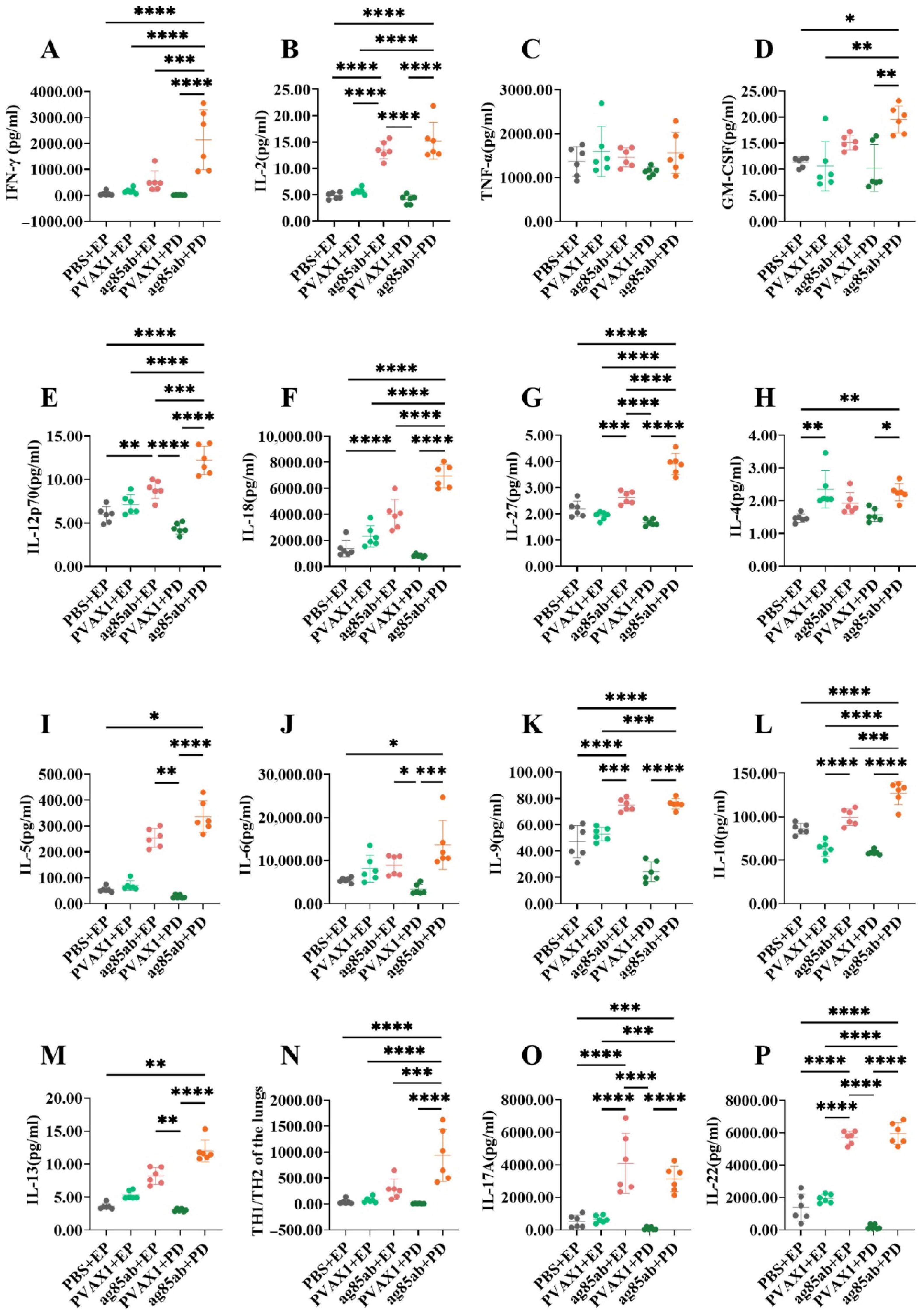
2.6. Determination of Th1, Th2, and Th17 Cytokine Levels in Lung and Spleen Lymphocyte Culture Supernatants Using the Luminex Method
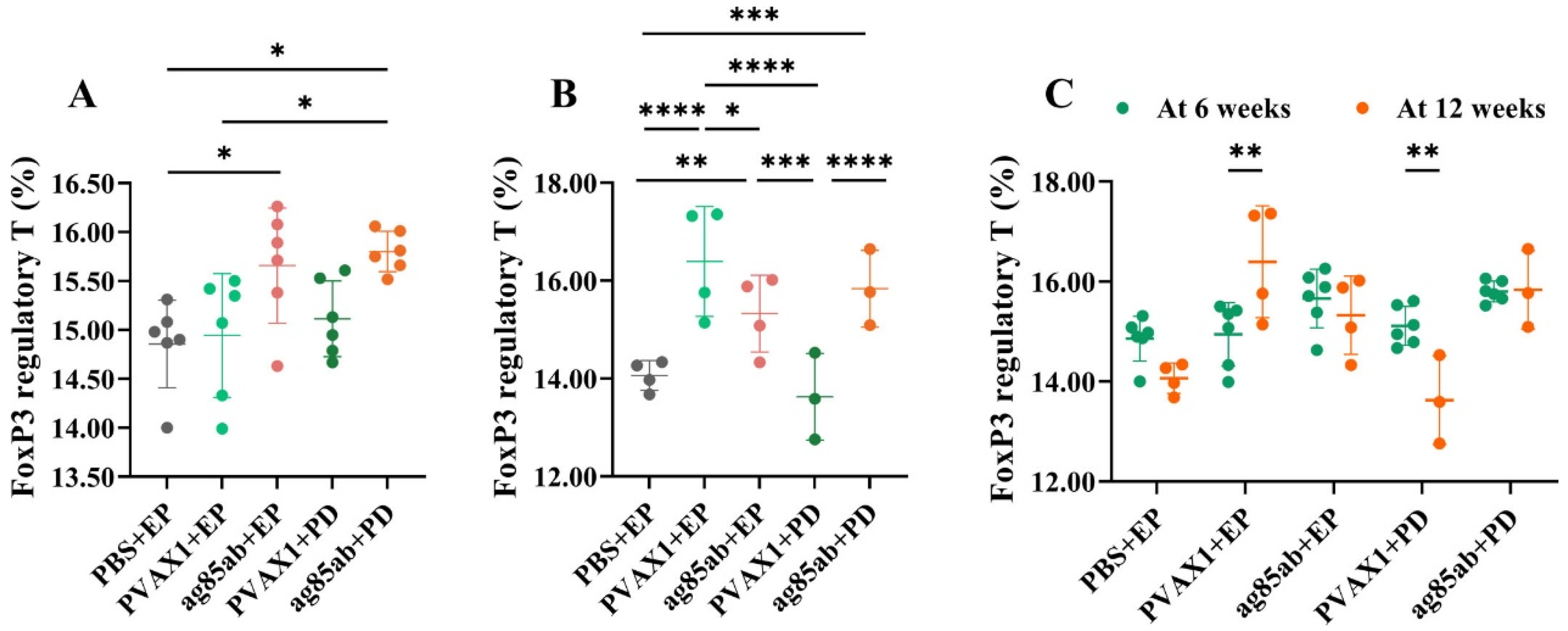
2.7. Proportion of FoxP3 Regulatory T Cells in Mouse Splenocytes Detected by Flow Cytometry
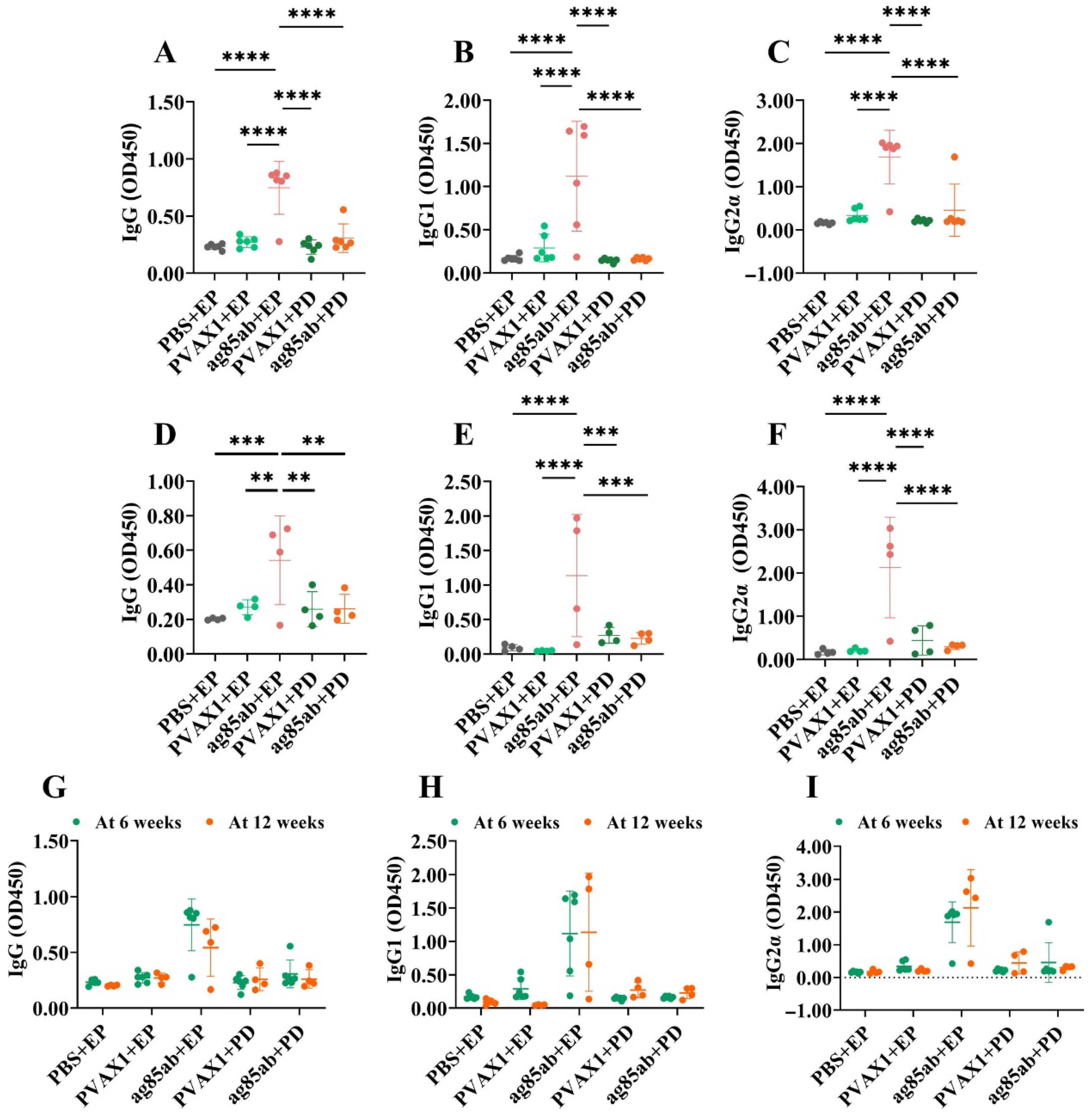
2.8. Detection of Antigen-Specific Antibodies IgG, IgG1, IgG2α in Mouse Plasma and IgA in Alveolar Lavage by ELISA Method
2.9. Experimental Data Processing and Statistical Analysis
3. Results
3.1. Detection of IFN-γ-Secreting T-Lymphocyte Spots in Lung and Spleen Lymphocytes by ELISPOT Assay
3.2. Determination of Th1, Th2, and Th17 Cytokine Levels in Lung and Spleen Lymphocyte Culture Supernatants by the Luminex Method
3.2.1. Th1, Th2, and Th17 Cytokine Levels in Lung Lymphocyte Culture Supernatants
3.2.2. Th1, Th2, and Th17 Cytokine Levels in Splenic Lymphocyte Culture Supernatants
3.3. Proportions of FoxP3 Regulatory T Cells in Mouse Splenocytes Detected by Flow Cytometry
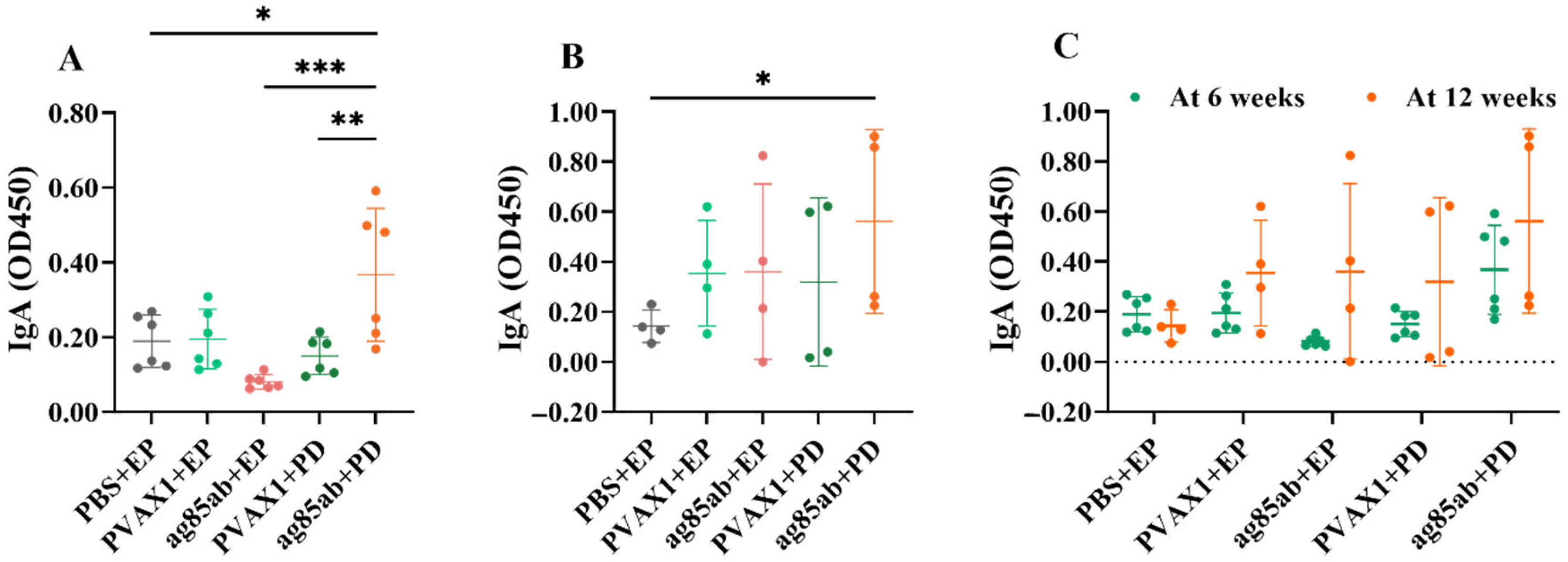
3.4. Detection of Specific Antibodies IgG, IgG1, and IgG2α in Mouse Plasma and IgA in Alveolar Lavage Fluid by ELISA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2024; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Yamazaki-Nakashimada, M.A.; Unzueta, A.; Berenise Gámez-González, L.; González-Saldaña, N.; Sorensen, R.U. BCG: A vaccine with multiple faces. Hum. Vaccin. Immunother. 2020, 16, 1841–1850. [Google Scholar] [CrossRef] [PubMed]
- Fine, P.E. The BCG story: Lessons from the past and implications for the future. Rev. Infect. Dis. 1989, 11 (Suppl. S2), S353–S359. [Google Scholar] [CrossRef] [PubMed]
- Huygen, K.; Content, J.; Denis, O.; Montgomery, D.L.; Yawman, A.M.; Deck, R.R.; DeWitt, C.M.; Orme, I.M.; Baldwin, S.; D’Souza, C.; et al. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat. Med. 1996, 2, 893–898. [Google Scholar] [CrossRef]
- Denis, O.; Tanghe, A.; Palfliet, K.; Jurion, F.; van den Berg, T.P.; Vanonckelen, A.; Ooms, J.; Saman, E.; Ulmer, J.B.; Content, J.; et al. Vaccination with plasmid DNA encoding mycobacterial antigen 85A stimulates a CD4+ and CD8+ T-cell epitopic repertoire broader than that stimulated by Mycobacterium tuberculosis H37Rv infection. Infect. Immun. 1998, 66, 1527–1533. [Google Scholar] [CrossRef]
- Ha, S.J.; Jeon, B.Y.; Youn, J.I.; Kim, S.C.; Cho, S.N.; Sung, Y.C. Protective effect of DNA vaccine during chemotherapy on reactivation and reinfection of Mycobacterium tuberculosis. Gene Ther. 2005, 12, 634–638. [Google Scholar] [CrossRef]
- Liang, Y.; Wu, X.; Zhang, J.; Li, N.; Yu, Q.; Yang, Y.; Bai, X.; Liu, C.; Shi, Y.; Liu, Q.; et al. The treatment of mice infected with multi-drug-resistant Mycobacterium tuberculosis using DNA vaccines or in combination with rifampin. Vaccine 2008, 26, 4536–4540. [Google Scholar] [CrossRef]
- Liang, Y.; Wu, X.; Zhang, J.; Yang, Y.; Wang, L.; Bai, X.; Yu, Q.; Li, N.; Li, Z. Treatment of multi-drug-resistant tuberculosis in mice with DNA vaccines alone or in combination with chemotherapeutic drugs. Scand. J. Immunol. 2011, 74, 42–46. [Google Scholar] [CrossRef]
- Zhu, D.; Jiang, S.; Luo, X. Therapeutic effects of Ag85B and MPT64 DNA vaccines in a murine model of Mycobacterium tuberculosis infection. Vaccine 2005, 23, 4619–4624. [Google Scholar] [CrossRef]
- Liang, Y.; Wu, X.; Zhang, J.; Xiao, L.; Yang, Y.; Bai, X.; Yu, Q.; Li, Z.; Bi, L.; Li, N.; et al. Immunogenicity and therapeutic effects of Ag85A/B chimeric DNA vaccine in mice infected with Mycobacterium tuberculosis. FEMS Immunol. Med. Microbiol. 2012, 66, 419–426. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, Y.; Bai, X.; Xiao, L.; Yang, Y.; Zhang, J.; Wang, L.; Cui, L.; Wang, T.; Shi, Y.; et al. Immunotherapeutic effects of Mycobacterium tuberculosis rv3407 DNA vaccine in mice. Autoimmunity 2018, 51, 417–422. [Google Scholar] [CrossRef]
- Alamri, S.S.; Alluhaybi, K.A.; Alhabbab, R.Y.; Basabrain, M.; Algaissi, A.; Almahboub, S.; Alfaleh, M.A.; Abujamel, T.S.; Abdulaal, W.H.; ElAssouli, M.Z.; et al. Synthetic SARS-CoV-2 Spike-Based DNA Vaccine Elicits Robust and Long-Lasting Th1 Humoral and Cellular Immunity in Mice. Front. Microbiol. 2021, 12, 727455. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Kita, Y. Tuberculosis vaccine development: The development of novel (preclinical) DNA vaccine. Hum. Vaccin. 2010, 6, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Kutzler, M.A.; Weiner, D.B. DNA vaccines: Ready for prime time? Nat. Rev. Genet. 2008, 9, 776–788. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, S.L.; Bertholet, S.; Kahn, M.; Zharkikh, I.; Ireton, G.C.; Vedvick, T.S.; Reed, S.G.; Coler, R.N. Intradermal immunization improves protective efficacy of a novel TB vaccine candidate. Vaccine 2009, 27, 3063–3071. [Google Scholar] [CrossRef]
- Carter, E.; Morton, B.; ElSafadi, D.; Jambo, K.; Kenny-Nyazika, T.; Hyder-Wright, A.; Chiwala, G.; Chikaonda, T.; Chirwa, A.E.; Gonzalez Sanchez, J.; et al. A feasibility study of controlled human infection with intradermal Bacillus Calmette-Guérin (BCG) injection: Pilot BCG controlled human infection model. Wellcome Open Res. 2023, 8, 424. [Google Scholar] [CrossRef]
- Buddle, B.M.; Keen, D.; Thomson, A.; Jowett, G.; McCarthy, A.R.; Heslop, J.; De Lisle, G.W.; Stanford, J.L.; Aldwell, F.E. Protection of cattle from bovine tuberculosis by vaccination with BCG by the respiratory or subcutaneous route, but not by vaccination with killed Mycobacterium vaccae. Res. Vet. Sci. 1995, 59, 10–16. [Google Scholar] [CrossRef]
- Meyer, J.; Harris, S.A.; Satti, I.; Poulton, I.D.; Poyntz, H.C.; Tanner, R.; Rowland, R.; Griffiths, K.L.; Fletcher, H.A.; McShane, H. Comparing the safety and immunogenicity of a candidate TB vaccine MVA85A administered by intramuscular and intradermal delivery. Vaccine 2013, 31, 1026–1033. [Google Scholar] [CrossRef]
- Zhang, X.; Divangahi, M.; Ngai, P.; Santosuosso, M.; Millar, J.; Zganiacz, A.; Wang, J.; Bramson, J.; Xing, Z. Intramuscular immunization with a monogenic plasmid DNA tuberculosis vaccine: Enhanced immunogenicity by electroporation and co-expression of GM-CSF transgene. Vaccine 2007, 25, 1342–1352. [Google Scholar] [CrossRef]
- Qu, M.; Zhou, X.; Li, H. BCG vaccination strategies against tuberculosis: Updates and perspectives. Hum. Vaccin. Immunother. 2021, 17, 5284–5295. [Google Scholar] [CrossRef]
- Jeyanathan, M.; Fritz, D.K.; Afkhami, S.; Aguirre, E.; Howie, K.J.; Zganiacz, A.; Dvorkin-Gheva, A.; Thompson, M.R.; Silver, R.F.; Cusack, R.P.; et al. Aerosol delivery, but not intramuscular injection, of adenovirus-vectored tuberculosis vaccine induces respiratory-mucosal immunity in humans. JCI Insight 2022, 7, e155655. [Google Scholar] [CrossRef]
- Darrah, P.A.; Zeppa, J.J.; Maiello, P.; Hackney, J.A.; Wadsworth, M.H., 2nd; Hughes, T.K.; Pokkali, S.; Swanson, P.A., 2nd; Grant, N.L.; Rodgers, M.A.; et al. Prevention of tuberculosis in macaques after intravenous BCG immunization. Nature 2020, 577, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, J.; Tian, J.; Zhu, B.; Zhang, Y.; Yang, K.; Ling, Y.; Hu, Y. IL-17 and IFN-γ production in peripheral blood following BCG vaccination and Mycobacterium tuberculosis infection in human. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 2029–2036. [Google Scholar] [PubMed]
- Black, G.F.; Weir, R.E.; Floyd, S.; Bliss, L.; Warndorff, D.K.; Crampin, A.C.; Ngwira, B.; Sichali, L.; Nazareth, B.; Blackwell, J.M.; et al. BCG-induced increase in interferon-gamma response to mycobacterial antigens and efficacy of BCG vaccination in Malawi and the UK: Two randomised controlled studies. Lancet 2002, 359, 1393–1401. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Cui, L.; Xiao, L.; Liu, X.; Yang, Y.; Ling, Y.; Wang, T.; Wang, L.; Wang, J.; Wu, X. Immunotherapeutic Effects of Different Doses of Mycobacterium tuberculosis ag85a/b DNA Vaccine Delivered by Electroporation. Front. Immunol. 2022, 13, 876579. [Google Scholar] [CrossRef]
- Vasilyev, K.; Shurygina, A.P.; Zabolotnykh, N.; Sergeeva, M.; Romanovskaya-Romanko, E.; Pulkina, A.; Buzitskaya, J.; Dogonadze, M.Z.; Vinogradova, T.I.; Stukova, M.A. Enhancement of the Local CD8(+) T-Cellular Immune Response to Mycobacterium tuberculosis in BCG-Primed Mice after Intranasal Administration of Influenza Vector Vaccine Carrying TB10.4 and HspX Antigens. Vaccines 2021, 9, 1273. [Google Scholar] [CrossRef]
- Perrett, S.; Lesellier, S.; Rogers, F.; Williams, G.A.; Gowtage, S.; Palmer, S.; Dalley, D.; Davé, D.; Weyer, U.; Wood, E.; et al. Assessment of the safety of Bacillus Calmette-Guérin vaccine administered orally to badgers (Meles meles). Vaccine 2018, 36, 1990–1995. [Google Scholar] [CrossRef]
- Mata, E.; Tarancon, R.; Guerrero, C.; Moreo, E.; Moreau, F.; Uranga, S.; Gomez, A.B.; Marinova, D.; Domenech, M.; Gonzalez-Camacho, F.; et al. Pulmonary BCG induces lung-resident macrophage activation and confers long-term protection against tuberculosis. Sci. Immunol. 2021, 6, eabc2934. [Google Scholar] [CrossRef]
- Gupta, A.; Geetha, N.; Mani, J.; Upadhyay, P.; Katoch, V.M.; Natrajan, M.; Gupta, U.D.; Bhaskar, S. Immunogenicity and protective efficacy of “Mycobacterium w” against Mycobacterium tuberculosis in mice immunized with live versus heat-killed M. w by the aerosol or parenteral route. Infect. Immun. 2009, 77, 223–231. [Google Scholar] [CrossRef]
- Stylianou, E.; Paul, M.J.; Reljic, R.; McShane, H. Mucosal delivery of tuberculosis vaccines: A review of current approaches and challenges. Expert. Rev. Vaccines 2019, 18, 1271–1284. [Google Scholar] [CrossRef]
- Stylianou, E.; Satti, I. Inhaled aerosol viral-vectored vaccines against tuberculosis. Curr. Opin. Virol. 2024, 66, 101408. [Google Scholar] [CrossRef]
- Satti, I.; Marshall, J.L.; Harris, S.A.; Wittenberg, R.; Tanner, R.; Lopez Ramon, R.; Wilkie, M.; Ramos Lopez, F.; Riste, M.; Wright, D.; et al. Safety of a controlled human infection model of tuberculosis with aerosolised, live-attenuated Mycobacterium bovis BCG versus intradermal BCG in BCG-naive adults in the UK: A dose-escalation, randomised, controlled, phase 1 trial. Lancet Infect. Dis. 2024, 24, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhao, H.; Li, P.; Wu, X.; Liang, Y. Research Progress on Liposome Pulmonary Delivery of Mycobacterium tuberculosis Nucleic Acid Vaccine and Its Mechanism of Action. J. Aerosol Med. Pulm. Drug Deliv. 2024, 37, 284–298. [Google Scholar] [CrossRef] [PubMed]
- Carrigy, N.B.; Larsen, S.E.; Reese, V.; Pecor, T.; Harrison, M.; Kuehl, P.J.; Hatfull, G.F.; Sauvageau, D.; Baldwin, S.L.; Finlay, W.H.; et al. Prophylaxis of Mycobacterium tuberculosis H37Rv Infection in a Preclinical Mouse Model via Inhalation of Nebulized Bacteriophage D29. Antimicrob. Agents Chemother. 2019, 63, e00871-19. [Google Scholar] [CrossRef]
- Ernst, J.D. Antigenic Variation and Immune Escape in the MTBC. Adv. Exp. Med. Biol. 2017, 1019, 171–190. [Google Scholar]
- Korompis, M.; De Voss, C.J.; Li, S.; Richard, A.; Almujri, S.S.; Ateere, A.; Frank, G.; Lemoine, C.; McShane, H.; Stylianou, E. Strong immune responses and robust protection following a novel protein in adjuvant tuberculosis vaccine candidate. Sci. Rep. 2025, 15, 1886. [Google Scholar] [CrossRef]
- Setiabudiawan, T.P.; Reurink, R.K.; Hill, P.C.; Netea, M.G.; van Crevel, R.; Koeken, V. Protection against tuberculosis by Bacillus Calmette-Guérin (BCG) vaccination: A historical perspective. Med 2022, 3, 6–24. [Google Scholar] [CrossRef]
- Fröberg, J.; Diavatopoulos, D.A. Mucosal immunity to severe acute respiratory syndrome coronavirus 2 infection. Curr. Opin. Infect. Dis. 2021, 34, 181–186. [Google Scholar] [CrossRef]
- Oh, J.E.; Song, E.; Moriyama, M.; Wong, P.; Zhang, S.; Jiang, R.; Strohmeier, S.; Kleinstein, S.H.; Krammer, F.; Iwasaki, A. Intranasal priming induces local lung-resident B cell populations that secrete protective mucosal antiviral IgA. Sci. Immunol. 2021, 6, eabj5129. [Google Scholar] [CrossRef]
- Marking, U.; Bladh, O.; Havervall, S.; Svensson, J.; Greilert-Norin, N.; Aguilera, K.; Kihlgren, M.; Salomonsson, A.C.; Månsson, M.; Gallini, R.; et al. 7-month duration of SARS-CoV-2 mucosal immunoglobulin-A responses and protection. Lancet Infect. Dis. 2023, 23, 150–152. [Google Scholar] [CrossRef]
- Zhou, X.; Wu, Y.; Zhu, Z.; Lu, C.; Zhang, C.; Zeng, L.; Xie, F.; Zhang, L.; Zhou, F. Mucosal immune response in biology, disease prevention and treatment. Signal Transduct. Target. Ther. 2025, 10, 7. [Google Scholar] [CrossRef]
- Ko, K.H.; Bae, H.S.; Park, J.W.; Lee, J.S.; Park, S.; Heo, J.; Park, H.; Choi, J.; Bae, E.; Na, W.; et al. A vaccine platform targeting lung-resident memory CD4(+) T-cells provides protection against heterosubtypic influenza infections in mice and ferrets. Nat. Commun. 2024, 15, 10368. [Google Scholar] [CrossRef] [PubMed]
- Forbes, E.K.; Sander, C.; Ronan, E.O.; McShane, H.; Hill, A.V.; Beverley, P.C.; Tchilian, E.Z. Multifunctional, high-level cytokine-producing Th1 cells in the lung, but not spleen, correlate with protection against Mycobacterium tuberculosis aerosol challenge in mice. J. Immunol. 2008, 181, 4955–4964. [Google Scholar] [CrossRef] [PubMed]
- Jeyanathan, M.; Heriazon, A.; Xing, Z. Airway luminal T cells: A newcomer on the stage of TB vaccination strategies. Trends Immunol. 2010, 31, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Santosuosso, M.; Zhang, X.; McCormick, S.; Wang, J.; Hitt, M.; Xing, Z. Mechanisms of mucosal and parenteral tuberculosis vaccinations: Adenoviral-based mucosal immunization preferentially elicits sustained accumulation of immune protective CD4 and CD8 T cells within the airway lumen. J. Immunol. 2005, 174, 7986–7994. [Google Scholar] [CrossRef]
- Santosuosso, M.; McCormick, S.; Roediger, E.; Zhang, X.; Zganiacz, A.; Lichty, B.D.; Xing, Z. Mucosal luminal manipulation of T cell geography switches on protective efficacy by otherwise ineffective parenteral genetic immunization. J. Immunol. 2007, 178, 2387–2395. [Google Scholar] [CrossRef]
- Ghanavi, J.; Farnia, P.; Farnia, P.; Velayati, A.A. The role of interferon-gamma and interferon-gamma receptor in tuberculosis and nontuberculous mycobacterial infections. Int. J. Mycobacteriol 2021, 10, 349–357. [Google Scholar] [CrossRef]
- Hu, X.; Ivashkiv, L.B. Cross-regulation of signaling pathways by interferon-gamma: Implications for immune responses and autoimmune diseases. Immunity 2009, 31, 539–550. [Google Scholar] [CrossRef]
- Wu, M.; Zhao, H.; Li, M.; Yue, Y.; Xiong, S.; Xu, W. Intranasal Vaccination with Mannosylated Chitosan Formulated DNA Vaccine Enables Robust IgA and Cellular Response Induction in the Lungs of Mice and Improves Protection against Pulmonary Mycobacterial Challenge. Front. Cell Infect. Microbiol. 2017, 7, 445. [Google Scholar] [CrossRef]
- Sheng, L.; Li, X.; Weng, F.; Wu, S.; Chen, Y.; Lou, L. Efficacy and Safety of Adjunctive Recombinant Human Interleukin-2 for Patients with Pulmonary Tuberculosis: A Meta-Analysis. J. Trop. Med. 2022, 2022, 5071816. [Google Scholar] [CrossRef]
- Liu, X.; Li, F.; Niu, H.; Ma, L.; Chen, J.; Zhang, Y.; Peng, L.; Gan, C.; Ma, X.; Zhu, B. IL-2 Restores T-Cell Dysfunction Induced by Persistent Mycobacterium tuberculosis Antigen Stimulation. Front. Immunol. 2019, 10, 2350. [Google Scholar] [CrossRef]
- Quesniaux, V.F.; Jacobs, M.; Allie, N.; Grivennikov, S.; Nedospasov, S.A.; Garcia, I.; Olleros, M.L.; Shebzukhov, Y.; Kuprash, D.; Vasseur, V.; et al. TNF in host resistance to tuberculosis infection. Curr. Dir. Autoimmun. 2010, 11, 157–179. [Google Scholar] [PubMed]
- Benmerzoug, S.; Marinho, F.V.; Rose, S.; Mackowiak, C.; Gosset, D.; Sedda, D.; Poisson, E.; Uyttenhove, C.; Van Snick, J.; Jacobs, M.; et al. GM-CSF targeted immunomodulation affects host response to M. tuberculosis infection. Sci. Rep. 2018, 8, 8652. [Google Scholar] [CrossRef] [PubMed]
- Trinchieri, G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 2003, 3, 133–146. [Google Scholar] [CrossRef]
- Kaplanski, G. Interleukin-18: Biological properties and role in disease pathogenesis. Immunol. Rev. 2018, 281, 138–153. [Google Scholar] [CrossRef]
- Harel, M.; Fauteux-Daniel, S.; Girard-Guyonvarc’h, C.; Gabay, C. Balance between Interleukin-18 and Interleukin-18 binding protein in auto-inflammatory diseases. Cytokine 2022, 150, 155781. [Google Scholar] [CrossRef]
- Laverdure, S.; Wang, Z.; Yang, J.; Yamamoto, T.; Thomas, T.; Sato, T.; Nagashima, K.; Imamichi, T. Interleukin-27 promotes autophagy in human serum-induced primary macrophages via an mTOR- and LC3-independent pathway. Sci. Rep. 2021, 11, 14898. [Google Scholar] [CrossRef]
- Zhai, W.; Wu, F.; Zhang, Y.; Fu, Y.; Liu, Z. The Immune Escape Mechanisms of Mycobacterium Tuberculosis. Int. J. Mol. Sci. 2019, 20, 340. [Google Scholar] [CrossRef]
- Dalvi, S.M.; Ramraje, N.N.; Patil, V.W.; Hegde, R.; Yeram, N. Study of IL-6 and vitamin D3 in patients of pulmonary tuberculosis. Indian. J. Tuberc. 2019, 66, 337–345. [Google Scholar] [CrossRef]
- Boni, F.G.; Hamdi, I.; Koundi, L.M.; Shrestha, K.; Xie, J. Cytokine storm in tuberculosis and IL-6 involvement. Infect. Genet. Evol. 2022, 97, 105166. [Google Scholar] [CrossRef]
- Xu, J.C.; Li, Z.Y.; Chen, X.N.; Shi, C.L.; Wu, M.Y.; Chen, H.; Zhu, X.Y.; Song, H.F.; Wu, M.J.; Xu, P. More significance of TB-IGRA except for the diagnose of tuberculosis. J. Clin. Lab. Anal. 2018, 32, e22183. [Google Scholar] [CrossRef]
- Dalimi, A.; Nasiri, V. Design, Construction and Immunogenicity Assessment of pEGFP-N1-KMP11-GP96 (Fusion) as a DNA Vaccine Candidate against Leishmania major Infection in BALB/c Mice. Iran. J. Parasitol. 2020, 15, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Wu, Y.; Chen, S.; Yu, M.; Zeng, Y.; You, X.; Xiao, J.; Wang, S. Protective immune responses in mice induced by intramuscular and intranasal immunization with a Mycoplasma pneumoniae P1C DNA vaccine. Can. J. Microbiol. 2012, 58, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekar, S.S.; Phanse, Y.; Hildebrand, R.E.; Hanafy, M.; Wu, C.W.; Hansen, C.H.; Osorio, J.E.; Suresh, M.; Talaat, A.M. Localized and Systemic Immune Responses against SARS-CoV-2 Following Mucosal Immunization. Vaccines 2021, 9, 132. [Google Scholar] [CrossRef]
- Khader, S.A.; Bell, G.K.; Pearl, J.E.; Fountain, J.J.; Rangel-Moreno, J.; Cilley, G.E.; Shen, F.; Eaton, S.M.; Gaffen, S.L.; Swain, S.L.; et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat. Immunol. 2007, 8, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Lyadova, I.V.; Panteleev, A.V. Th1 and Th17 Cells in Tuberculosis: Protection, Pathology, and Biomarkers. Mediat. Inflamm. 2015, 2015, 854507. [Google Scholar] [CrossRef]
- Sun, M.; Phan, J.M.; Kieswetter, N.S.; Huang, H.; Yu, K.K.Q.; Smith, M.T.; Liu, Y.E.; Wang, C.; Gupta, S.; Obermoser, G.; et al. Specific CD4(+) T cell phenotypes associate with bacterial control in people who ‘resist’ infection with Mycobacterium tuberculosis. Nat. Immunol. 2024, 25, 1411–1421. [Google Scholar] [CrossRef]
- Ronacher, K.; Sinha, R.; Cestari, M. IL-22: An Underestimated Player in Natural Resistance to Tuberculosis? Front. Immunol. 2018, 9, 2209. [Google Scholar] [CrossRef]
- Devalraju, K.P.; Neela, V.S.K.; Ramaseri, S.S.; Chaudhury, A.; Van, A.; Krovvidi, S.S.; Vankayalapati, R.; Valluri, V.L. IL-17 and IL-22 production in HIV+ individuals with latent and active tuberculosis. BMC Infect. Dis. 2018, 18, 321. [Google Scholar] [CrossRef]
- Bhuju, S.; Aranday-Cortes, E.; Villarreal-Ramos, B.; Xing, Z.; Singh, M.; Vordermeier, H.M. Global gene transcriptome analysis in vaccinated cattle revealed a dominant role of IL-22 for protection against bovine tuberculosis. PLoS Pathog. 2012, 8, e1003077. [Google Scholar] [CrossRef][Green Version]
- Cardona, P.; Cardona, P.J. Regulatory T Cells in Mycobacterium tuberculosis Infection. Front. Immunol. 2019, 10, 2139. [Google Scholar] [CrossRef]
- Jaron, B.; Maranghi, E.; Leclerc, C.; Majlessi, L. Effect of attenuation of Treg during BCG immunization on anti-mycobacterial Th1 responses and protection against Mycobacterium tuberculosis. PLoS ONE 2008, 3, e2833. [Google Scholar] [CrossRef] [PubMed]
- Fedatto, P.F.; Sérgio, C.A.; Paula, M.O.; Gembre, A.F.; Franco, L.H.; Wowk, P.F.; Ramos, S.G.; Horn, C.; Marchal, G.; Turato, W.M.; et al. Protection conferred by heterologous vaccination against tuberculosis is dependent on the ratio of CD4(+) /CD4(+) Foxp3(+) cells. Immunology 2012, 137, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Javid, B. Antibodies and tuberculosis: Finally coming of age? Nat. Rev. Immunol. 2018, 18, 591–596. [Google Scholar] [CrossRef]
- Chen, T.; Blanc, C.; Eder, A.Z.; Prados-Rosales, R.; Souza, A.C.; Kim, R.S.; Glatman-Freedman, A.; Joe, M.; Bai, Y.; Lowary, T.L.; et al. Association of Human Antibodies to Arabinomannan With Enhanced Mycobacterial Opsonophagocytosis and Intracellular Growth Reduction. J. Infect. Dis. 2016, 214, 300–310. [Google Scholar] [CrossRef]
- Phuah, J.; Wong, E.A.; Gideon, H.P.; Maiello, P.; Coleman, M.T.; Hendricks, M.R.; Ruden, R.; Cirrincione, L.R.; Chan, J.; Lin, P.L.; et al. Effects of B Cell Depletion on Early Mycobacterium tuberculosis Infection in Cynomolgus Macaques. Infect. Immun. 2016, 84, 1301–1311. [Google Scholar] [CrossRef]
- Thakur, A.; Pinto, F.E.; Hansen, H.S.; Andersen, P.; Christensen, D.; Janfelt, C.; Foged, C. Intrapulmonary (i.pulmon.) Pull Immunization With the Tuberculosis Subunit Vaccine Candidate H56/CAF01 After Intramuscular (i.m.) Priming Elicits a Distinct Innate Myeloid Response and Activation of Antigen-Presenting Cells Than i.m. or i.pulmon. Prime Immunization Alone. Front. Immunol. 2020, 11, 803. [Google Scholar]
- Ai, W.; Yue, Y.; Xiong, S.; Xu, W. Enhanced protection against pulmonary mycobacterial challenge by chitosan-formulated polyepitope gene vaccine is associated with increased pulmonary secretory IgA and gamma-interferon(+) T cell responses. Microbiol. Immunol. 2013, 57, 224–235. [Google Scholar] [CrossRef]
- Muranishi, K.; Kinoshita, M.; Inoue, K.; Ohara, J.; Mihara, T.; Sudo, K.; Ishii, K.J.; Sawa, T.; Ishikura, H. Antibody Response Following the Intranasal Administration of SARS-CoV-2 Spike Protein-CpG Oligonucleotide Vaccine. Vaccines 2023, 12, 5. [Google Scholar] [CrossRef]
- Torrieri-Dramard, L.; Lambrecht, B.; Ferreira, H.L.; Van den Berg, T.; Klatzmann, D.; Bellier, B. Intranasal DNA vaccination induces potent mucosal and systemic immune responses and cross-protective immunity against influenza viruses. Mol. Ther. 2011, 19, 602–611. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Zhang, Z.; Xue, Y.; Wang, N.; Liu, Y.; Ma, X.; Wang, L.; Wang, X.; Zhang, D.; Zhang, J.; et al. Evaluation of Immunogenicity of Mycobacterium tuberculosis ag85ab DNA Vaccine Delivered by Pulmonary Administration. Vaccines 2025, 13, 442. https://doi.org/10.3390/vaccines13050442
Zhao H, Zhang Z, Xue Y, Wang N, Liu Y, Ma X, Wang L, Wang X, Zhang D, Zhang J, et al. Evaluation of Immunogenicity of Mycobacterium tuberculosis ag85ab DNA Vaccine Delivered by Pulmonary Administration. Vaccines. 2025; 13(5):442. https://doi.org/10.3390/vaccines13050442
Chicago/Turabian StyleZhao, Haimei, Zhen Zhang, Yong Xue, Nan Wang, Yinping Liu, Xihui Ma, Lan Wang, Xiaoou Wang, Danyang Zhang, Junxian Zhang, and et al. 2025. "Evaluation of Immunogenicity of Mycobacterium tuberculosis ag85ab DNA Vaccine Delivered by Pulmonary Administration" Vaccines 13, no. 5: 442. https://doi.org/10.3390/vaccines13050442
APA StyleZhao, H., Zhang, Z., Xue, Y., Wang, N., Liu, Y., Ma, X., Wang, L., Wang, X., Zhang, D., Zhang, J., Wu, X., & Liang, Y. (2025). Evaluation of Immunogenicity of Mycobacterium tuberculosis ag85ab DNA Vaccine Delivered by Pulmonary Administration. Vaccines, 13(5), 442. https://doi.org/10.3390/vaccines13050442




