Immune Protection Gap Between Porcine Reproductive and Respiratory Syndrome Subunit Vaccine (N Protein) and Live Vaccine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell, and Strain
2.2. Protein Expression
2.3. Animal Experiment Process
2.4. Serological Examination
2.5. RT-qPCR Detection
2.6. Histopathology and Immunohistochemistry
2.7. Statistical Analysis
3. Results
3.1. The Immune Effect of PRRSV N Protein on Piglets
3.2. Clinical Symptoms
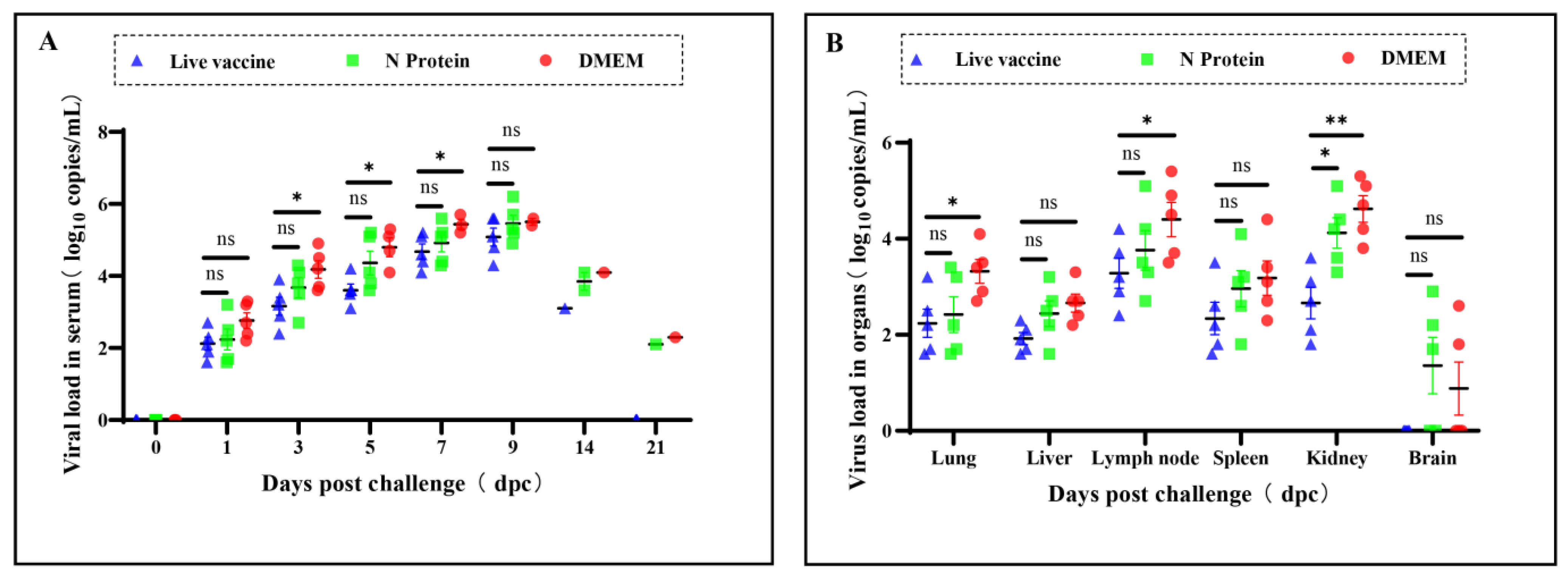
3.3. Virus Loads in Serum and Tissues of the Piglets
3.4. Pathological Damage to the Lungs
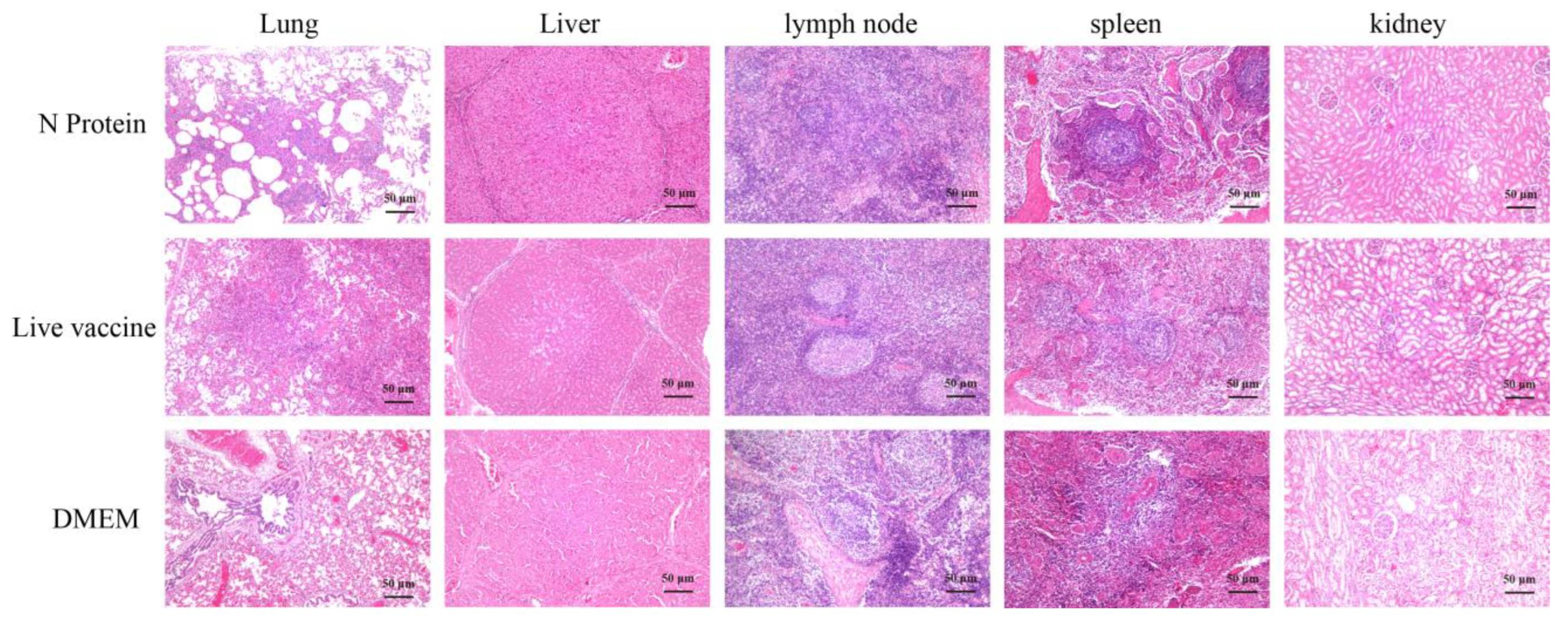
3.5. Histopathological Sections of Piglets
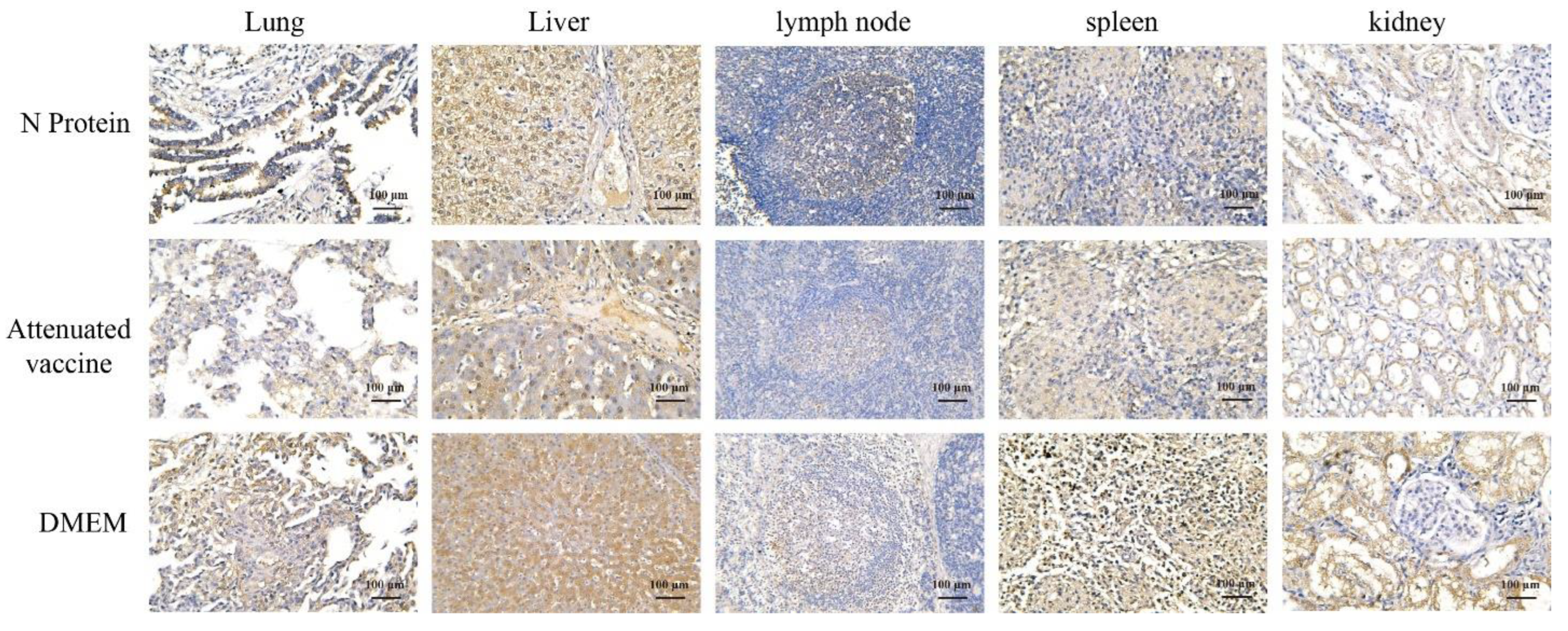
3.6. Immunohistochemistry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lunney, J.K.; Benfield, D.A.; Rowland, R.R.R. Porcine Reproductive and Respiratory Syndrome Virus: An Update on an Emerging and Re-Emerging Viral Disease of Swine. Virus Res. 2010, 154, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Bo, K.; Wang, X.; Tang, B.; Yang, B.; Jiang, W.; Jiang, P. Emergence of a Highly Pathogenic Porcine Reproductive and Respiratory Syndrome Virus in the Mid-Eastern Region of China. Vet. J. 2007, 174, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Linhares, D.C.L.; Betlach, C.; Morrison, R.B. Effect of Immunologic Solutions on Sows and Gilts on Time to Stability, and Production Losses in Breeding Herds Infected with 1-7-4 PRRSV. Prev. Vet. Med. 2017, 144, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Baron, T.; Albina, E.; Leforban, Y.; Madec, F.; Guilmoto, H.; Duran, J.P.; Vannier, P. Report on the First Outbreaks of the Porcine Reproductive and Respiratory Syndrome (PRRS) in France. Diagnosis and Viral Isolation. Ann. Rech. Vet. 1992, 2, 161–166. [Google Scholar] [PubMed]
- Bøtner, A.; Nielsen, J.; Bille-Hansen, V. Isolation of Porcine Reproductive and Respiratory Syndrome (PRRS) Virus in a Danish Swine Herd and Experimental Infection of Pregnant Gilts with the Virus. Vet. Microbiol. 1994, 40, 351–360. [Google Scholar] [CrossRef]
- Tong, G.-Z.; Zhou, Y.-J.; Hao, X.-F.; Tian, Z.-J.; An, T.-Q.; Qiu, H.-J. Highly Pathogenic Porcine Reproductive and Respiratory Syndrome, China. Emerg. Infect. Dis. 2007, 13, 1434–1436. [Google Scholar] [CrossRef]
- Tian, K.; Yu, X.; Zhao, T.; Feng, Y.; Cao, Z.; Wang, C.; Hu, Y.; Chen, X.; Hu, D.; Tian, X.; et al. Emergence of Fatal PRRSV Variants: Unparalleled Outbreaks of Atypical PRRS in China and Molecular Dissection of the Unique Hallmark. PLoS ONE 2007, 2, e526. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, X.; Li, R.; Qiao, S.; Zhang, G. The Prevalent Status and Genetic Diversity of Porcine Reproductive and Respiratory Syndrome Virus in China: A Molecular Epidemiological Perspective. Virol. J. 2018, 15, 2. [Google Scholar] [CrossRef]
- Bao, H.; Li, X. Emergence and Spread of NADC34-like PRRSV in China. Transbound. Emerg. Dis. 2021, 68, 3005–3008. [Google Scholar] [CrossRef]
- Chang, H.; Gao, X.; Wu, Y.; Wang, F.; Lai, M.; Zheng, J.; Qiu, Y.; He, Y.; Liang, X.; Yuan, K.; et al. Genomic and Pathogenicity Analysis of Two Novel Highly Pathogenic Recombinant NADC30-like PRRSV Strains in China, in 2023. Microbiol. Spectr. 2024, 12, e00368-24. [Google Scholar] [CrossRef]
- Gong, B.; Xu, H.; Sun, Q.; Li, C.; Xiang, L.; Zhao, J.; Li, W.; Guo, Z.; Li, J.; Wang, Q.; et al. Dissecting Genetic Diversity and Evolutionary Trends of Chinese PRRSV-1 Based on Whole-Genome Analysis. Transbound. Emerg. Dis. 2024, 2024, 9705539. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, Z.; Ding, Y.; Ge, X.; Guo, X.; Yang, H. NADC30-like Strain of Porcine Reproductive and Respiratory Syndrome Virus, China. Emerg. Infect. Dis. 2015, 21, 2256–2257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Luo, Q.; Zheng, Y.; Sha, H.; Li, G.; Kong, W.; Huang, L.; Zhao, M. Genetic Variability and Recombination of the NSP2 Gene of PRRSV-2 Strains in China from 1996 to 2021. Vet. Sci. 2023, 10, 325. [Google Scholar] [CrossRef] [PubMed]
- Madsen, K.G.; Hansen, C.M.; Madsen, E.S.; Strandbygaard, B.; Bøtner, A.; Sørensen, K.J. Sequence Analysis of Porcine Reproductive and Respiratory Syndrome Virus of the American Type Collected from Danish Swine Herds. Arch. Virol. 1998, 143, 1683–1700. [Google Scholar] [CrossRef]
- Nelsen, C.J.; Murtaugh, M.P.; Faaberg, K.S. Porcine Reproductive and Respiratory Syndrome Virus Comparison: Divergent Evolution on Two Continents. J. Virol. 1999, 73, 270–280. [Google Scholar] [CrossRef]
- Barfoed, A.M.; Blixenkrone-Møller, M.; Jensen, M.H.; Bøtner, A.; Kamstrup, S. DNA Vaccination of Pigs with Open Reading Frame 1–7 of PRRS Virus. Vaccine 2004, 22, 3628–3641. [Google Scholar] [CrossRef]
- Duy, D.T.; Kim, H.; Jeong, J.; Park, K.H.; Yang, S.; Oh, T.; Kim, S.; Kang, I.; Chae, C. Comparative Evaluation of the Efficacy of Commercial and Prototype PRRS Subunit Vaccines against an HP-PRRSV Challenge. J. Vet. Med. Sci. 2018, 80, 1463–1467. [Google Scholar] [CrossRef]
- Ma, H.; Li, X.; Li, J.; Zhao, Z.; Zhang, H.; Hao, G.; Chen, H.; Qian, P. Immunization with a Recombinant Fusion of Porcine Reproductive and Respiratory Syndrome Virus Modified GP5 and Ferritin Elicits Enhanced Protective Immunity in Pigs. Virology 2021, 552, 112–120. [Google Scholar] [CrossRef]
- Wootton, S.K.; Rowland, R.R.R.; Yoo, D. Phosphorylation of the Porcine Reproductive and Respiratory Syndrome Virus Nucleocapsid Protein. J. Virol. 2002, 76, 10569–10576. [Google Scholar] [CrossRef]
- Mardassi, H.; Athanassious, R.; Mounir, S.; Dea, S. Porcine Reproductive and Respiratory Syndrome Virus: Morphological, Biochemical and Serological Characteristics of Quebec Isolates Associated with Acute and Chronic Outbreaks of Porcine Reproductive and Respiratory Syndrome. Can. J. Vet. Res. 1994, 1, 55–64. [Google Scholar] [PubMed]
- Ke, H.; Yoo, D. The Viral Innate Immune Antagonism and an Alternative Vaccine Design for PRRS Virus. Vet. Microbiol. 2017, 209, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Li, L.-W.; Jiang, Y.-F.; Gao, F.; Zhang, Y.-J.; Zhao, W.-Y.; Li, G.-X.; Yu, L.-X.; Zhou, Y.-J.; Tong, G.-Z. Nucleocapsid Protein of Porcine Reproductive and Respiratory Syndrome Virus Antagonizes the Antiviral Activity of TRIM25 by Interfering with TRIM25-Mediated RIG-I Ubiquitination. Vet. Microbiol. 2019, 233, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Li, X.; Sun, R.; Shi, P.; Cao, A.; Zhang, L.; Guo, Y.; Huang, J. The C/EBPβ-Dependent Induction of TFDP2 Facilitates Porcine Reproductive and Respiratory Syndrome Virus Proliferation. Virol. Sin. 2021, 36, 1341–1351. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, G.; Luo, Q.; Sha, H.; Zhang, H.; Wang, R.; Kong, W.; Liao, J.; Zhao, M. Research Progress on the N Protein of Porcine Reproductive and Respiratory Syndrome Virus. Front. Microbiol. 2024, 15, 1391697. [Google Scholar] [CrossRef]
- Music, N.; Gagnon, C.A. The Role of Porcine Reproductive and Respiratory Syndrome (PRRS) Virus Structural and Non-Structural Proteins in Virus Pathogenesis. Anim. Health. Res. Rev. 2010, 11, 135–163. [Google Scholar] [CrossRef]
- Ren, X.; Wang, M.; Yin, J.; Li, G. Phages Harboring Specific Peptides That Recognize the N Protein of the Porcine Reproductive and Respiratory Syndrome Virus Distinguish the Virus from Other Viruses. J. Clin. Microbiol. 2010, 48, 1875–1881. [Google Scholar] [CrossRef]
- Yu, D.; Han, Z.; Xu, J.; Shao, Y.; Li, H.; Kong, X.; Liu, S. A Novel B-Cell Epitope of Avian Infectious Bronchitis Virus N Protein. Viral Immunol. 2010, 23, 189–199. [Google Scholar] [CrossRef]
- Ding, Y.; Wubshet, A.K.; Ding, X.; Zhang, Z.; Li, Q.; Dai, J.; Hou, Q.; Hu, Y.; Zhang, J. Evaluation of Four Commercial Vaccines for the Protection of Piglets against the Highly Pathogenic Porcine Reproductive and Respiratory Syndrome Virus (Hp-PRRSV) QH-08 Strain. Vaccines 2021, 9, 1020. [Google Scholar] [CrossRef]
- Nan, Y.; Wu, C.; Gu, G.; Sun, W.; Zhang, Y.-J.; Zhou, E.-M. Improved Vaccine against PRRSV: Current Progress and Future Perspective. Front. Microbiol. 2017, 8, 1635. [Google Scholar] [CrossRef]
- Chae, C. Commercial PRRS Modified-Live Virus Vaccines. Vaccines 2021, 9, 185. [Google Scholar] [CrossRef]
- Ni, Y.-Y.; Huang, Y.-W.; Cao, D.; Opriessnig, T.; Meng, X.-J. Establishment of a DNA-Launched Infectious Clone for a Highly Pneumovirulent Strain of Type 2 Porcine Reproductive and Respiratory Syndrome Virus: Identification and in Vitro and in Vivo Characterization of a Large Spontaneous Deletion in the Nsp2 Region. Virus Res. 2011, 160, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Chen, J.; Luo, S.; Yan, R.; Zhang, P.; Ren, Z.; Chen, X.; Wang, G.; Xiang, H.; Cai, R.; et al. Pseudorabies gD Protein Protects Mice and Piglets against Lethal Doses of Pseudorabies Virus. Front. Microbiol. 2023, 14, 1288458. [Google Scholar] [CrossRef] [PubMed]
- Halbur, P.G.; Paul, P.S.; Frey, M.L.; Landgraf, J.; Eernisse, K.; Meng, X.-J.; Lum, M.A.; Andrews, J.J.; Rathje, J.A. Comparison of the Pathogenicity of Two US Porcine Reproductive and Respiratory Syndrome Virus Isolates with That of the Lelystad Virus. Vet. Pathol. 1995, 32, 648–660. [Google Scholar] [CrossRef] [PubMed]
- Chand, R.J.; Trible, B.R.; Rowland, R.R. Pathogenesis of Porcine Reproductive and Respiratory Syndrome Virus. Curr. Opin. Virol. 2012, 2, 256–263. [Google Scholar] [CrossRef]
- Yu, L.; Wang, X.; Yu, H.; Jiang, Y.; Gao, F.; Tong, W.; Li, L.; Li, H.; Yang, S.; Chen, P.; et al. The Emergence of a Highly Pathogenic Porcine Reproductive and Respiratory Syndrome Virus with Additional 120aa Deletion in Nsp2 Region in Jiangxi, China. Transbound. Emerg. Dis. 2018, 65, 1740–1748. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Z.; Li, H.; Yang, S.; Ren, F.; Bian, T.; Sun, L.; Zhou, B.; Zhou, L.; Qu, X. The Economic Impact of Porcine Reproductive and Respiratory Syndrome Outbreak in Four Chinese Farms: Based on Cost and Revenue Analysis. Front. Vet. Sci. 2022, 9, 1024720. [Google Scholar] [CrossRef]
- Nieuwenhuis, N.; Duinhof, T.F.; Van Nes, A. Economic Analysis of Outbreaks of Porcine Reproductive and Respiratory Syndrome Virus in Nine Sow Herds. Vet. Rec. 2012, 170, 225. [Google Scholar] [CrossRef]
- Kim, H.S.; Kwang, J.; Yoon, I.J.; Joo, H.S.; Frey, M.L. Enhanced Replication of Porcine Reproductive and Respiratory Syndrome (PRRS) Virus in a Homogeneous Subpopulation of MA-104 Cell Line. Arch. Virol. 1993, 133, 477–483. [Google Scholar] [CrossRef]
- Kishimoto, C.; Hiraoka, Y.; Takada, H. T Cell-Mediated Immune Response Enhances the Severity of Myocarditis in Secondary Cardiotropic Virus Infection in Mice. Basic. Res. Cardiol. 2001, 96, 439–445. [Google Scholar] [CrossRef]
- Holtkamp, D.; Torremorell, M.; Corzo, C.; Linhares, D.; Almeida, M.; Yeske, P.; Polson, D.; Becton, L.; Snelson, H.; Donovan, T.; et al. Proposed Modifications to Porcine Reproductive and Respiratory Syndrome Virus Herd Classification. J. Swine Health Prod. 2021, 29, 261–270. [Google Scholar] [CrossRef]
- Song, S.; Xu, H.; Zhao, J.; Leng, C.; Xiang, L.; Li, C.; Fu, J.; Tang, Y.; Peng, J.; Wang, Q.; et al. Pathogenicity of NADC34-like PRRSV HLJDZD32-1901 Isolated in China. Vet. Microbiol. 2020, 246, 108727. [Google Scholar] [CrossRef] [PubMed]
- Lager, K.M.; Mengeling, W.L.; Brockmeier, S.L. Homologous Challenge of Porcine Reproductive and Respiratory Syndrome Virus Immunity in Pregnant Swine. Vet. Microbiol. 1997, 58, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Lager, K.M.; Mengeling, W.L.; Brockmeier, S.L. Duration of Homologous Porcine Reproductive and Respiratory Syndrome Virus Immunity in Pregnant Swine. Vet. Microbiol. 1997, 58, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Kang, I.; Cho, H.; Oh, T.; Park, K.H.; Min, K.-D.; Chae, C. A Modified-Live Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) Vaccine Protects Late-Term Pregnancy Gilts against a Heterologous PRRSV-2 Challenge. Can. J. Vet. Res. 2020, 3, 172–180. [Google Scholar] [PubMed]
- Wang, A.; Chen, Q.; Wang, L.; Madson, D.; Harmon, K.; Gauger, P.; Zhang, J.; Li, G. Recombination between Vaccine and Field Strains of Porcine Reproductive and Respiratory Syndrome Virus. Emerg. Infect. Dis. 2019, 25, 2335–2337. [Google Scholar] [CrossRef]
- Chen, Y.; He, S.; Sun, L.; Luo, Y.; Sun, Y.; Xie, J.; Zhou, P.; Su, S.; Zhang, G. Genetic Variation, Pathogenicity, and Immunogenicity of Highly Pathogenic Porcine Reproductive and Respiratory Syndrome Virus Strain XH-GD at Different Passage Levels. Arch. Virol. 2016, 161, 77–86. [Google Scholar] [CrossRef]
- Zhou, Y.-J.; An, T.-Q.; Liu, J.-X.; Qiu, H.-J.; Wang, Y.-F.; Tong, G.-Z. Identification of a Conserved Epitope Cluster in the N Protein of Porcine Reproductive and Respiratory Syndrome Virus. Viral Immunol. 2006, 19, 383–390. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, Z.; Yi, H.; Wei, Y.; Han, X.; Li, Q.; Ji, C.; Huang, J.; Deng, Q.; Liu, Y.; et al. The Phosphorylation of the N Protein Could Affect PRRSV Virulence in Vivo. Vet. Microbiol. 2019, 231, 226–231. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, S.; Cui, Z.; Li, W.; Xu, P.; Liu, H.; Miao, X.; Chen, Y.; Han, F.; Zhang, H.; et al. MicroRNA-376b-3p Promotes Porcine Reproductive and Respiratory Syndrome Virus Replication by Targeting Viral Restriction Factor TRIM22. J. Virol. 2022, 96, e01597-21. [Google Scholar] [CrossRef]
- Zhao, P.; Jing, H.; Dong, W.; Duan, E.; Ke, W.; Tao, R.; Li, Y.; Cao, S.; Wang, H.; Zhang, Y.; et al. TRIM26-Mediated Degradation of Nucleocapsid Protein Limits Porcine Reproductive and Respiratory Syndrome Virus-2 Infection. Vet. Rec. 2022, 311, 198690. [Google Scholar] [CrossRef]
- Sagong, M.; Lee, C. Porcine Reproductive and Respiratory Syndrome Virus Nucleocapsid Protein Modulates Interferon-β Production by Inhibiting IRF3 Activation in Immortalized Porcine Alveolar Macrophages. Arch. Virol. 2011, 156, 2187–2195. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, Q.; Bai, J.; Zhao, Y.; Wang, X.; Wang, H.; Jiang, P. The Nucleocapsid Protein and Nonstructural Protein 10 of Highly Pathogenic Porcine Reproductive and Respiratory Syndrome Virus Enhance CD83 Production via NF-κB and Sp1 Signaling Pathways. J. Virol. 2017, 91, e00986-17. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Zhou, Y.; Fang, L.; Ding, Z.; Wang, D.; Ke, W.; Chen, H.; Xiao, S. DExD/H-Box Helicase 36 Signaling via Myeloid Differentiation Primary Response Gene 88 Contributes to NF-κB Activation to Type 2 Porcine Reproductive and Respiratory Syndrome Virus Infection. Front. Immunol. 2017, 8, 1365. [Google Scholar] [CrossRef]
- Han, K.; Seo, H.W.; Park, C.; Chae, C. Vaccination of Sows against Type 2 Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) before Artificial Insemination Protects against Type 2 PRRSV Challenge but Does Not Protect against Type 1 PRRSV Challenge in Late Gestation. Vet. Res. 2014, 45, 12. [Google Scholar] [CrossRef]
- Dea, S.; Gagnon, C.A.; Mardassi, H.; Pirzadeh, B.; Rogan, D. Current Knowledge on the Structural Proteins of Porcine Reproductive and Respiratory Syndrome (PRRS) Virus: Comparison of the North American and European Isolates. Arch. Virol. 2000, 145, 659–688. [Google Scholar] [CrossRef]
- Li, Z.; Wang, G.; Wang, Y.; Zhang, C.; Huang, B.; Li, Q.; Li, L.; Xue, B.; Ding, P.; Cai, X.; et al. Immune Responses of Pigs Immunized with a Recombinant Porcine Reproductive and Respiratory Syndrome Virus Expressing Porcine GM-CSF. Vet. Immunol. Immunopathol. 2015, 168, 40–48. [Google Scholar] [CrossRef]
- Luckow, V.A.; Lee, S.C.; Barry, G.F.; Olins, P.O. Efficient Generation of Infectious Recombinant Baculoviruses by Site-Specific Transposon-Mediated Insertion of Foreign Genes into a Baculovirus Genome Propagated in Escherichia Coli. J. Virol. 1993, 67, 4566–4579. [Google Scholar] [CrossRef]
- Wang, H.; Feng, W. Current Status of Porcine Reproductive and Respiratory Syndrome Vaccines. Vaccines 2024, 12, 1387. [Google Scholar] [CrossRef]
- Pandey, K.K.; Sahoo, B.R.; Pattnaik, A.K. Protein Nanoparticles as Vaccine Platforms for Human and Zoonotic Viruses. Viruses 2024, 16, 936. [Google Scholar] [CrossRef]
- Ahmadivand, S.; Fux, R.; Palić, D. Ferritin Vaccine Platform for Animal and Zoonotic Viruses. Vaccines 2024, 12, 1112. [Google Scholar] [CrossRef]
- Chang, X.; Ma, J.; Zhou, Y.; Xiao, S.; Xiao, X.; Fang, L. Development of a Ferritin Protein Nanoparticle Vaccine with PRRSV GP5 Protein. Viruses 2024, 16, 991. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Chen, Y. Epitope Screening and Vaccine Molecule Design of PRRSV GP3 and GP5 Protein Based on Immunoinformatics. J. Cell Mol. Med. 2024, 28, e18103. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Zhang, P.; Zhang, X.; Luo, S.; Yuan, Z.; Huang, Y.; Wang, G.; Xiang, H.; Huang, Y.; Jin, Y.; et al. Immune Protection Gap Between Porcine Reproductive and Respiratory Syndrome Subunit Vaccine (N Protein) and Live Vaccine. Vaccines 2025, 13, 441. https://doi.org/10.3390/vaccines13050441
Zhao M, Zhang P, Zhang X, Luo S, Yuan Z, Huang Y, Wang G, Xiang H, Huang Y, Jin Y, et al. Immune Protection Gap Between Porcine Reproductive and Respiratory Syndrome Subunit Vaccine (N Protein) and Live Vaccine. Vaccines. 2025; 13(5):441. https://doi.org/10.3390/vaccines13050441
Chicago/Turabian StyleZhao, Mengpo, Pian Zhang, Xiaoxiao Zhang, Shengjun Luo, Ziguo Yuan, Yanju Huang, Gang Wang, Hua Xiang, Yuan Huang, Yuzhu Jin, and et al. 2025. "Immune Protection Gap Between Porcine Reproductive and Respiratory Syndrome Subunit Vaccine (N Protein) and Live Vaccine" Vaccines 13, no. 5: 441. https://doi.org/10.3390/vaccines13050441
APA StyleZhao, M., Zhang, P., Zhang, X., Luo, S., Yuan, Z., Huang, Y., Wang, G., Xiang, H., Huang, Y., Jin, Y., Chen, J., & Wang, X. (2025). Immune Protection Gap Between Porcine Reproductive and Respiratory Syndrome Subunit Vaccine (N Protein) and Live Vaccine. Vaccines, 13(5), 441. https://doi.org/10.3390/vaccines13050441






