Maternal Immunization: Current Evidence, Progress, and Challenges
Abstract
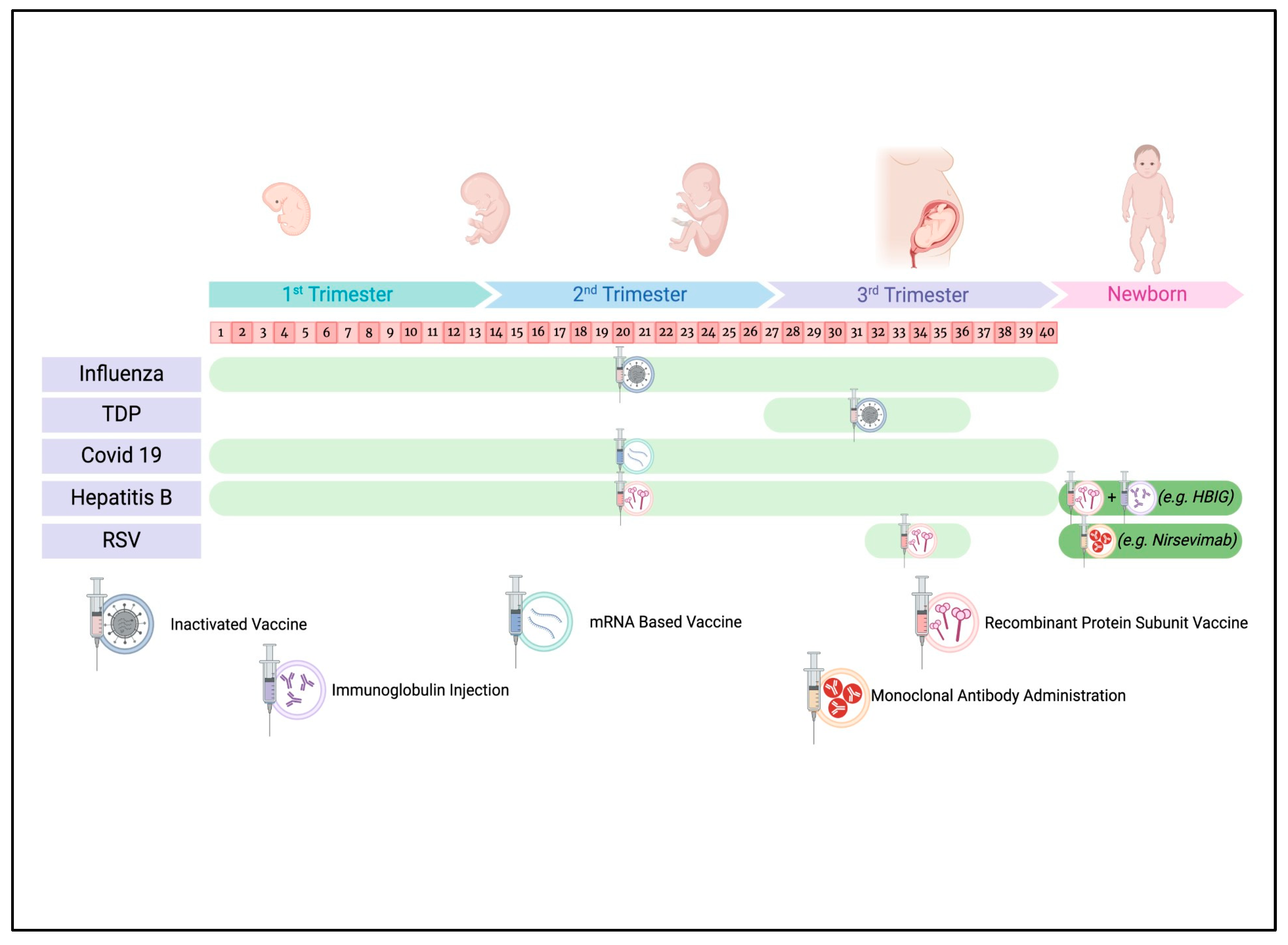
:1. Introduction
2. Mechanisms and Benefits of Maternal Immunization
3. Current Recommendations and Safety Profile of Maternal Vaccines
3.1. Influenza Vaccine
3.1.1. Safety
3.1.2. Efficacy and Effectiveness
3.2. Tetanus, Diphtheria, and Acellular Pertussis (Tdap) Vaccine
3.2.1. Safety
3.2.2. Efficacy and Effectiveness
3.3. COVID-19 Vaccine
3.3.1. Safety
3.3.2. Efficacy and Effectiveness
3.4. Hepatitis B Vaccine
3.5. Respiratory Syncytial Virus (RSV) Vaccine
3.5.1. Safety
3.5.2. Efficacy and Effectiveness
4. Barriers to Maternal Immunization
5. Recent Innovations and Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACIP | Advisory Committee on Immunization Practices |
| CISA | Clinical Immunization Safety Assessment |
| ccIIV | cell culture-based inactivated influenza vaccine |
| IIV | inactivated influenza vaccines |
| RCTs | randomized controlled trials |
| RIV | recombinant influenza vaccine |
| RSV | respiratory syncytial virus |
| Tdap | tetanus-diphtheria-acellular pertussis |
| Th2 | T-helper 2 |
| VAERS | Vaccine Adverse Event Reporting System |
| CMO | extracorporeal membrane oxygenation |
| FHA | filamentous hemagglutinin |
| GBS | Group B Streptococcus |
| HBIG | hepatitis B immunoglobulin |
| HBV | hepatitis B virus |
| LRTD | lower respiratory tract disease (diseases of the lungs such as bronchitis or pneumonia) |
| MTCT | mother-to-child transmission (of hepatitis B virus) |
| NNT | neonatal tetanus |
| PT | pertussis toxin |
| PRN | pertactin |
| TT | tetanus toxoid |
| WHO | World Health Organization |
References
- de Bruin, O.; Phijffer, E.; Ahmadizar, F.; van der Maas, N.; Wildenbeest, J.; Sturkenboom, M.M.; Bont, L.; Bloemenkamp, K. Are maternal vaccines effective and safe for mothers and infants? A systematic review and meta-analysis of randomized controlled trials. BMJ Glob. Health 2023, 8, e012376. [Google Scholar] [CrossRef] [PubMed]
- Engmann, C.; Fleming, J.A.; Khan, S.; Innis, B.L.; Smith, J.M.; Hombach, J.; Sobanjo-ter Meulen, A. Closer and closer? Maternal immunization: Current promise, future horizons. J. Perinatol. 2020, 40, 844–857. [Google Scholar] [CrossRef] [PubMed]
- Langel, S.N.; Blasi, M.; Permar, S.R. Maternal immune protection against infectious diseases. Cell Host Microbe 2022, 30, 660–674. [Google Scholar] [CrossRef]
- Semmes, E.C.; Coyne, C.B. Innate immune defenses at the maternal-fetal interface. Curr. Opin. Immunol. 2022, 74, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Clements, T.; Rice, T.F.; Vamvakas, G.; Barnett, S.; Barnes, M.; Donaldson, B.; Jones, C.E.; Kampmann, B.; Holder, B. Update on Transplacental Transfer of IgG Subclasses: Impact of Maternal and Fetal Factors. Front. Immunol. 2020, 11, 1920. [Google Scholar] [CrossRef]
- Wessel, R.E.; Dolatshahi, S. Quantitative mechanistic model reveals key determinants of placental IgG transfer and informs prenatal immunization strategies. PLoS Comput. Biol. 2023, 19, e1011109. [Google Scholar] [CrossRef]
- Wilcox, C.R.; Holder, B.; Jones, C.E. Factors Affecting the FcRn-Mediated Transplacental Transfer of Antibodies and Implications for Vaccination in Pregnancy. Front. Immunol. 2017, 8, 1294. [Google Scholar] [CrossRef]
- Calvert, A.; Jones, C.E. Placental transfer of antibody and its relationship to vaccination in pregnancy. Curr. Opin. Infect. Dis. 2017, 30, 268–273. [Google Scholar] [CrossRef]
- Chu, H.Y.; Englund, J.A. Maternal immunization. Clin. Infect. Dis. 2014, 59, 560–568. [Google Scholar] [CrossRef]
- Oguti, B.; Ali, A.; Andrews, N.; Barug, D.; Anh Dang, D.; Halperin, S.A.; Blanchard-Rohner, G.; Lemaître, B.; Boukrid, M.; Combescure, C.; et al. The half-life of maternal transplacental antibodies against diphtheria, tetanus, and pertussis in infants: An individual participant data meta-analysis. Vaccine 2022, 40, 450–458. [Google Scholar] [CrossRef]
- Shook, L.L.; Atyeo, C.G.; Yonker, L.M.; Fasano, A.; Gray, K.J.; Alter, G.; Edlow, A.G. Durability of Anti-Spike Antibodies in Infants After Maternal COVID-19 Vaccination or Natural Infection. JAMA 2022, 327, 1087–1089. [Google Scholar] [CrossRef]
- Lopez, P.A.; Nziza, N.; Chen, T.; Shook, L.L.; Burns, M.D.; Demidkin, S.; Jasset, O.; Akinwunmi, B.; Yonker, L.M.; Gray, K.J.; et al. Placental transfer dynamics and durability of maternal COVID-19 vaccine-induced antibodies in infants. iScience 2024, 27, 109273. [Google Scholar] [CrossRef] [PubMed]
- Hurley, W.L.; Theil, P.K. Perspectives on immunoglobulins in colostrum and milk. Nutrients 2011, 3, 442–474. [Google Scholar] [CrossRef]
- Marchant, A.; Sadarangani, M.; Garand, M.; Dauby, N.; Verhasselt, V.; Pereira, L.; Jones, C.E.; Kampmann, B.; Holder, B.; Kollmann, T.R.; et al. Maternal immunisation: Collaborating with mother nature. Lancet Infect. Dis. 2017, 17, e197–e208. [Google Scholar] [CrossRef]
- Kollmann, T.R.; Marchant, A.; Way, S.S. Vaccination strategies to enhance immunity in neonates. Science 2020, 368, 612–615. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, M.; Arck, P.C. Vertically Transferred Immunity in Neonates: Mothers, Mechanisms and Mediators. Front. Immunol. 2020, 11, 555. [Google Scholar] [CrossRef] [PubMed]
- Pasetti, M.F.; Ackerman, M.E.; Hoen, A.G.; Alter, G.; Tsang, J.S.; Marchant, A. Maternal determinants of infant immunity: Implications for effective immunization and maternal-child health. Vaccine 2020, 38, 4491–4494. [Google Scholar] [CrossRef]
- Ding, X.; Zhao, F.; Liu, Z.; Yao, J.; Yu, H.; Zhang, X. Original antigenic sin: A potential double-edged effect for vaccine improvement. Biomed. Pharmacother. 2024, 178, 117187. [Google Scholar] [CrossRef]
- Malinská, N.; Grobárová, V.; Knížková, K.; Černý, J. Maternal-Fetal Microchimerism: Impacts on Offspring’s Immune Development and Transgenerational Immune Memory Transfer. Physiol. Res. 2024, 73, 315–332. [Google Scholar] [CrossRef]
- Peroni, D.G.; Chirumbolo, S.; Veneri, D.; Piacentini, G.L.; Tenero, L.; Vella, A.; Salvatori, G.; Spanevello, A.; Boner, A.L.; Bodini, A.; et al. Colostrum-derived B and T cells as an extra-lymphoid compartment of effector cell populations in humans. J. Matern. Fetal Neonatal Med. 2013, 26, 137–142. [Google Scholar] [CrossRef]
- Armistead, B.; Jiang, Y.; Carlson, M.; Ford, E.S.; Jani, S.; Houck, J.; Schilling, M.; Burnham, C.D.; Wiedeman, A.; Permar, S.R.; et al. Spike-specific T cells are enriched in breastmilk following SARS-CoV-2 mRNA vaccination. Mucosal Immunol. 2023, 16, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Dulfer, E.A.; Domínguez-Andrés, J. Mechanisms involved in the transmission of trained immunity to offspring. J. Allergy Clin. Immunol. 2024, 154, 1117–1119. [Google Scholar] [CrossRef]
- Nunes, M.C.; Cutland, C.L.; Jones, S.; Hugo, A.; Madimabe, R.; Simões, E.A.; Klugman, K.P.; Moloi, M.; Baillie, V.; Darlow, C.; et al. Maternal Flu Trial Team. Duration of Infant Protection Against Influenza Illness Conferred by Maternal Immunization: Secondary Analysis of a Randomized Clinical Trial. JAMA Pediatr. 2016, 170, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Grohskopf, L.A.; Ferdinands, J.M.; Blanton, L.H.; Broder, K.R.; Loehr, J. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2024–2025 Influenza Season. MMWR Recomm. Rep. 2024, 73, 1–25. [Google Scholar] [CrossRef]
- Riley, L.E.; Silverman, N.S.; Swamy, G.K.; Thompson, J.L. Influenza in Pregnancy: Prevention and Treatment: ACOG Committee Statement No. 7. Obstet. Gynecol. 2024, 143, e24–e30. [Google Scholar] [CrossRef]
- Madhi, S.A.; Cutland, C.L.; Kuwanda, L.; Weinberg, A.; Hugo, A.; Jones, S.; Klugman, K.P.; Darlow, C.; Louw, C.; Moloi, M.; et al. Maternal Flu Trial (Matflu) Team. Influenza vaccination of pregnant women and protection of their infants. N. Engl. J. Med. 2014, 371, 918–931. [Google Scholar] [CrossRef] [PubMed]
- Cuningham, W.; Geard, N.; Fielding, J.E.; Braat, S.; Madhi, S.A.; Nunes, M.C.; Klugman, K.P.; Darlow, C.; Jones, S.; Moloi, M.; et al. Optimal timing of influenza vaccine during pregnancy: A systematic review and meta-analysis. Influenza Other Respir. Viruses 2019, 13, 438–452. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tamma, P.D.; Ault, K.A.; del Rio, C.; Steinhoff, M.C.; Halsey, N.A.; Omer, S.B. Safety of influenza vaccination during pregnancy. Am. J. Obstet. Gynecol. 2009, 201, 547–552. [Google Scholar] [CrossRef]
- Moro, P.L.; Marquez, P. Reports of cell-based influenza vaccine administered during pregnancy in the Vaccine Adverse Event Reporting System (VAERS), 2013–2020. Vaccine 2021, 39, 678–681. [Google Scholar] [CrossRef]
- Robinson, C.; van Boxmeer, J.; Tilson, H.; Scialli, A.; Vanchiere, J.A.; Ides, E.; Wood, D.; Johnson, S.; Hodges, M.; Freed, G.L.; et al. Outcomes in Pregnant Persons Immunized with a Cell-Based Quadrivalent Inactivated Influenza Vaccine: A Prospective Observational Cohort Study. Vaccines 2022, 10, 1600. [Google Scholar] [CrossRef]
- Swamy, G. Clinical trial to compare safety of recombinant influenza vaccine (RIV4) versus quadrivalent inactivated influenza vaccine (IIV4) in pregnancy (ClinicalTrials.gov NCT03969641). In Proceedings of the ACIP Meeting, Atlanta, GA, USA, 19–20 October 2022; Volume 202211. [Google Scholar]
- Hsiao, A.; Yee, A.; Izikson, R.; Fireman, B.; Hansen, J.; Lewis, N.; Daley, M.F.; Williams, J.; Thompson, M.G.; Klein, N.P.; et al. Safety of quadrivalent recombinant influenza vaccine in pregnant persons and their infants. AJOG Glob. Rep. 2024, 4, 100395. [Google Scholar] [CrossRef] [PubMed]
- Getahun, D.; Liu, I.A.; Sy, L.S.; Glanz, J.M.; Zerbo, O.; Vazquez-Benitez, G.; Tseng, H.F.; DeSilva, M.; Naleway, A.; Klein, N.P.; et al. Safety of the Seasonal Influenza Vaccine in 2 Successive Pregnancies. JAMA Netw. Open 2024, 7, e2434857. [Google Scholar] [CrossRef] [PubMed]
- Duque, J.; Howe, S.A.; Azziz-Baumgartner, E.; Petousis-Harris, H. Multi-decade national cohort identifies adverse pregnancy and birth outcomes associated with acute respiratory illness hospitalisations during the influenza season. Influenza Other Respir. Viruses 2023, 17, e13063. [Google Scholar] [CrossRef]
- Tiwari, T.S.; Baughman, A.L.; Clark, T.A. First pertussis vaccine dose and prevention of infant mortality. Pediatrics 2015, 135, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, M.; Liang, J.L.; Messonnier, N.; Clark, T.A. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in pregnant women—Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morb. Mortal. Wkly. Rep. 2013, 62, 131–135. [Google Scholar]
- Simayi, A.; Zhu, L.; Jin, H. Safety and Immunogenicity of Pertussis Vaccine Immunization during Pregnancy: A Meta-Analysis of Randomized Clinical Trials. J. Trop. Med. 2022, 2022, 4857872. [Google Scholar] [CrossRef]
- Murphy, T.V.; Slade, B.A.; Broder, K.R.; Kretsinger, K.; Tiwari, T.; Joyce, P.M.; Clark, T.A.; Iskander, J.; Mootrey, G.T.; Liang, J.L.; et al. Prevention of pertussis, tetanus, and diphtheria among pregnant and postpartum women and their infants recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2008, 57, 1–51. [Google Scholar]
- Centers for Disease Control and Prevention. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis (Tdap) vaccine from the Advisory Committee on Immunization Practices, 2010. MMWR 2011, 60, 13–15. [Google Scholar] [PubMed]
- Sukumaran, L.; McCarthy, N.L.; Kharbanda, E.O.; Weintraub, E.S.; Vazquez-Benitez, G.; McNeil, M.M.; Li, R.; Klein, N.P.; Hambidge, S.J.; Naleway, A.L.; et al. Safety of Tetanus Toxoid, Reduced Diphtheria Toxoid, and Acellular Pertussis and Influenza Vaccinations in Pregnancy. Obstet. Gynecol. 2015, 126, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Sukumaran, L.; McCarthy, N.L.; Kharbanda, E.O.; McNeil, M.M.; Naleway, A.L.; Klein, N.P.; Hesse, E.M.; Weintraub, E.S.; DeStefano, F.; Zheteyeva, Y.A.; et al. Association of Tdap Vaccination With Acute Events and Adverse Birth Outcomes Among Pregnant Women With Prior Tetanus-Containing Immunizations. JAMA 2015, 314, 1581–1587. [Google Scholar] [CrossRef]
- Chaithongwongwatthana, S.; Wijagkanalan, W.; Wanlapakorn, N.; Fortuna, L.; Yuwaree, V.; Kerdsomboon, C.; Kamolratanakul, S.; Ussing, N.; Mekmullica, J.; Puthanakit, T. Transplacental transfer of maternal antibodies following immunization with recombinant pertussis vaccines during pregnancy: Real-world evidence. Int. J. Infect. Dis. 2024, 144, 107047. [Google Scholar] [CrossRef] [PubMed]
- Perrett, K.P.; Halperin, S.A.; Nolan, T.; Martínez Pancorbo, C.; Tapiero, B.; Martinón-Torres, F.; Smolenov, I.; Leroux-Roels, G.; Vandermeulen, C.; Kieninger, D. Immunogenicity, transplacental transfer of pertussis antibodies and safety following pertussis immunization during pregnancy: Evidence from a randomized, placebo-controlled trial. Vaccine 2020, 38, 2095–2104. [Google Scholar] [CrossRef] [PubMed]
- Abu Raya, B.; Srugo, I.; Kessel, A.; Peterman, M.; Bader, D.; Gonen, R.; Shlomo, E.; Levin, G.; Wolf, D.G.; Prajgrod, M. The effect of timing of maternal tetanus, diphtheria, and acellular pertussis (Tdap) immunization during pregnancy on newborn pertussis antibody levels—A prospective study. Vaccine 2014, 32, 5787–5793. [Google Scholar] [CrossRef] [PubMed]
- Baxter, R.; Bartlett, J.; Fireman, B.; Lewis, E.; Klein, N.P. Effectiveness of Vaccination During Pregnancy to Prevent Infant Pertussis. Pediatrics 2017, 139, e20164091. [Google Scholar] [CrossRef]
- Skoff, T.H.; Deng, L.; Bozio, C.H.; Hariri, S. US Infant Pertussis Incidence Trends Before and After Implementation of the Maternal Tetanus, Diphtheria, and Pertussis Vaccine. JAMA Pediatr. 2023, 177, 395–400. [Google Scholar] [CrossRef]
- Abu-Raya, B.; Maertens, K.; Munoz, F.M.; Zimmermann, P.; Curtis, N.; Halperin, S.A.; Leuridan, E.; Marchant, A.; Giles, M.L.; Edwards, K.M.; et al. The Effect of Tetanus-Diphtheria-Acellular-Pertussis Immunization During Pregnancy on Infant Antibody Responses: Individual-Participant Data Meta-Analysis. Front. Immunol. 2021, 12, 689394. [Google Scholar] [CrossRef]
- Caboré, R.N.; Maertens, K.; Dobly, A.; Leuridan, E.; Van Damme, P.; Huyge, K. Influence of maternal vaccination against diphtheria, tetanus, and pertussis on the avidity of infant antibody responses to a pertussis containing vaccine in Belgium. Virulence 2017, 8, 1245–1254. [Google Scholar] [CrossRef]
- Saso, A.; Kampmann, B. What Is the Impact of Maternal Pertussis Immunization in Pregnancy on the Quantity, Quality and Longevity of Infant Vaccine Responses?: A Review of the Current Evidence. Pediatr. Infect. Dis. J. 2025, 44, S49–S55. [Google Scholar] [CrossRef]
- Zimmermann, P.; Perrett, K.P.; Messina, N.L.; Donath, S.; Ritz, N.; van der Klis, F.R.M.; Curtis, N. The Effect of Maternal Immunisation During Pregnancy on Infant Vaccine Responses. eClinicalMedicine 2019, 13, 21–30. [Google Scholar] [CrossRef]
- Briga, M.; Goult, E.; Brett, T.S.; Rohani, P.; Domenech de Cellès, M. Maternal pertussis immunization and the blunting of routine vaccine effectiveness: A meta-analysis and modeling study. Nat. Commun. 2024, 15, 921. [Google Scholar] [CrossRef]
- Principi, N.; Bianchini, S.; Esposito, S. Pertussis Epidemiology in Children: The Role of Maternal Immunization. Vaccines 2024, 12, 1030. [Google Scholar] [CrossRef] [PubMed]
- Roper, M.H.; Vandelaer, J.H.; Gasse, F. Maternal and neonatal tetanus. Lancet 2007, 370, 1947–1959. [Google Scholar] [CrossRef] [PubMed]
- Schofield, F.D.; Tucker, V.M.; Westbrook, G.R. Neonatal tetanus in New Guinea. Effect of active immunization in pregnancy. BMJ 1961, 5255, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Newell, K.W.; Duenas Lehmann, A.; LeBlanc, D.R.; Garces Osorio, N. The use of toxoid for the prevention of tetanus neonatorum. Final report of a double-blind controlled field trial. Bull. World Health Organ. 1966, 35, 863–871. [Google Scholar]
- Yusuf, B.; Solter, S.; Bertsch, D.; Arnold, R.B. Impact of a tetanus toxoid immunization mass campaign on neonatal tetanus mortality in Aceh Province, Indonesia. Southeast Asian J. Trop. Med. Public Health 1991, 22, 351–356. [Google Scholar]
- Arnold, R.B.; Soewarso, T.I.; Karyadi, A. Mortality from neonatal tetanus in Indonesia: Results of two surveys. Bull. World Health Organ. 1986, 64, 259–262. [Google Scholar]
- Zambrano, L.D.; Ellington, S.; Strid, P.; Galang, R.R.; Oduyebo, T.; Tong, V.T.; Woodworth, K.R.; Nahabedian, J.F.; Azziz-Baumgartner, E.; Meaney-Delman, D.; et al. Update: Characteristics of Symptomatic Women of Reproductive Age with Laboratory-Confirmed SARS-CoV-2 Infection by Pregnancy Status—United States, January 22–October 3, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1641–1647. [Google Scholar] [CrossRef]
- Panagiotakopoulos, L.; Moulia, D.L.; Godfrey, M.; Link-Gelles, R.; Roper, L.; Havers, F.P.; Oliveira, C.R.; Daley, M.F.; Woodworth, K.R.; Oliver, S.E. Use of COVID-19 Vaccines for Persons Aged ≥6 Months: Recommendations of the Advisory Committee on Immunization Practices—United States, 2024. MMWR Morb. Mortal. Wkly. Rep. 2024, 73, 819–824. [Google Scholar] [CrossRef]
- Hawkins, S.S. Current Evidence to Guide Practice, Policy, and Research: COVID-19 Vaccination During Pregnancy. J. Obstet. Gynecol. Neonatal Nurs. 2023, 52, 159–167. [Google Scholar] [CrossRef]
- Norman, M.; Magnus, M.C.; Söderling, J.; Juliusson, P.B.; Navér, L.; Örtqvist, A.K.; Pasternak, B.; Hviid, A.; Fell, D.B.; Skajaa, N. Neonatal Outcomes After COVID-19 Vaccination in Pregnancy. JAMA 2024, 331, 396–407. [Google Scholar] [CrossRef]
- Jorgensen, S.C.J.; Drover, S.S.M.; Fell, D.B.; Austin, P.C.; D’Souza, R.; Guttmann, A.; McArthur, M.A.; Gershon, A.S.; Ray, J.G.; Daneman, N. Newborn and Early Infant Outcomes Following Maternal COVID-19 Vaccination During Pregnancy. JAMA Pediatr. 2023, 177, 1314–1323. [Google Scholar] [CrossRef] [PubMed]
- Dagan, N.; Barda, N.; Biron-Shental, T.; Makov-Assif, M.; Key, C.; Kohane, I.S.; Alroy-Preis, S.; Balicer, R.D. Effectiveness of the BNT162b2 mRNA COVID-19 vaccine in pregnancy. Nat. Med. 2021, 27, 1693–1695. [Google Scholar] [CrossRef] [PubMed]
- Toussia-Cohen, S.; Nir, O.; Peretz-Machluf, R.; Bookstein-Peretz, S.; Segal, O.; Asraf, K.; Shapira, M.; Kalka, I.N.; Bar-On, Y.; Dicker, D.; et al. Maternal and Neonatal Immune Responses Following COVID-19 Infection and Vaccinations in Pregnancy. Vaccines 2022, 10, 2019. [Google Scholar] [CrossRef] [PubMed]
- Marchand, G.; Masoud, A.T.; Grover, S.; King, A.; Brazil, G.; Ulibarri, H.; Sainz, K.; Parise, C.; Ulibarri, A.; Ruther, S.; et al. Maternal and neonatal outcomes of COVID-19 vaccination during pregnancy, a systematic review and meta-analysis. NPJ Vaccines 2023, 8, 103. [Google Scholar] [CrossRef]
- Simeone, R.M.; Zambrano, L.D.; Halasa, N.B.; Fleming-Dutra, K.E.; Newhams, M.M.; Wu, M.J.; Britton, A.; Olsen, S.J.; Gaglani, M.; Reese, S.E.; et al. Effectiveness of Maternal mRNA COVID-19 Vaccination During Pregnancy Against COVID-19-Associated Hospitalizations in Infants Aged <6 Months During SARS-CoV-2 Omicron Predominance—20 States, March 9, 2022–May 31, 2023. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Eke, A.C.; Eleje, G.U.; Eke, U.A.; Xia, Y.; Liu, J. Hepatitis B immunoglobulin during pregnancy for prevention of mother-to-child transmission of hepatitis B virus. Cochrane Database Syst. Rev. 2017, 2, CD008545. [Google Scholar] [CrossRef]
- Chen, Z.; Zeng, M.; Liu, D.; Wu, L.; Zhang, L. Antenatal administration of hepatitis B immunoglobulin and hepatitis B vaccine to prevent mother to child transmission in hepatitis B virus surface antigen positive pregnant women: A systematic review and meta-analysis. Medicine 2020, 99, e19886. [Google Scholar] [CrossRef]
- Liu, S.; Wang, J.; Li, Y.; Wang, M.; Du, P.; Zhang, Z.; Zhang, X.; Li, X.; Liu, J.; Zhang, Y. A Multivalent mRNA Therapeutic Vaccine Exhibits Breakthroughs in Immune Tolerance and Virological Suppression of HBV by Stably Presenting the Pre-S Antigen on the Cell Membrane. Pharmaceutics 2025, 17, 211. [Google Scholar] [CrossRef]
- Prabhu, M.; Susich, M.K.; Packer, C.H.; Hersch, A.R.; Riley, L.E.; Caughey, A.B. Universal Hepatitis B Antibody Screening and Vaccination in Pregnancy: A Cost-Effectiveness Analysis. Obstet Gynecol. 2022, 139, 357–367. [Google Scholar] [CrossRef]
- Wildenbeest, J.G.; Billard, M.N.; Zuurbier, R.P.; Korsten, K.; Langedijk, A.C.; van de Ven, P.M.; Verhagen, J.; de Vos, A.; Hoeben, P.; Nijkamp, J. RESCEU Investigators. The burden of respiratory syncytial virus in healthy term-born infants in Europe: A prospective birth cohort study. Lancet Respir. Med. 2023, 11, 341–353. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. FDA News Release: FDA Approves First Vaccine for Pregnant Individuals to Prevent RSV in Infants. 2023. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-vaccine-pregnant-individuals-prevent-rsv-infants (accessed on 16 April 2025).
- Buchwald, A.G.; Graham, B.S.; Traore, A.; Haidara, F.C.; Chen, M.; Morabito, K.; Zhao, C.; Wu, R.; Jung, D.; Lee, J. Respiratory Syncytial Virus (RSV) Neutralizing Antibodies at Birth Predict Protection from RSV Illness in Infants in the First 3 Months of Life. Clin. Infect. Dis. 2021, 73, e4421–e4427. [Google Scholar] [CrossRef] [PubMed]
- Kampmann, B.; Madhi, S.A.; Munjal, I.; Simões, E.A.F.; Pahud, B.A.; Llapur, C.; Han, Y.; Garg, A.; Patel, M.; Gawande, M. MATISSE Study Group. Bivalent Prefusion F Vaccine in Pregnancy to Prevent RSV Illness in Infants. N. Engl. J. Med. 2023, 388, 1451–1464. [Google Scholar] [CrossRef] [PubMed]
- Hong-Nguyen, Y.K.; Toerner, J.; Lee, L.; Allende, M.C.; Kaslow, D.C. Regulatory review of benefits and risks of preventing infant RSV disease through maternal immunization. NPJ Vaccines 2024, 9, 210. [Google Scholar] [CrossRef] [PubMed]
- Pecenka, C.; Sparrow, E.; Feikin, D.R.; Srikantiah, P.; Darko, D.M.; Karikari-Boateng, E.; Patel, S.; D’Souza, M.; Zhang, W.; Liu, J. Respiratory syncytial virus vaccination and immunoprophylaxis: Realising the potential for protection of young children. Lancet 2024, 404, 1157–1170. [Google Scholar] [CrossRef]
- European Medicines Agency. Abrysvo (Respiratory Syncytial Virus Vaccine [Bivalent, Recombinant]) Risk Management Plan. 2023. Available online: www.ema.europa.eu/en/documents/rmp-summary/abrysvo-epar-risk-management-plan_en.pdf (accessed on 23 July 2024).
- Son, M.; Riley, L.E.; Staniczenko, A.P.; Cron, J.; Yen, S.; Thomas, C.; Sholle, E.; Patel, K.; Li, M.; Miller, D. Nonadjuvanted Bivalent Respiratory Syncytial Virus Vaccination and Perinatal Outcomes. JAMA Netw. Open. 2024, 7, e2419268. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. RSV Immunization Guidance for Infants and Young Children. Available online: https://www.cdc.gov/rsv/hcp/vaccine-clinical-guidance/infants-young-children.html?utm_source=chatgpt.com (accessed on 16 April 2025).
- Vilca, L.M.; Cesari, E.; Tura, A.M.; Di Stefano, A.; Vidiri, A.; Cavaliere, A.F.; Cetin, I. Barriers and facilitators regarding influenza and pertussis maternal vaccination uptake: A multi-center survey of pregnant women in Italy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 247, 10–15. [Google Scholar] [CrossRef]
- Ferrari, A.; Moretti, G.; Corazza, I.; Mannella, P.; Simoncini, T.; Bonciani, M. Pregnancy vaccination predictive factors and uptake profiles among Italian women: A cross-sectional survey study on a large population. Int. J. Gynaecol. Obstet. 2023, 162, 105–115. [Google Scholar] [CrossRef]
- Kelly, S.M.; Bracken, O.; Bholah, T.; Crosby, D.A. Uptake rates and attitudes to influenza and COVID-19 vaccination in pregnancy—A prospective cohort study. Ir. J. Med. Sci. 2024, 193, 289–293. [Google Scholar] [CrossRef]
- Rand, C.M.; Olson-Chen, C. Maternal Vaccination and Vaccine Hesitancy. Pediatr. Clin. N. Am. 2023, 70, 259–269. [Google Scholar] [CrossRef]
- Razzaghi, H.; Garacci, E.; Kahn, K.E.; Lindley, M.C.; Jones, J.M.; Stokley, S.; Calhoun, K.; Black, C.L. Maternal Respiratory Syncytial Virus Vaccination and Receipt of Respiratory Syncytial Virus Antibody (Nirsevimab) by Infants Aged <8 Months—United States, April 2024. MMWR Morb. Mortal. Wkly. Rep. 2024, 73, 837–843. [Google Scholar] [CrossRef]
- Cinicola, B.; Conti, M.G.; Terrin, G.; Sgrulletti, M.; Elfeky, R.; Carsetti, R.; Salinas, A.F.; Mortari, E.P.; Brindisi, G.; De Curtis, M.; et al. The Protective Role of Maternal Immunization in Early Life. Front. Pediatr. 2021, 9, 638871. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moschese, V.; de Angelis, L.; Capogna, M.V.; Graziani, S.; Baglivo, F.; Pietropolli, A.; Del Giudice, M.M.; Rizzo, C.; Italian Society of Pediatric Allergology and Immunology (SIAIP) Vaccine Committee. Vaccine hesitancy and knowledge regarding maternal immunization among reproductive age women in central Italy: A cross sectional study. Front. Glob. Women’s Health 2023, 4, 1237064. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tucker Edmonds, B.M.; Coleman, J.; Armstrong, K.; Shea, J.A. Risk perceptions, worry, or distrust: What drives pregnant women’s decisions to accept the H1N1 vaccine? Matern. Child Health J. 2011, 15, 1203–1209. [Google Scholar] [CrossRef] [PubMed]
- Moniz, M.H.; Beigi, R.H. Maternal immunization. Clinical experiences, challenges, and opportunities in vaccine acceptance. Hum. Vaccines Immunother. 2014, 10, 2562–2570. [Google Scholar] [CrossRef]
- Fisher, B.M.; Scott, J.; Hart, J.; Winn, V.D.; Gibbs, R.S.; Lynch, A.M. Behaviors and perceptions regarding seasonal and H1N1 influenza vaccination during pregnancy. Am. J. Obstet. Gynecol. 2011, 4 (Suppl. S1), S107–S111. [Google Scholar] [CrossRef] [PubMed]
- Agricola, E.; Gesualdo, F.; Alimenti, L.; Pandolfi, E.; Carloni, E.; D’Ambrosio, A.; Russo, L.; Campagna, I.; Ferretti, B.; Tozzi, A.E. Knowledge attitude and practice toward pertussis vaccination during pregnancy among pregnant and postpartum Italian women. Hum. Vaccines Immunother. 2016, 12, 1982–1988. [Google Scholar] [CrossRef]
- Geoghegan, S.; Shuster, S.; Butler, K.M.; Feemster, K.A. Understanding Barriers and Facilitators to Maternal Immunization: A Systematic Narrative Synthesis of the Published Literature. Matern. Child Health J. 2022, 26, 2198–2209. [Google Scholar] [CrossRef]
- Kilich, E.; Dada, S.; Francis, M.R.; Tazare, J.; Chico, R.M.; Paterson, P.; J Larson, H. Factors that influence vaccination decision-making among pregnant women: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0234827. [Google Scholar] [CrossRef]
- Barber, A.; Muscoplat, M.H.; Fedorowicz, A. Coverage with Tetanus, Diphtheria, and Acellular Pertussis Vaccine and Influenza Vaccine Among Pregnant Women—Minnesota, March 2013–December 2014. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 56–59. [Google Scholar] [CrossRef]
- Anatea, M.D.; Mekonnen, T.H.; Dachew, B.A. Determinants and perceptions of the utilization of tetanus toxoid immunization among reproductive-age women in Dukem Town, Eastern Ethiopia: A community-based cross-sectional study. BMC Int. Health Hum. Rights 2018, 18, 27. [Google Scholar] [CrossRef]
- Arriola, C.S.; Suntarattiwong, P.; Dawood, F.S.; Soto, G.; Das, P.; Hunt, D.R.; Sinthuwattanawibool, C.; Kurhe, K.; Thompson, M.G.; Wesley, M.G.; et al. What do pregnant women think about influenza disease and vaccination practices in selected countries. Hum. Vaccines Immunother. 2021, 17, 2176–2184. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Ali, I.; Ekmekcioglu, C.; Kundi, M. Increasing Frequency of Antenatal Care Visits May Improve Tetanus Toxoid Vaccination Coverage in Pregnant Women in Pakistan. Hum. Vaccines Immunother. 2020, 16, 1529–1532. [Google Scholar] [CrossRef] [PubMed]
- Fleming, J.A.; Baltrons, R.; Rowley, E.; Quintanilla, I.; Crespin, E.; Ropero, A.M.; Ortiz, J.R.; Lambach, P.; Neuzil, K.M.; Stepanchack, M.; et al. Implementation of maternal influenza immunization in El Salvador: Experiences and lessons learned from a mixed-methods study. Vaccine 2018, 36, 4054–4061. [Google Scholar] [CrossRef] [PubMed]
- Yaya, S.; Kota, K.; Buh, A.; Bishwajit, G. Antenatal visits are positively associated with uptake of tetanus toxoid and intermittent preventive treatment in pregnancy in Ivory Coast. BMC Public Health 2019, 19, 1467. [Google Scholar] [CrossRef]
- Yaya, S.; Kota, K.; Buh, A.; Bishwajit, G. Prevalence and predictors of taking tetanus toxoid vaccine in pregnancy: A cross-sectional study of 8,722 women in Sierra Leone. BMC Public Health 2020, 20, 855. [Google Scholar] [CrossRef]
- Doraivelu, K.; Boulet, S.; Biswas, H.H.; Adams, J.C.; Haddad, L.B.; Jamieson, D.J. Predictors of tetanus, diphtheria, acellular pertussis and influenza vaccination during pregnancy among full-term deliveries in a medically underserved population. Vaccine 2019, 37, 6054–6059. [Google Scholar] [CrossRef]
- Merritt, T.A.; Rasmussen, S.A.; Bright, M.A.; Roussos-Ross, D.; Sims, S.M.; Gurka, M.J.; Thompson, L.A. Variation in Tdap and Influenza Vaccination Coverage Among Pregnant Women by Insurance Type—Florida, 2016–2018. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 72–76. [Google Scholar] [CrossRef]
- Koerner, J.; Forinash, A.B.; Yancey, A.M.; Brinkmeyer, J.; Dingman, S.; Miller, C.; Thompson, J.; Bergin, L.; López, J.D.; Ravin, A. Administration rates of the Tdap vaccine in obstetric patients. Ann. Pharmacother. 2018, 52, 655–661. [Google Scholar] [CrossRef]
- New, S.; Winter, K.; Boyte, R.; Harriman, K.; Gutman, A.; Christiansen, A.; Royce, S. Barriers to Receipt of Prenatal Tetanus Toxoid, Reduced Diphtheria Toxoid, and Acellular Pertussis Vaccine Among Mothers of Infants Aged <4 Months with Pertussis—California, 2016. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 1068–1071. [Google Scholar] [CrossRef]
- Regan, A.K.; Mak, D.B.; Hauck, Y.L.; Gibbs, R.; Tracey, L.; Effler, P.V. Trends in seasonal influenza vaccine uptake during pregnancy in Western Australia: Implications for midwives. Women Birth 2016, 29, 423–429. [Google Scholar] [CrossRef]
- Wales, D.P.; Khan, S.; Suresh, D.; Ata, A.; Morris, B. Factors associated with Tdap vaccination receipt during pregnancy: A cross-sectional study. Public Health 2020, 179, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Crosby, D.A.; Delau, D.; Brophy, C.; McAuliffe, F.M.; Mahony, R. Uptake of the Influenza Vaccination in Pregnancy. Ir. Med. J. 2016, 109, 449. [Google Scholar] [PubMed]
- Moschese, V.; Graziani, S.; Spadea, A.; D’Amore, M.; Mosco, R.; Ciampini, S.; Di Giorgio, N.; Arcano, S.; Ceccarelli, S.; Chianca, M.; et al. Vaccinations in children of non-European origin: The Vax4globe survey. Vaccine 2024, 42, 126466. [Google Scholar] [CrossRef]
- Giersing, B.K.; Karron, R.A.; Vekemans, J.; Kaslow, D.C.; Moorthy, V.S. Meeting report: WHO consultation on respiratory syncytial virus (RSV) vaccine development, Geneva, 25–26 April 2016. Vaccine 2019, 37, 7355–7362. [Google Scholar] [CrossRef]
- McCormack, S.; Thompson, C.; Nolan, M.; Imcha, M.; Dee, A.; Saunders, J.; Philip, R.K. Maternal awareness, acceptability and willingness towards respiratory syncytial virus (RSV) vaccination during pregnancy in Ireland. Immun. Inflamm. Dis. 2024, 12, e1257. [Google Scholar] [CrossRef]
- Dubé, E.; Gagnon, D.; Kaminsky, K.; Green, C.R.; Ouakki, M.; Bettinger, J.A.; Brousseau, N.; Castillo, E.; Crowcroft, N.S.; Driedger, S.M.; et al. Canadian Immunization Network. Vaccination Against Influenza in Pregnancy: A Survey of Canadian Maternity Care Providers. J. Obstet. Gynaecol. Can. 2019, 41, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Vishram, B.; Letley, L.; Jan Van Hoek, A.; Silverton, L.; Donovan, H.; Adams, C.; Green, D.; Edwards, A.; Yarwood, J.; Bedford, H.; et al. Vaccination in pregnancy: Attitudes of nurses, midwives and health visitors in England. Hum. Vaccines Immunother. 2018, 14, 179–188. [Google Scholar] [CrossRef]
- Frawley, J.E.; McKenzie, K.; Sinclair, L.; Cummins, A.; Wardle, J.; Hall, H. Midwives’ knowledge, attitudes and confidence in discussing maternal and childhood immunisation with parents: A national study. Vaccine 2020, 38, 366–371. [Google Scholar] [CrossRef]
- Nanduri, S.A.; Petit, S.; Smelser, C.; Apostol, M.; Alden, N.B.; Harrison, L.H.; Lynfield, R.; Vagnone, P.S.; Burzlaff, K.; Spina, N.L.; et al. Epidemiology of Invasive Early-Onset and Late-Onset Group B Streptococcal Disease in the United States, 2006 to 2015: Multistate Laboratory and Population-Based Surveillance. JAMA Pediatr. 2019, 173, 224–233. [Google Scholar] [CrossRef]
- Madhi, S.A.; Anderson, A.S.; Absalon, J.; Radley, D.; Simon, R.; Jongihlati, B.; Strehlau, R.; Van Niekerk, A.M.; Izu, A.; Naidoo, N.; et al. Potential for Maternally Administered Vaccine for Infant Group B Streptococcus. N. Engl. J. Med. 2023, 389, 215–227. [Google Scholar] [CrossRef]
- Seale, A.C.; Bianchi-Jassir, F.; Russell, N.J.; Kohli-Lynch, M.; Tann, C.J.; Hall, J.; Madrid, L.; Blencowe, H.; Cousens, S.; Baker, C.J.; et al. Estimates of the Burden of Group B Streptococcal Disease Worldwide for Pregnant Women, Stillbirths, and Children. Clin. Infect. Dis. 2017, 65 (Suppl. S2), S200–S219. [Google Scholar] [CrossRef] [PubMed]
- Schleiss, M.R.; Permar, S.R.; Plotkin, S.A. Progress toward Development of a Vaccine against Congenital Cytomegalovirus Infection. Clin. Vaccine Immunol. 2017, 24, e00268-17. [Google Scholar] [CrossRef] [PubMed]
- Gamain, B.; Chêne, A.; Viebig, N.K.; Ndam, N.T.; Nielsen, M.A. Progress and Insights Toward an Effective Placental Malaria Vaccine. Front. Immunol. 2021, 12, 634508. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santilli, V.; Sgrulletti, M.; Costagliola, G.; Beni, A.; Mastrototaro, M.F.; Montin, D.; Rizzo, C.; Martire, B.; Miraglia del Giudice, M.; Moschese, V., on behalf of the Italian Society of Pediatric Allergy and Immunology (SIAIP) Vaccine Committee. Maternal Immunization: Current Evidence, Progress, and Challenges. Vaccines 2025, 13, 450. https://doi.org/10.3390/vaccines13050450
Santilli V, Sgrulletti M, Costagliola G, Beni A, Mastrototaro MF, Montin D, Rizzo C, Martire B, Miraglia del Giudice M, Moschese V on behalf of the Italian Society of Pediatric Allergy and Immunology (SIAIP) Vaccine Committee. Maternal Immunization: Current Evidence, Progress, and Challenges. Vaccines. 2025; 13(5):450. https://doi.org/10.3390/vaccines13050450
Chicago/Turabian StyleSantilli, Veronica, Mayla Sgrulletti, Giorgio Costagliola, Alessandra Beni, Maria Felicia Mastrototaro, Davide Montin, Caterina Rizzo, Baldassarre Martire, Michele Miraglia del Giudice, and Viviana Moschese on behalf of the Italian Society of Pediatric Allergy and Immunology (SIAIP) Vaccine Committee. 2025. "Maternal Immunization: Current Evidence, Progress, and Challenges" Vaccines 13, no. 5: 450. https://doi.org/10.3390/vaccines13050450
APA StyleSantilli, V., Sgrulletti, M., Costagliola, G., Beni, A., Mastrototaro, M. F., Montin, D., Rizzo, C., Martire, B., Miraglia del Giudice, M., & Moschese, V., on behalf of the Italian Society of Pediatric Allergy and Immunology (SIAIP) Vaccine Committee. (2025). Maternal Immunization: Current Evidence, Progress, and Challenges. Vaccines, 13(5), 450. https://doi.org/10.3390/vaccines13050450





