A Cyclic-di-AMP Adjuvanted CPAF Protein Vaccine Is Immunogenic in Swine, but It Fails to Reduce Genital Chlamydia trachomatis Burden
Abstract
1. Introduction
2. Materials and Methods
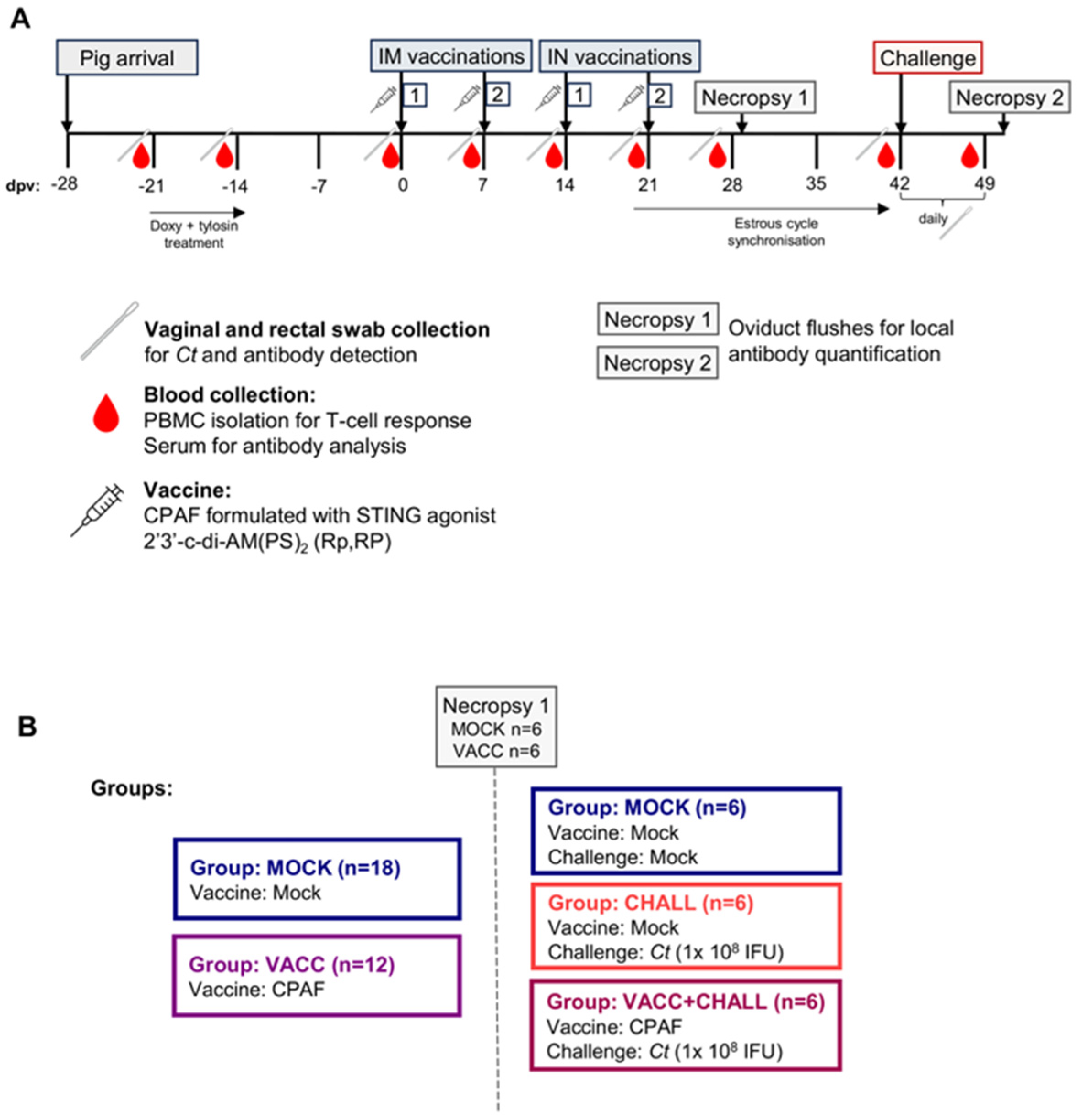
2.1. Animal Trial
2.2. Vaccine Antigen Production and Formulation
2.3. Cell Isolation, Swabs and Sera
2.4. IFN-γ and IL-17A ELISpots
2.5. In Vitro Stimulation and Flow Cytometry Staining
2.6. Anti-CPAF IgG and IgA ELISAs
2.7. Detection of Chlamydiaceae via qPCR
2.8. Statistical Analysis
3. Results
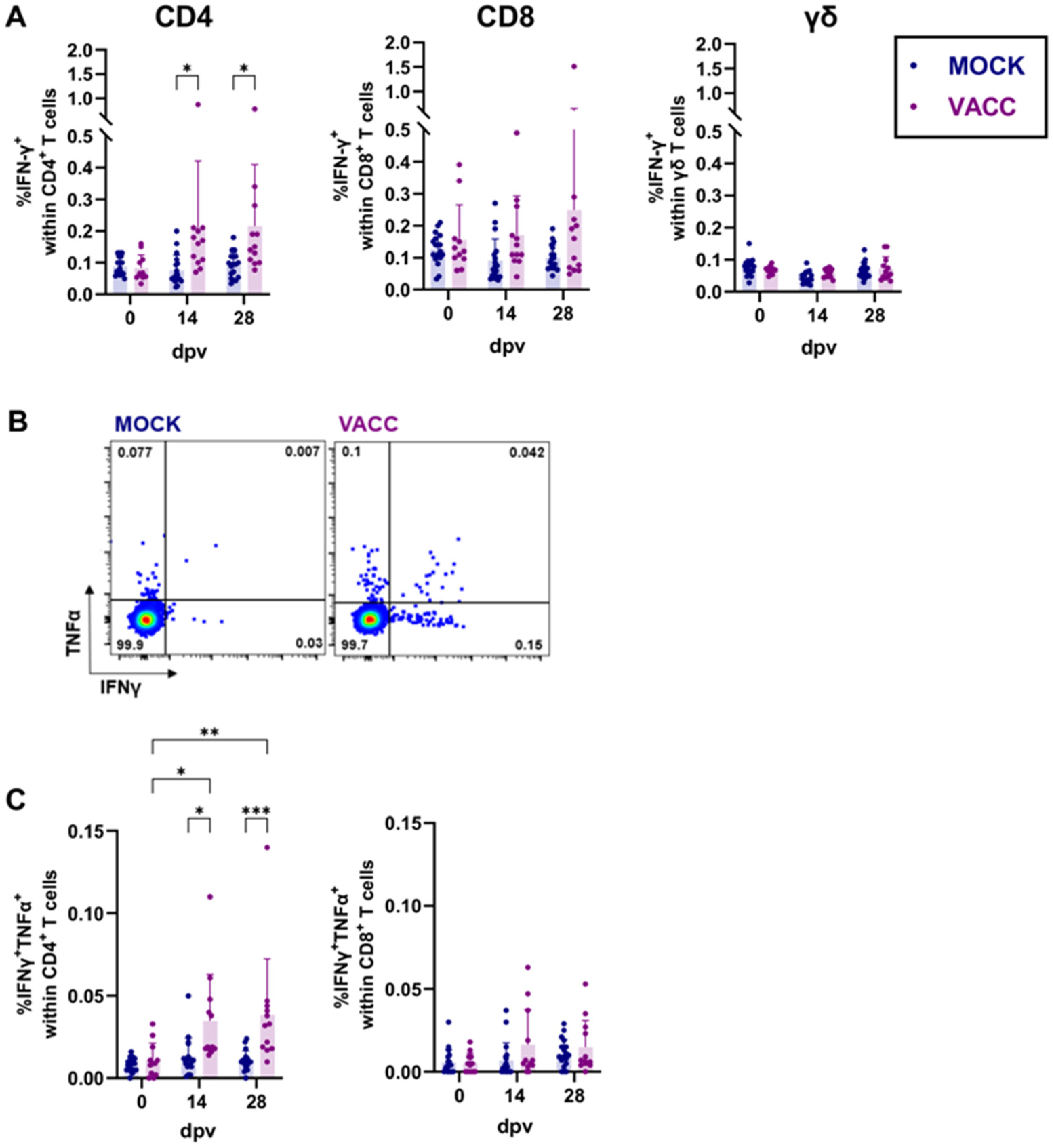
3.1. Vaccination with CPAF/c-di-AMP Elicits a Systemic IFN-γ and IL-17A Response

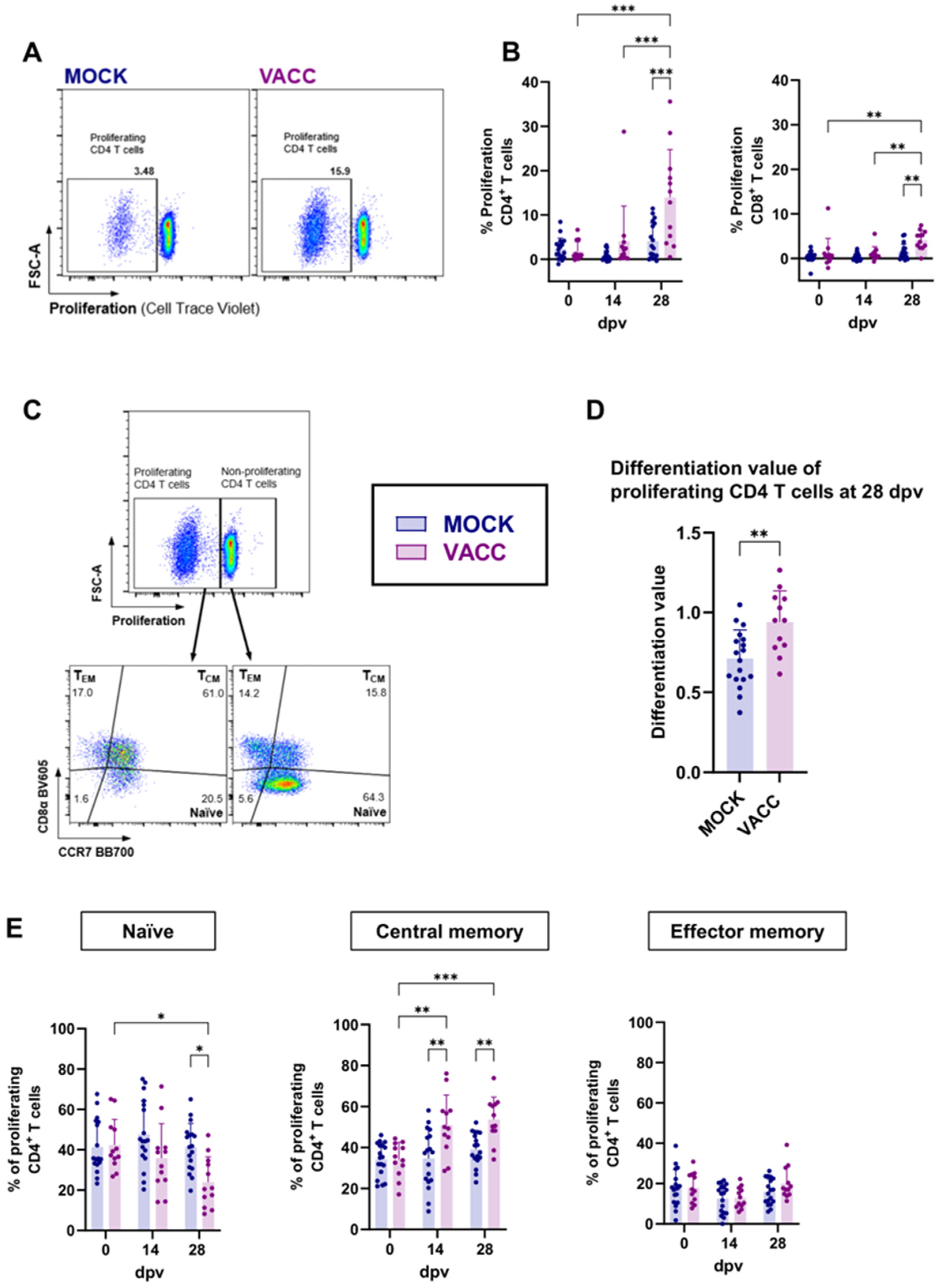
3.2. CPAF/c-di-AMP Induces Cytokine Production, Proliferation, and Differentiation in CD4 T Cells
3.3. The CPAF/c-di-AMP Vaccine Induced Systemic and Local IgG Responses
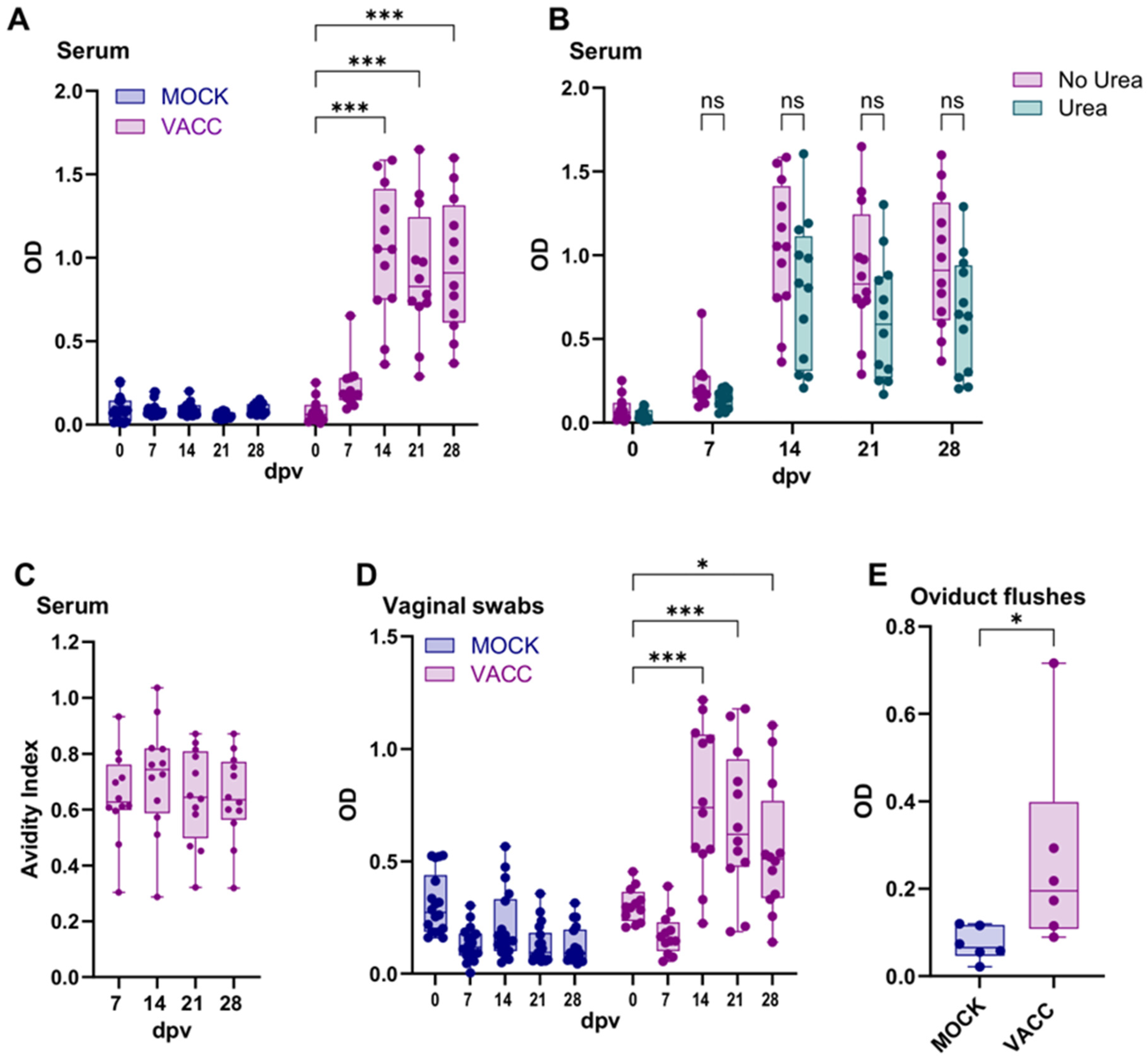
3.4. CPAF/c-di-AMP-Vaccinated Pigs Are Not Protected from Ct Challenge but Show a Boosted Systemic CPAF-Specific IFN-γ and Local CPAF-Specific IgG Response

4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stelzner, K.; Vollmuth, N.; Rudel, T. Intracellular Lifestyle of Chlamydia Trachomatis and Host–Pathogen Interactions. Nat. Rev. Microbiol. 2023, 21, 448–462. [Google Scholar] [CrossRef] [PubMed]
- den Heijer, C.D.J.; Hoebe, C.J.P.A.; Driessen, J.H.M.; Wolffs, P.; van den Broek, I.V.F.; Hoenderboom, B.M.; Williams, R.; de Vries, F.; Dukers-Muijrers, N.H.T.M. Chlamydia Trachomatis and the Risk of Pelvic Inflammatory Disease, Ectopic Pregnancy, and Female Infertility: A Retrospective Cohort Study Among Primary Care Patients. Clin. Infect. Dis. 2019, 69, 1517–1525. [Google Scholar] [CrossRef]
- Hillier, S.L.; Bernstein, K.T.; Aral, S. A Review of the Challenges and Complexities in the Diagnosis, Etiology, Epidemiology, and Pathogenesis of Pelvic Inflammatory Disease. J. Infect. Dis. 2021, 224, S23–S28. [Google Scholar] [CrossRef]
- Kiekens, C.; Morré, S.A.; Vanrompay, D. Advances in Chlamydia Trachomatis Vaccination: Unveiling the Potential of Major Outer Membrane Protein Derivative Constructs. Microorganisms 2024, 12, 1196. [Google Scholar] [CrossRef]
- Abraham, S.; Juel, H.B.; Bang, P.; Cheeseman, H.M.; Dohn, R.B.; Cole, T.; Kristiansen, M.P.; Korsholm, K.S.; Lewis, D.; Olsen, A.W.; et al. Safety and Immunogenicity of the Chlamydia Vaccine Candidate CTH522 Adjuvanted with CAF01 Liposomes or Aluminium Hydroxide: A First-in-Human, Randomised, Double-Blind, Placebo-Controlled, Phase 1 Trial. Lancet Infect. Dis. 2019, 19, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Pollock, K.M.; Borges, Á.H.; Cheeseman, H.M.; Rosenkrands, I.; Schmidt, K.L.; Søndergaard, R.E.; Day, S.; Evans, A.; McFarlane, L.R.; Joypooranachandran, J.; et al. An Investigation of Trachoma Vaccine Regimens by the Chlamydia Vaccine CTH522 Administered with Cationic Liposomes in Healthy Adults (CHLM-02): A Phase 1, Double-Blind Trial. Lancet Infect. Dis. 2024, 24, 829–844. [Google Scholar] [CrossRef] [PubMed]
- Käser, T. Swine as Biomedical Animal Model for T-Cell Research—Success and Potential for Transmittable and Non-Transmittable Human Diseases. Mol. Immunol. 2021, 135, 95–115. [Google Scholar] [CrossRef]
- Pabst, R. The Pig as a Model for Immunology Research. Cell Tissue Res. 2020, 380, 287–304. [Google Scholar] [CrossRef]
- Lunney, J.K.; Van Goor, A.; Walker, K.E.; Hailstock, T.; Franklin, J.; Dai, C. Importance of the Pig as a Human Biomedical Model. Sci. Transl. Med. 2021, 13, eabd5758. [Google Scholar] [CrossRef]
- Schautteet, K.; Stuyven, E.; Beeckman, D.S.A.; Van Acker, S.; Carlon, M.; Chiers, K.; Cox, E.; Vanrompay, D. Protection of Pigs against Chlamydia Trachomatis Challenge by Administration of a MOMP-Based DNA Vaccine in the Vaginal Mucosa. Vaccine 2011, 29, 1399–1407. [Google Scholar] [CrossRef]
- Erneholm, K.; Lorenzen, E.; Bøje, S.; Olsen, A.W.; Jungersen, G.; Jensen, H.E.; Cassidy, J.P.; Andersen, P.; Agerholm, J.S.; Follmann, F. Genital Infiltrations of CD4+ and CD8+ T Lymphocytes, IgA+ and IgG+ Plasma Cells and Intra-Mucosal Lymphoid Follicles Associate With Protection Against Genital Chlamydiatrachomatis Infection in Minipigs Intramuscularly Immunized With UV-Inactivated Bacteria Adjuvanted With CAF01. Front. Microbiol. 2019, 10, 197. [Google Scholar] [CrossRef]
- Bøje, S.; Olsen, A.W.; Erneholm, K.; Agerholm, J.S.; Jungersen, G.; Andersen, P.; Follmann, F. A Multi-Subunit Chlamydia Vaccine Inducing Neutralizing Antibodies and Strong IFN-Γ+ CMI Responses Protects against a Genital Infection in Minipigs. Immunol. Cell Biol. 2016, 94, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Proctor, J.; Stadler, M.; Cortes, L.M.; Brodsky, D.; Poisson, L.; Gerdts, V.; Smirnov, A.I.; Smirnova, T.I.; Barua, S.; Leahy, D.; et al. A TriAdj-Adjuvanted Chlamydia Trachomatis CPAF Protein Vaccine Is Highly Immunogenic in Pigs. Vaccines 2024, 12, 423. [Google Scholar] [CrossRef] [PubMed]
- Dimond, Z.E.; Hefty, P.S. Comprehensive Genome Analysis and Comparisons of the Swine Pathogen, Chlamydia Suis Reveals Unique ORFs and Candidate Host-Specificity Factors. Pathog. Dis. 2020, 79, ftaa035. [Google Scholar] [CrossRef]
- Häcker, G. Chlamydia in Pigs: Intriguing Bacteria Associated with Sub-Clinical Carriage and Clinical Disease, and with Zoonotic Potential. Front. Cell Dev. Biol. 2024, 12, 1301892. [Google Scholar] [CrossRef] [PubMed]
- Schautteet, K.; Vanrompay, D. Chlamydiaceae Infections in Pig. Vet. Res. 2011, 42, 29. [Google Scholar] [CrossRef]
- Käser, T.; Pasternak, J.A.; Delgado-Ortega, M.; Hamonic, G.; Lai, K.; Erickson, J.; Walker, S.; Dillon, J.R.; Gerdts, V.; Meurens, F. Chlamydia Suis and Chlamydia Trachomatis Induce Multifunctional CD4 T Cells in Pigs. Vaccine 2017, 35, 91–100. [Google Scholar] [CrossRef]
- Amaral, A.F.; Rahman, K.S.; Kick, A.R.; Cortes, L.M.; Robertson, J.; Kaltenboeck, B.; Gerdts, V.; O’Connell, C.M.; Poston, T.B.; Zheng, X.; et al. Mucosal Vaccination with UV-Inactivated Chlamydia Suis in Pre-Exposed Outbred Pigs Decreases Pathogen Load and Induces CD4 T-Cell Maturation into IFN-Γ+ Effector Memory Cells. Vaccines 2020, 8, 353. [Google Scholar] [CrossRef]
- Johansson, M.; Schön, K.; Ward, M.; Lycke, N. Genital Tract Infection with Chlamydia Trachomatis Fails to Induce Protective Immunity in Gamma Interferon Receptor-Deficient Mice despite a Strong Local Immunoglobulin A Response. Infect. Immun. 1997, 65, 1032–1044. [Google Scholar] [CrossRef]
- Helble, J.D.; Gonzalez, R.J.; von Andrian, U.H.; Starnbach, M.N. Gamma Interferon Is Required for Chlamydia Clearance but Is Dispensable for T Cell Homing to the Genital Tract. mBio 2020, 11, e00191-20. [Google Scholar] [CrossRef]
- Bakshi, R.K.; Gupta, K.; Jordan, S.J.; Chi, X.; Lensing, S.Y.; Press, C.G.; Geisler, W.M. An Adaptive Chlamydia Trachomatis-Specific IFN-γ-Producing CD4+ T Cell Response Is Associated With Protection Against Chlamydia Reinfection in Women. Front. Immunol. 2018, 9, 1981. [Google Scholar] [CrossRef]
- Yu, H.; Geisler, W.M.; Dai, C.; Gupta, K.; Cutter, G.; Brunham, R.C. Antibody Responses to Chlamydia Trachomatis Vaccine Candidate Antigens in Chlamydia-Infected Women and Correlation with Antibody-Mediated Phagocytosis of Elementary Bodies. Front. Cell. Infect. Microbiol. 2024, 14, 1342621. [Google Scholar] [CrossRef]
- Darville, T.; Albritton, H.L.; Zhong, W.; Dong, L.; O’Connell, C.M.; Poston, T.B.; Quayle, A.J.; Goonetilleke, N.; Wiesenfeld, H.C.; Hillier, S.L.; et al. Anti-Chlamydia IgG and IgA Are Insufficient to Prevent Endometrial Chlamydia Infection in Women, and Increased Anti-Chlamydia IgG Is Associated with Enhanced Risk for Incident Infection. Am. J. Reprod. Immunol. 2019, 81, e13103. [Google Scholar] [CrossRef] [PubMed]
- El Hakim, E.A.; Gordon, U.D.; Akande, V.A. The Relationship between Serum Chlamydia Antibody Levels and Severity of Disease in Infertile Women with Tubal Damage. Arch. Gynecol. Obstet. 2010, 281, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.G.; Morrison, R.P. Resolution of Secondary Chlamydia Trachomatis Genital Tract Infection in Immune Mice with Depletion of Both CD4+ and CD8+ T Cells. Infect. Immun. 2001, 69, 2643–2649. [Google Scholar] [CrossRef]
- Morrison, S.G.; Morrison, R.P. A Predominant Role for Antibody in Acquired Immunity to Chlamydial Genital Tract Reinfection1. J. Immunol. 2005, 175, 7536–7542. [Google Scholar] [CrossRef]
- Li, Y.; Warren, J.A.; Poston, T.B.; Clutton, G.; Shaw, F.R.; Conrad, S.Z.; Xu, Y.; Zheng, X.; Yount, K.S.; O’Connell, C.M.; et al. Low-Frequency, Sustained CD4 T-Cell Responses Chlamydia Trachomatis in Women: Predominant Targeting of Chlamydial Proteaselike Activity Factor (CPAF). J. Infect. Dis. 2024, 231, e385–e395. [Google Scholar] [CrossRef]
- Bastidas, R.J.; Elwell, C.A.; Engel, J.N.; Valdivia, R.H. Chlamydial Intracellular Survival Strategies. Cold Spring Harb. Perspect. Med. 2013, 3, a010256. [Google Scholar] [CrossRef] [PubMed]
- Rajeeve, K.; Das, S.; Prusty, B.K.; Rudel, T. Chlamydia Trachomatis Paralyses Neutrophils to Evade the Host Innate Immune Response. Nat. Microbiol. 2018, 3, 824–835. [Google Scholar] [CrossRef]
- Patton, M.J.; McCorrister, S.; Grant, C.; Westmacott, G.; Fariss, R.; Hu, P.; Zhao, K.; Blake, M.; Whitmire, B.; Yang, C.; et al. Chlamydial Protease-Like Activity Factor and Type III Secreted Effectors Cooperate in Inhibition of P65 Nuclear Translocation. mBio 2016, 7, e01427-16. [Google Scholar] [CrossRef]
- Schott, B.H.; Antonia, A.L.; Wang, L.; Pittman, K.J.; Sixt, B.S.; Barnes, A.B.; Valdivia, R.H.; Ko, D.C. Modeling of Variables in Cellular Infection Reveals CXCL10 Levels Are Regulated by Human Genetic Variation and the Chlamydia-Encoded CPAF Protease. Sci. Rep. 2020, 10, 18269. [Google Scholar] [CrossRef]
- Van Herck, S.; Feng, B.; Tang, L. Delivery of STING Agonists for Adjuvanting Subunit Vaccines. Adv. Drug Deliv. Rev. 2021, 179, 114020. [Google Scholar] [CrossRef] [PubMed]
- Volckmar, J.; Knop, L.; Stegemann-Koniszewski, S.; Schulze, K.; Ebensen, T.; Guzmán, C.A.; Bruder, D. The STING Activator C-Di-AMP Exerts Superior Adjuvant Properties than the Formulation Poly(I:C)/CpG after Subcutaneous Vaccination with Soluble Protein Antigen or DEC-205-Mediated Antigen Targeting to Dendritic Cells. Vaccine 2019, 37, 4963–4974. [Google Scholar] [CrossRef]
- Van Dis, E.; Sogi, K.M.; Rae, C.S.; Sivick, K.E.; Surh, N.H.; Leong, M.L.; Kanne, D.B.; Metchette, K.; Leong, J.J.; Bruml, J.R.; et al. STING-Activating Adjuvants Elicit a Th17 Immune Response and Protect against Mycobacterium Tuberculosis Infection. Cell Rep. 2018, 23, 1435–1447. [Google Scholar] [CrossRef] [PubMed]
- Poston, T.B.; Girardi, J.; Kim, M.; Zwarycz, P.; Polson, A.G.; Yount, K.S.; Hanlan, C.; Jaras Salas, I.; Lammert, S.M.; Arroyo, D.; et al. Intranasal Immunization with CPAF Combined with ADU-S100 Induces an Effector CD4 T Cell Response and Reduces Bacterial Burden Following Intravaginal Infection with Chlamydia Muridarum. Vaccine 2025, 43, 126526. [Google Scholar] [CrossRef] [PubMed]
- Pantchev, A.; Sting, R.; Bauerfeind, R.; Tyczka, J.; Sachse, K. Detection of All Chlamydophila and Chlamydia Spp. of Veterinary Interest Using Species-Specific Real-Time PCR Assays. Comp. Immunol. Microbiol. Infect. Dis. 2010, 33, 473–484. [Google Scholar] [CrossRef]
- Blumer, S.; Greub, G.; Waldvogel, A.; Hässig, M.; Thoma, R.; Tschuor, A.; Pospischil, A.; Borel, N. Waddlia, Parachlamydia and Chlamydiaceae in Bovine Abortion. Vet. Microbiol. 2011, 152, 385–393. [Google Scholar] [CrossRef]
- Tian, X.; Ai, J.; Tian, X.; Wei, X. cGAS-STING Pathway Agonists Are Promising Vaccine Adjuvants. Med. Res. Rev. 2024, 44, 1768–1799. [Google Scholar] [CrossRef]
- Garg, R.; Babiuk, L.; van Drunen Littel-van den Hurk, S.; Gerdts, V. A Novel Combination Adjuvant Platform for Human and Animal Vaccines. Vaccine 2017, 35, 4486–4489. [Google Scholar] [CrossRef]
- Garg, R.; Brownlie, R.; Latimer, L.; Gerdts, V.; Potter, A.; van Drunen Littel-van den Hurk, S. A Chimeric Glycoprotein Formulated with a Combination Adjuvant Induces Protective Immunity against Both Human Respiratory Syncytial Virus and Parainfluenza Virus Type 3. Antivir. Res. 2018, 158, 78–87. [Google Scholar] [CrossRef]
- Lu, Y.; Landreth, S.; Liu, G.; Brownlie, R.; Gaba, A.; Littel-van den Hurk, S.v.D.; Gerdts, V.; Zhou, Y. Innate Immunemodulator Containing Adjuvant Formulated HA Based Vaccine Protects Mice from Lethal Infection of Highly Pathogenic Avian Influenza H5N1 Virus. Vaccine 2020, 38, 2387–2395. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Ning, J.; Xu, X.; Zhou, X. The Role of Bacterial Cyclic Di-Adenosine Monophosphate in the Host Immune Response. Front. Microbiol. 2022, 13, 958133. [Google Scholar] [CrossRef]
- Snider, M.; Garg, R.; Brownlie, R.; van den Hurk, J.V.; van Drunen Littel-van den Hurk, S. The Bovine Viral Diarrhea Virus E2 Protein Formulated with a Novel Adjuvant Induces Strong, Balanced Immune Responses and Provides Protection from Viral Challenge in Cattle. Vaccine 2014, 32, 6758–6764. [Google Scholar] [CrossRef] [PubMed]
- Ebensen, T.; Delandre, S.; Prochnow, B.; Guzmán, C.A.; Schulze, K. The Combination Vaccine Adjuvant System Alum/c-Di-AMP Results in Quantitative and Qualitative Enhanced Immune Responses Post Immunization. Front. Cell. Infect. Microbiol. 2019, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Madhun, A.S.; Haaheim, L.R.; Nøstbakken, J.K.; Ebensen, T.; Chichester, J.; Yusibov, V.; Guzman, C.A.; Cox, R.J. Intranasal C-Di-GMP-Adjuvanted Plant-Derived H5 Influenza Vaccine Induces Multifunctional Th1 CD4+ Cells and Strong Mucosal and Systemic Antibody Responses in Mice. Vaccine 2011, 29, 4973–4982. [Google Scholar] [CrossRef]
- Wang, J.; Li, P.; Wu, M.X. Natural STING Agonist as an “Ideal” Adjuvant for Cutaneous Vaccination. J. Investig. Dermatol. 2016, 136, 2183–2191. [Google Scholar] [CrossRef]
- Bartsch, T.; Becker, M.; Rolf, J.; Rosenthal, K.; Lütz, S. Biotechnological Production of Cyclic Dinucleotides—Challenges and Opportunities. Biotechnol. Bioeng. 2022, 119, 677–684. [Google Scholar] [CrossRef]
- Halbritter, A.-L.J.; Gärtner, Y.V.; Nabiev, J.; Hernichel, F.; Ganazzoli, G.; Özdemir, D.; Pappa, A.; Veth, S.; Stazzoni, S.; Müller, M.; et al. A Phosphotriester-Masked Dideoxy-cGAMP Derivative as a Cell-Permeable STING Agonist. Angew. Chem. Int. Ed. 2024, 63, e202416353. [Google Scholar] [CrossRef]
- Yan, H.; Chen, W. The Promise and Challenges of Cyclic Dinucleotides as Molecular Adjuvants for Vaccine Development. Vaccines 2021, 9, 917. [Google Scholar] [CrossRef]
- Ning, H.; Zhang, W.; Kang, J.; Ding, T.; Liang, X.; Lu, Y.; Guo, C.; Sun, W.; Wang, H.; Bai, Y.; et al. Subunit Vaccine ESAT-6:C-Di-AMP Delivered by Intranasal Route Elicits Immune Responses and Protects Against Mycobacterium Tuberculosis Infection. Front. Cell. Infect. Microbiol. 2021, 11, 647220. [Google Scholar] [CrossRef]
- Ebensen, T.; Libanova, R.; Schulze, K.; Yevsa, T.; Morr, M.; Guzmán, C.A. Bis-(3′,5′)-Cyclic Dimeric Adenosine Monophosphate: Strong Th1/Th2/Th17 Promoting Mucosal Adjuvant. Vaccine 2011, 29, 5210–5220. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.V.; Ebensen, T.; Schulze, K.; Cargnelutti, D.; Blazejewska, P.; Scodeller, E.A.; Guzmán, C.A. Intranasal Delivery of Influenza rNP Adjuvanted with C-Di-AMP Induces Strong Humoral and Cellular Immune Responses and Provides Protection against Virus Challenge. PLoS ONE 2014, 9, e104824. [Google Scholar] [CrossRef] [PubMed]
- Longet, S.; Paul, S. Pivotal Role of Tissue-Resident Memory Lymphocytes in the Control of Mucosal Infections: Can Mucosal Vaccination Induce Protective Tissue-Resident Memory T and B Cells? Front. Immunol. 2023, 14, 1216402. [Google Scholar] [CrossRef] [PubMed]
- Stary, G.; Olive, A.; Radovic-Moreno, A.F.; Gondek, D.; Alvarez, D.; Basto, P.A.; Perro, M.; Vrbanac, V.D.; Tager, A.M.; Shi, J.; et al. A Mucosal Vaccine against Chlamydia Trachomatis Generates Two Waves of Protective Memory T Cells. Science 2015, 348, aaa8205. [Google Scholar] [CrossRef]
- Pepper, M.; Jenkins, M.K. Origins of CD4+ Effector and Central Memory T Cells. Nat. Immunol. 2011, 12, 467–471. [Google Scholar] [CrossRef]
- Morrison, S.G.; Su, H.; Caldwell, H.D.; Morrison, R.P. Immunity to Murine Chlamydia Trachomatis Genital Tract Reinfection Involves B Cells and CD4+ T Cells but Not CD8+ T Cells. Infect. Immun. 2000, 68, 6979–6987. [Google Scholar] [CrossRef]
- Olsen, A.W.; Follmann, F.; Erneholm, K.; Rosenkrands, I.; Andersen, P. Protection Against Chlamydia Trachomatis Infection and Upper Genital Tract Pathological Changes by Vaccine-Promoted Neutralizing Antibodies Directed to the VD4 of the Major Outer Membrane Protein. J. Infect. Dis. 2015, 212, 978–989. [Google Scholar] [CrossRef]
- Moore, T.; Ananaba, G.A.; Bolier, J.; Bowers, S.; Belay, T.; Eko, F.O.; Igietseme, J.U. Fc Receptor Regulation of Protective Immunity against Chlamydia Trachomatis. Immunology 2002, 105, 213–221. [Google Scholar] [CrossRef]
- Poston, T.B.; Girardi, J.; Polson, A.G.; Bhardwaj, A.; Yount, K.S.; Jaras Salas, I.; Trim, L.K.; Li, Y.; O’Connell, C.M.; Leahy, D.; et al. Viral-Vectored Boosting of OmcB- or CPAF-Specific T-Cell Responses Fail to Enhance Protection from Chlamydia Muridarum in Infection-Immune Mice and Elicits a Non-Protective CD8-Dominant Response in Naïve Mice. Mucosal Immunol. 2024, 17, 1005–1018. [Google Scholar] [CrossRef]
- Murthy, A.K.; Chaganty, B.K.R.; Li, W.; Guentzel, M.N.; Chambers, J.P.; Seshu, J.; Zhong, G.; Arulanandam, B.P. A Limited Role for Antibody in Protective Immunity Induced by rCPAF and CpG Vaccination Against Primary Genital Chlamydia Muridarum Challenge. FEMS Immunol. Med. Microbiol. 2009, 55, 271–279. [Google Scholar] [CrossRef]
- Sharma, J.; Dong, F.; Pirbhai, M.; Zhong, G. Inhibition of Proteolytic Activity of a Chlamydial Proteasome/Protease-Like Activity Factor by Antibodies from Humans Infected with Chlamydia Trachomatis. Infect. Immun. 2005, 73, 4414–4419. [Google Scholar] [CrossRef]
- Liu, C.; Hufnagel, K.; O’Connell, C.M.; Goonetilleke, N.; Mokashi, N.; Waterboer, T.; Tollison, T.S.; Peng, X.; Wiesenfeld, H.C.; Hillier, S.L.; et al. Reduced Endometrial Ascension and Enhanced Reinfection Associated With Immunoglobulin G Antibodies to Specific Chlamydia Trachomatis Proteins in Women at Risk for Chlamydia. J. Infect. Dis. 2022, 225, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Tang, L.; Zhou, Z.; Zhong, G. Neutralizing Antichlamydial Activity of Complement by Chlamydia-Secreted Protease CPAF. Microbes Infect. 2016, 18, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Chen, J.; Zhou, Z.; Yu, P.; Yang, Z.; Zhong, G. Chlamydia-Secreted Protease CPAF Degrades Host Antimicrobial Peptides. Microbes Infect. 2015, 17, 402–408. [Google Scholar] [CrossRef]
- Pedersen, G.K.; Wørzner, K.; Andersen, P.; Christensen, D. Vaccine Adjuvants Differentially Affect Kinetics of Antibody and Germinal Center Responses. Front. Immunol. 2020, 11, 579761. [Google Scholar] [CrossRef] [PubMed]
- Lorenzen, E.; Follmann, F.; Secher, J.O.; Goericke-Pesch, S.; Hansen, M.S.; Zakariassen, H.; Olsen, A.W.; Andersen, P.; Jungersen, G.; Agerholm, J.S. Intrauterine Inoculation of Minipigs with Chlamydia Trachomatis during Diestrus Establishes a Longer Lasting Infection Compared to Vaginal Inoculation during Estrus. Microbes Infect. 2017, 19, 334–342. [Google Scholar] [CrossRef][Green Version]
- Dirks, J.A.M.C.; van Liere, G.A.F.S.; Bogers, S.; Dukers-Muijrers, N.H.T.M.; Wolffs, P.F.G.; Hoebe, C.J.P.A. Natural Course of Chlamydia trachomatis Bacterial Load in the Time Interval between Screening and Treatment in Anogenital Samples. PLoS ONE 2015, 10, e0145693. [Google Scholar] [CrossRef][Green Version]
- Al-Mously, N.; Cross, N.A.; Eley, A.; Pacey, A.A. Real-Time Polymerase Chain Reaction Shows That Density Centrifugation Does Not Always Remove Chlamydia Trachomatis from Human Semen. Fertil. Steril. 2009, 92, 1606–1615. [Google Scholar] [CrossRef]
| Antigen | Clone | Isotype | Fluorochrome | Labeling Strategy | Primary Antibody Source | Secondary Antibody Source |
|---|---|---|---|---|---|---|
| Anti-CPAF T cell response: Cytokine production | ||||||
| CD3 | PPT3 | mIgG1 | FITC | Directly conjugated | Southern Biotech (Birmingham, AL, USA) | - |
| CD4 | 74-12-4 | mIgG2b | BV421 | Secondary antibody | In house | Jackson ImmunoResearch (West Grove, PA, USA) |
| CD8α | 76-2-11 | mIgG2a | BUV395 | Biotin-Streptavidin | Thermo Fisher | Biolegend |
| TCRγδ | PGBL22A | mIgG1 | AF647 * | Directly conjugated | Kingfisher Biotech (Saint Paul, MN, USA) | Thermo Fisher |
| Live/Dead | - | - | eFlour780 | - | Invitrogen (Waltham, MA, USA) | - |
| TNFα | Mab11 | mIgG2a | BV605 | Directly conjugated | Biolegend (San Diego, CA, USA) | - |
| IFNγ | P2G10 | mIgG1 | PE | Directly conjugated | BD Biosciences | - |
| Anti-CPAF T cell response: Proliferation and differentiation | ||||||
| CD3 | PPT3 | mIgG1 | FITC | Directly conjugated | Southern Biotech | - |
| CD4 | 74-12-4 | mIgG2b | PE | Secondary antibody | In house | Southern Biotech |
| CD8α | 76-2-11 | mIgG2a | BV605 | Biotin-Streptavidin | Thermo Fisher | Biolegend |
| TCRγδ | PGBL22A | mIgG1 | AF647 * | Directly conjugated | Kingfisher | Thermo Fisher |
| CCR7 | 3D12 | rIgG2a | BB700 | Directly conjugated | BD Biosciences | - |
| Proliferation | - | - | Cell Trace Violet | - | Thermo Fisher | - |
| Live/Dead | - | - | eFlour780 | - | Invitrogen | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bettin, L.; Stadler, M.; Unterweger, C.; Dippel, M.; Harris, J.M.; Buzanich-Ladinig, A.; Poston, T.B.; Darville, T.; Käser, T. A Cyclic-di-AMP Adjuvanted CPAF Protein Vaccine Is Immunogenic in Swine, but It Fails to Reduce Genital Chlamydia trachomatis Burden. Vaccines 2025, 13, 468. https://doi.org/10.3390/vaccines13050468
Bettin L, Stadler M, Unterweger C, Dippel M, Harris JM, Buzanich-Ladinig A, Poston TB, Darville T, Käser T. A Cyclic-di-AMP Adjuvanted CPAF Protein Vaccine Is Immunogenic in Swine, but It Fails to Reduce Genital Chlamydia trachomatis Burden. Vaccines. 2025; 13(5):468. https://doi.org/10.3390/vaccines13050468
Chicago/Turabian StyleBettin, Leonie, Maria Stadler, Christine Unterweger, Maximiliane Dippel, Jonathan M. Harris, Andrea Buzanich-Ladinig, Taylor B. Poston, Toni Darville, and Tobias Käser. 2025. "A Cyclic-di-AMP Adjuvanted CPAF Protein Vaccine Is Immunogenic in Swine, but It Fails to Reduce Genital Chlamydia trachomatis Burden" Vaccines 13, no. 5: 468. https://doi.org/10.3390/vaccines13050468
APA StyleBettin, L., Stadler, M., Unterweger, C., Dippel, M., Harris, J. M., Buzanich-Ladinig, A., Poston, T. B., Darville, T., & Käser, T. (2025). A Cyclic-di-AMP Adjuvanted CPAF Protein Vaccine Is Immunogenic in Swine, but It Fails to Reduce Genital Chlamydia trachomatis Burden. Vaccines, 13(5), 468. https://doi.org/10.3390/vaccines13050468







