Clinical Translation Challenges and Strategies for Tumour Vaccines Considering Multiple Delivery Routes
Abstract
1. Introduction
2. Traditional Delivery of Oncology Vaccines
2.1. Nasal Spray for Vaccination
2.2. Inhalation Vaccination
2.3. Oral Vaccination
2.4. Vaccination by Routine Injection
2.4.1. Intramuscular Injection
2.4.2. Hypodermic Injection
2.4.3. Intracutaneous Injection
2.4.4. Intravenous Injection
2.4.5. Intratumoural Injection
3. Novel Delivery Modes for Oncology Vaccines
3.1. Novel Needle-Free Thermal Release-Driven Jet Injector
3.2. Tumour Vaccine Tattoo
3.3. Nanogel Delivery of Vaccines
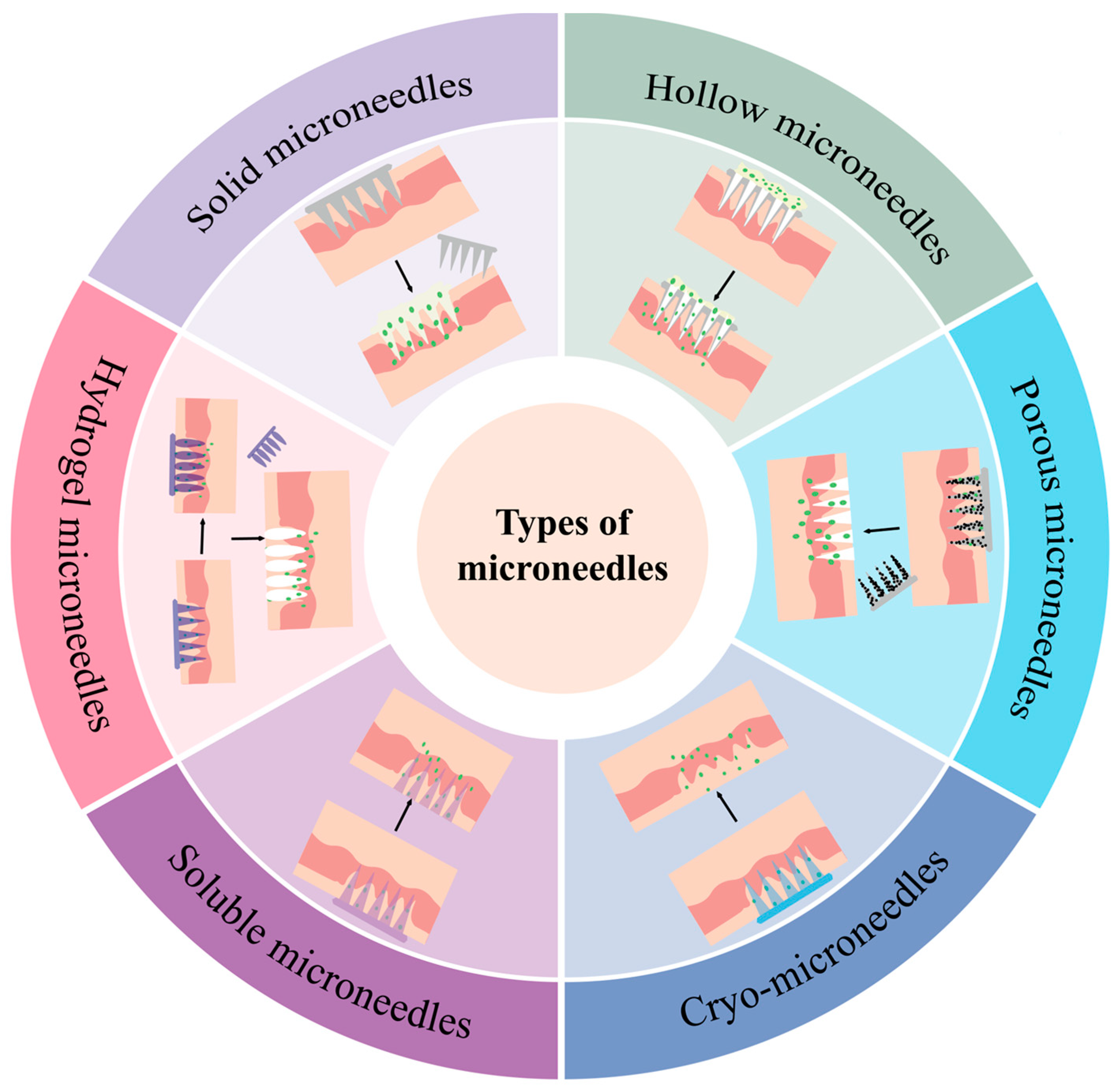
3.4. Microneedle Load Tumour Vaccine
4. Challenges in the Clinical Translation of Oncology Vaccine Delivery Systems
5. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Cao, Z.; Prettner, K.; Kuhn, M.; Yang, J.; Jiao, L.; Wang, Z.; Li, W.; Geldsetzer, P.; Bärnighausen, T.; et al. Estimates and Projections of the Global Economic Cost of 29 Cancers in 204 Countries and Territories from 2020 to 2050. JAMA Oncol. 2023, 9, 465–472. [Google Scholar] [CrossRef] [PubMed]
- de Visser, K.E.; Joyce, J.A. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef]
- Fan, X. Recent highlights of cancer immunotherapy. Holist. Integr. Oncol. 2023, 2, 37. [Google Scholar] [CrossRef]
- Anassi, E.; Ndefo, U.A. Sipuleucel-T (provenge) injection: The first immunotherapy agent (vaccine) for hormone-refractory prostate cancer. Pharm. Ther. 2011, 36, 197–202. [Google Scholar]
- Reuschenbach, M.; Doorbar, J.; Del Pino, M.; Joura, E.A.; Walker, C.; Drury, R.; Rauscher, A.; Saah, A.J. Prophylactic HPV vaccines in patients with HPV-associated diseases and cancer. Vaccine 2023, 41, 6194–6205. [Google Scholar] [CrossRef]
- Liu, Y.; Li, S.; Chen, L.; Lin, L.; Xu, C.; Qiu, H.; Li, X.; Cao, H.; Liu, K. Global trends in tumor microenvironment-related research on tumor vaccine: A review and bibliometric analysis. Front. Immunol. 2024, 15, 1341596. [Google Scholar] [CrossRef]
- Stoitzner, P.; Romani, N.; Rademacher, C.; Probst, H.C.; Mahnke, K. Antigen targeting to dendritic cells: Still a place in future immunotherapy? Eur. J. Immunol. 2022, 52, 1909–1924. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, X.; Bao, W.; Liu, G.; Wei, W.; Ping, Y. An oncolytic virus-T cell chimera for cancer immunotherapy. Nat. Biotechnol. 2024, 42, 1876–1887. [Google Scholar] [CrossRef]
- Lin, X.; Tang, S.; Guo, Y.; Tang, R.; Li, Z.; Pan, X.; Chen, G.; Qiu, L.; Dong, X.; Zhang, L.; et al. Personalized neoantigen vaccine enhances the therapeutic efficacy of bevacizumab and anti-PD-1 antibody in advanced non-small cell lung cancer. Cancer Immunol. Immunother. 2024, 73, 26. [Google Scholar] [CrossRef]
- Buonaguro, L.; Tagliamonte, M. Peptide-based vaccine for cancer therapies. Front. Immunol. 2023, 14, 1210044. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yan, Q.; Zeng, Z.; Fan, C.; Xiong, W. Advances and prospects of mRNA vaccines in cancer immunotherapy. Biochim. Biophys. Acta Rev. Cancer 2024, 1879, 189068. [Google Scholar] [CrossRef] [PubMed]
- Schnyder, J.L.; Garcia Garrido, H.M.; De Pijper, C.A.; Daams, J.G.; Stijnis, C.; Goorhuis, A.; Grobusch, M.P. Comparison of equivalent fractional vaccine doses delivered by intradermal and intramuscular or subcutaneous routes: A systematic review. Travel. Med. Infect. Dis. 2021, 41, 102007. [Google Scholar] [CrossRef]
- Waltz, E. How nasal-spray vaccines could change the pandemic. Nature 2022, 609, 240–242. [Google Scholar] [CrossRef]
- Boboltz, A.; Kumar, S.; Duncan, G.A. Inhaled drug delivery for the targeted treatment of asthma. Adv. Drug Deliv. Rev. 2023, 198, 114858. [Google Scholar] [CrossRef] [PubMed]
- Heida, R.; Hinrichs, W.L.; Frijlink, H.W. Inhaled vaccine delivery in the combat against respiratory viruses: A 2021 overview of recent developments and implications for COVID-19. Expert. Rev. Vaccines 2022, 21, 957–974. [Google Scholar] [CrossRef]
- Tang, Z.; You, X.; Xiao, Y.; Chen, W.; Li, Y.; Huang, X.; Liu, H.; Xiao, F.; Liu, C.; Koo, S.; et al. Inhaled mRNA nanoparticles dual-targeting cancer cells and macrophages in the lung for effective transfection. Proc. Natl. Acad. Sci. USA 2023, 120, e2304966120. [Google Scholar] [CrossRef]
- Baker, P.J. Advantages of an Oral Vaccine to Control the COVID-19 Pandemic. Am. J. Med. 2022, 135, 133–134. [Google Scholar] [CrossRef]
- Hald Albertsen, C.; Kulkarni, J.A.; Witzigmann, D.; Lind, M.; Petersson, K.; Simonsen, J.B. The role of lipid components in lipid nanoparticles for vaccines and gene therapy. Adv. Drug Deliv. Rev. 2022, 188, 114416. [Google Scholar] [CrossRef]
- Luo, P.K.; Ho, H.M.; Chiang, M.C.; Chu, L.A.; Chuang, Y.H.; Lyu, P.C.; Hu, I.C.; Chang, W.A.; Peng, S.Y.; Jayakumar, J.; et al. pH-Responsive β-Glucans-Complexed mRNA in LNPs as an Oral Vaccine for Enhancing Cancer Immunotherapy. Adv. Mater. 2024, 36, e2404830. [Google Scholar] [CrossRef]
- Yue, Y.; Xu, J.; Li, Y.; Cheng, K.; Feng, Q.; Ma, X.; Ma, N.; Zhang, T.; Wang, X.; Zhao, X.; et al. Antigen-bearing outer membrane vesicles as tumour vaccines produced in situ by ingested genetically engineered bacteria. Nat. Biomed. Eng. 2022, 6, 898–909. [Google Scholar] [CrossRef] [PubMed]
- Ueki, H.; Kitagawa, K.; Kato, M.; Yanase, S.; Okamura, Y.; Bando, Y.; Hara, T.; Terakawa, T.; Furukawa, J.; Nakano, Y.; et al. An oral cancer vaccine using Bifidobacterium vector augments combination of anti-PD-1 and anti-CTLA-4 antibodies in mouse renal cell carcinoma model. Sci. Rep. 2023, 13, 9994. [Google Scholar] [CrossRef] [PubMed]
- Vela Ramirez, J.E.; Sharpe, L.A.; Peppas, N.A. Current state and challenges in developing oral vaccines. Adv. Drug Deliv. Rev. 2017, 114, 116–131. [Google Scholar] [CrossRef]
- Bitounis, D.; Jacquinet, E.; Rogers, M.A.; Amiji, M.M. Strategies to reduce the risks of mRNA drug and vaccine toxicity. Nat. Rev. Drug Discov. 2024, 23, 281–300. [Google Scholar] [CrossRef]
- Carter, J.J.; Smith, R.A.; Scherer, E.M.; Skibinski, D.A.G.; Sankaranarayanan, S.; Luxembourg, A.; Kollmann, T.; Marty, K.D.; Sadarangani, M.; Dobson, S.; et al. Term immune memory responses to human papillomavirus (HPV) vaccination following 2 versus 3 doses of HPV vaccine. Vaccine 2025, 50, 126817. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.Y.; Cun, Y.N. Current status of clinical trials of HPV therapeutic vaccines. Zhonghua Yu Fang Yi Xue Za Zhi 2023, 57, 1647–1654. [Google Scholar] [CrossRef]
- Chen, J.; Xu, Y.; Zhou, M.; Xu, S.; Varley, A.J.; Golubovic, A.; Lu, R.X.Z.; Wang, K.C.; Yeganeh, M.; Vosoughi, D.; et al. Combinatorial design of ionizable lipid nanoparticles for muscle-selective mRNA delivery with minimized off-target effects. Proc. Natl. Acad. Sci. USA 2023, 120, e2309472120. [Google Scholar] [CrossRef]
- Lee, D.H.; Lim, S.; Kwak, S.S.; Kim, J. Advancements in Skin-Mediated Drug Delivery: Mechanisms, Techniques, and Applications. Adv. Healthc. Mater. 2024, 13, e2302375. [Google Scholar] [CrossRef]
- Rahimi, E.; Gomez, H.; Ardekani, A.M. Transport and distribution of biotherapeutics in different tissue layers after subcutaneous injection. Int. J. Pharm. 2022, 626, 122125. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, B.; Yu, Z.; Zhao, K.; Zhang, Y.; He, K.; Seow, Y.; Yin, H. Complete remission of tumors in mice with neoantigen-painted exosomes and anti-PD-1 therapy. Mol. Ther. 2023, 31, 3579–3593. [Google Scholar] [CrossRef]
- Su, L.; Hao, Y.; Li, R.; Pan, W.; Ma, X.; Weng, J.; Min, Y. Red blood cell-based vaccines for ameliorating cancer chemoimmunotherapy. Acta Biomater. 2022, 154, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Si, Y.F.; Lan, G.P.; Wang, Z.; Zhou, L.; Tang, M.Z.; Sj, O.B.; Lan, J.; Zhou, X.Y.; Wang, Y.L.; et al. LMP2-DC Vaccine Elicits Specific EBV-LMP2 Response to Effectively Improve Immunotherapy in Patients with Nasopharyngeal Cancer. Biomed. Environ. Sci. 2020, 33, 849–856. [Google Scholar] [CrossRef]
- Spira, A.; Hansen, A.R.; Harb, W.A.; Curtis, K.K.; Koga-Yamakawa, E.; Origuchi, M.; Li, Z.; Ertik, B.; Shaib, W.L. Multicenter, Open-Label, Phase I Study of DSP-7888 Dosing Emulsion in Patients with Advanced Malignancies. Target. Oncol. 2021, 16, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Baharom, F.; Ramirez-Valdez, R.A.; Khalilnezhad, A.; Khalilnezhad, S.; Dillon, M.; Hermans, D.; Fussell, S.; Tobin, K.K.S.; Dutertre, C.A.; Lynn, G.M.; et al. Systemic vaccination induces CD8(+) T cells and remodels the tumor microenvironment. Cell 2022, 185, 4317–4332.e15. [Google Scholar] [CrossRef]
- Li, Q.; Teng, Z.; Tao, J.; Shi, W.; Yang, G.; Zhang, Y.; Su, X.; Chen, L.; Xiu, W.; Yuwen, L.; et al. Elastic Nanovaccine Enhances Dendritic Cell-Mediated Tumor Immunotherapy. Small 2022, 18, e2201108. [Google Scholar] [CrossRef]
- Peng, S.; Tan, M.; Li, Y.D.; Cheng, M.A.; Farmer, E.; Ferrall, L.; Gaillard, S.; Roden, R.B.S.; Hung, C.F.; Wu, T.C. PD-1 blockade synergizes with intratumoral vaccination of a therapeutic HPV protein vaccine and elicits regression of tumor in a preclinical model. Cancer Immunol. Immunother. 2021, 70, 1049–1062. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, J.; Zheng, X.; Liu, Z.; Zhang, X.; Li, Y.; Wilhelm, J.; Cao, J.; Huang, G.; Zhang, J.; et al. Intratumoral administration of STING-activating nanovaccine enhances T cell immunotherapy. J. Immunother. Cancer 2022, 10, e003960. [Google Scholar] [CrossRef]
- Zhu, F.; Huang, S.; Liu, X.; Chen, Q.; Zhuang, C.; Zhao, H.; Han, J.; Jaen, A.M.; Do, T.H.; Peter, J.G.; et al. Safety and efficacy of the intranasal spray SARS-CoV-2 vaccine dNS1-RBD: A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir. Med. 2023, 11, 1075–1088. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Migueles, S.A.; Huang, J.; Bolkhovitinov, L.; Stuccio, S.; Griesman, T.; Pullano, A.A.; Kang, B.H.; Ishida, E.; Zimmerman, M.; et al. A replication-competent adenovirus-vectored influenza vaccine induces durable systemic and mucosal immunity. J. Clin. Investig. 2021, 131, e140794. [Google Scholar] [CrossRef]
- Liu, S.; Wen, Y.; Shan, X.; Ma, X.; Yang, C.; Cheng, X.; Zhao, Y.; Li, J.; Mi, S.; Huo, H.; et al. Charge-assisted stabilization of lipid nanoparticles enables inhaled mRNA delivery for mucosal vaccination. Nat. Commun. 2024, 15, 9471. [Google Scholar] [CrossRef]
- Chinese Society of Peritoneal Oncology, China Anti-Cancer Association; Cui, S. China Anti-Cancer Association (CACA) guidelines for holistic integrative management of cancer-peritoneal tumours from gastrointestinal tract. Zhonghua Wei Chang Wai Ke Za Zhi 2023, 26, 111–120. [Google Scholar] [CrossRef]
- He, L.; Bai, Y.; Xia, L.; Pan, J.; Sun, X.; Zhu, Z.; Ding, J.; Qi, C.; Tang, C. Oral administration of a whole glucan particle (WGP)-based therapeutic cancer vaccine targeting macrophages inhibits tumor growth. Cancer Immunol. Immunother. 2022, 71, 2007–2028. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Shi, Y.; Liang, T.; Xing, H.; Ma, W.; Li, Y.M.; Wang, Y. Peptide vaccine against glioblastoma: From bench to bedside. Holist. Integr. Oncol. 2022, 1, 21. [Google Scholar] [CrossRef]
- Weller, M.; Butowski, N.; Tran, D.D.; Recht, L.D.; Lim, M.; Hirte, H.; Ashby, L.; Mechtler, L.; Goldlust, S.A.; Iwamoto, F.; et al. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): A randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017, 18, 1373–1385. [Google Scholar] [CrossRef]
- Hou, Y.-J.; Yang, X.-X.; Meng, H.-X. Pathological mechanisms and advances in neoadjuvant PD-1 blockade combined with chemotherapy for head and neck cancer. Holist. Integr. Oncol. 2024, 3, 63. [Google Scholar] [CrossRef]
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulières, D.; Tahara, M.; de Castro, G., Jr.; Psyrri, A.; Basté, N.; Neupane, P.; Bratland, Å.; et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef]
- Kang, N.; Zhang, S.; Wang, Y. A personalized mRNA vaccine has exhibited potential in the treatment of pancreatic cancer. Holist. Integr. Oncol. 2023, 2, 18. [Google Scholar] [CrossRef]
- Rojas, L.A.; Sethna, Z.; Soares, K.C.; Olcese, C.; Pang, N.; Patterson, E.; Lihm, J.; Ceglia, N.; Guasp, P.; Chu, A.; et al. Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer. Nature 2023, 618, 144–150. [Google Scholar] [CrossRef]
- Bouazzaoui, A.; Abdellatif, A.A.H. Vaccine delivery systems and administration routes: Advanced biotechnological techniques to improve the immunization efficacy. Vaccine X 2024, 19, 100500. [Google Scholar] [CrossRef]
- Inoue, S.; Mizoguchi, I.; Sonoda, J.; Sakamoto, E.; Katahira, Y.; Hasegawa, H.; Watanabe, A.; Furusaka, Y.; Xu, M.; Yoneto, T.; et al. Induction of potent antitumor immunity by intradermal DNA injection using a novel needle-free pyro-drive jet injector. Cancer Sci. 2023, 114, 34–47. [Google Scholar] [CrossRef]
- Chang, C.; Sun, J.; Hayashi, H.; Suzuki, A.; Sakaguchi, Y.; Miyazaki, H.; Nishikawa, T.; Nakagami, H.; Yamashita, K.; Kaneda, Y. Stable Immune Response Induced by Intradermal DNA Vaccination by a Novel Needleless Pyro-Drive Jet Injector. AAPS PharmSciTech 2019, 21, 19. [Google Scholar] [CrossRef] [PubMed]
- Geukes Foppen, M.H.; Rohaan, M.W.; Borgers, J.S.W.; Philips, D.; Vyth-Dreese, F.; Beijnen, J.H.; Nuijen, B.; van den Berg, J.H.; Haanen, J. Intradermal Naked DNA Vaccination by DNA Tattooing for Mounting Tumor-Specific Immunity in Stage IV Melanoma Patients: A Phase I Clinical Trial. Oncol. Res. Treat. 2024, 47, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Bakker, N.A.M.; Rotman, J.; van Beurden, M.; Zijlmans, H.J.M.; van Ruiten, M.; Samuels, S.; Nuijen, B.; Beijnen, J.; De Visser, K.; Haanen, J.; et al. HPV-16 E6/E7 DNA tattoo vaccination using genetically optimized vaccines elicit clinical and immunological responses in patients with usual vulvar intraepithelial neoplasia (uVIN): A phase I/II clinical trial. J. Immunother. Cancer 2021, 9, e002547. [Google Scholar] [CrossRef] [PubMed]
- Laurie, C.; Tota, J.E.; El-Zein, M.; Tellier, P.P.; Coutlée, F.; Burchell, A.N.; Franco, E.L. Design and methods for the Carrageenan-gel Against Transmission of Cervical Human papillomavirus (CATCH) study: A randomized controlled trial. Contemp. Clin. Trials 2021, 110, 106560. [Google Scholar] [CrossRef]
- Sabado, R.L.; Pavlick, A.; Gnjatic, S.; Cruz, C.M.; Vengco, I.; Hasan, F.; Spadaccia, M.; Darvishian, F.; Chiriboga, L.; Holman, R.M.; et al. Resiquimod as an immunologic adjuvant for NY-ESO-1 protein vaccination in patients with high-risk melanoma. Cancer Immunol. Res. 2015, 3, 278–287. [Google Scholar] [CrossRef]
- Adigweme, I.; Yisa, M.; Ooko, M.; Akpalu, E.; Bruce, A.; Donkor, S.; Jarju, L.B.; Danso, B.; Mendy, A.; Jeffries, D.; et al. A measles and rubella vaccine microneedle patch in The Gambia: A phase 1/2, double-blind, double-dummy, randomised, active-controlled, age de-escalation trial. Lancet 2024, 403, 1879–1892. [Google Scholar] [CrossRef]
- Chen, Z.; Guo, Z.; Hu, T.; Huang, B.; Zheng, Q.; Du, X.; Huang, L.; Hu, W. Double-layered microneedle patch loaded with bioinspired nano-vaccine for melanoma treatment and wound healing. Int. J. Biol. Macromol. 2024, 262, 129961. [Google Scholar] [CrossRef]
- Sonoda, J.; Mizoguchi, I.; Inoue, S.; Watanabe, A.; Sekine, A.; Yamagishi, M.; Miyakawa, S.; Yamaguchi, N.; Horio, E.; Katahira, Y.; et al. A Promising Needle-Free Pyro-Drive Jet Injector for Augmentation of Immunity by Intradermal Injection as a Physical Adjuvant. Int. J. Mol. Sci. 2023, 24, 9094. [Google Scholar] [CrossRef]
- Barber-Axthelm, I.M.; Kelly, H.G.; Esterbauer, R.; Wragg, K.M.; Gibbon, A.M.; Lee, W.S.; Wheatley, A.K.; Kent, S.J.; Tan, H.X.; Juno, J.A. Coformulation with Tattoo Ink for Immunological Assessment of Vaccine Immunogenicity in the Draining Lymph Node. J. Immunol. 2021, 207, 735–744. [Google Scholar] [CrossRef]
- Quaak, S.G.; van den Berg, J.H.; Toebes, M.; Schumacher, T.N.; Haanen, J.B.; Beijnen, J.H.; Nuijen, B. GMP production of pDERMATT for vaccination against melanoma in a phase I clinical trial. Eur. J. Pharm. Biopharm. 2008, 70, 429–438. [Google Scholar] [CrossRef]
- Moulin, V.; Morgan, M.E.; Eleveld-Trancikova, D.; Haanen, J.B.; Wielders, E.; Looman, M.W.; Janssen, R.A.; Figdor, C.G.; Jansen, B.J.; Adema, G.J. Targeting dendritic cells with antigen via dendritic cell-associated promoters. Cancer Gene Ther. 2012, 19, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Babiarova, K.; Kutinova, L.; Zurkova, K.; Krystofova, J.; Brabcova, E.; Hainz, P.; Musil, J.; Nemeckova, S. Immunization with WT1-derived peptides by tattooing inhibits the growth of TRAMP-C2 prostate tumor in mice. J. Immunother. 2012, 35, 478–487. [Google Scholar] [CrossRef] [PubMed]
- van de Wall, S.; Walczak, M.; van Rooij, N.; Hoogeboom, B.N.; Meijerhof, T.; Nijman, H.W.; Daemen, T. Tattoo Delivery of a Semliki Forest Virus-Based Vaccine Encoding Human Papillomavirus E6 and E7. Vaccines 2015, 3, 221–238. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.C.; Chang, C.C.; Chan, H.P.; Chung, T.W.; Shu, C.W.; Chuang, K.P.; Duh, T.H.; Yang, M.H.; Tyan, Y.C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef]
- Delitto, D.; Zabransky, D.J.; Chen, F.; Thompson, E.D.; Zimmerman, J.W.; Armstrong, T.D.; Leatherman, J.M.; Suri, R.; Lopez-Vidal, T.Y.; Huff, A.L.; et al. Implantation of a neoantigen-targeted hydrogel vaccine prevents recurrence of pancreatic adenocarcinoma after incomplete resection. Oncoimmunology 2021, 10, 2001159. [Google Scholar] [CrossRef]
- Nie, X.; Shi, C.; Chen, X.; Yu, C.; Jiang, Z.; Xu, G.; Lin, Y.; Tang, M.; Luan, Y. A single-shot prophylactic tumor vaccine enabled by an injectable biomembrane hydrogel. Acta Biomater. 2023, 169, 306–316. [Google Scholar] [CrossRef]
- Yang, X.; Huang, C.; Wang, H.; Yang, K.; Huang, M.; Zhang, W.; Yu, Q.; Wang, H.; Zhang, L.; Zhao, Y.; et al. Multifunctional Nanoparticle-Loaded Injectable Alginate Hydrogels with Deep Tumor Penetration for Enhanced Chemo-Immunotherapy of Cancer. ACS Nano 2024, 18, 18604–18621. [Google Scholar] [CrossRef]
- Liu, W.S.; Lu, Z.M.; Pu, X.H.; Li, X.Y.; Zhang, H.Q.; Zhang, Z.Z.; Zhang, X.Y.; Shi, T.; Jiang, X.H.; Zhou, J.S.; et al. A dendritic cell-recruiting, antimicrobial blood clot hydrogel for melanoma recurrence prevention and infected wound management. Biomaterials 2025, 313, 122776. [Google Scholar] [CrossRef]
- Shao, L.; Pan, B.; Hou, R.; Jin, Y.; Yao, Y. User-friendly microfluidic manufacturing of hydrogel microspheres with sharp needle. Biofabrication 2022, 14, 025017. [Google Scholar] [CrossRef]
- Liu, J.; Du, C.; Chen, H.; Huang, W.; Lei, Y. Nano-Micron Combined Hydrogel Microspheres: Novel Answer for Minimal Invasive Biomedical Applications. Macromol. Rapid Commun. 2024, 45, e2300670. [Google Scholar] [CrossRef]
- Zaman, R.U.; Gala, R.P.; Bansal, A.; Bagwe, P.; D’Souza, M.J. Preclinical evaluation of a microparticle-based transdermal vaccine patch against metastatic breast cancer. Int. J. Pharm. 2022, 627, 122249. [Google Scholar] [CrossRef] [PubMed]
- Faraji Rad, Z.; Prewett, P.D.; Davies, G.J. An overview of microneedle applications, materials, and fabrication methods. Beilstein J. Nanotechnol. 2021, 12, 1034–1046. [Google Scholar] [CrossRef] [PubMed]
- Price, S.L.; Oakes, R.S.; Gonzalez, R.J.; Edwards, C.; Brady, A.; DeMarco, J.K.; von Andrian, U.H.; Jewell, C.M.; Lawrenz, M.B. Microneedle array delivery of Yersinia pestis recapitulates bubonic plague. iScience 2024, 27, 108600. [Google Scholar] [CrossRef]
- Babu, M.R.; Vishwas, S.; Khursheed, R.; Harish, V.; Sravani, A.B.; Khan, F.; Alotaibi, B.; Binshaya, A.; Disouza, J.; Kumbhar, P.S.; et al. Unravelling the role of microneedles in drug delivery: Principle, perspectives, and practices. Drug Deliv. Transl. Res. 2024, 14, 1393–1431. [Google Scholar] [CrossRef]
- D’Amico, C.; Fusciello, M.; Hamdan, F.; D’Alessio, F.; Bottega, P.; Saklauskaite, M.; Russo, S.; Cerioni, J.; Elbadri, K.; Kemell, M.; et al. Transdermal delivery of PeptiCRAd cancer vaccine using microneedle patches. Bioact. Mater. 2025, 45, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Huang, J.; Xiang, Q.; Dong, H. Hollow microneedle microfluidic paper-based chip for biomolecules rapid sampling and detection in interstitial fluid. Anal. Chim. Acta 2023, 1255, 341101. [Google Scholar] [CrossRef]
- van der Maaden, K.; Heuts, J.; Camps, M.; Pontier, M.; Terwisscha van Scheltinga, A.; Jiskoot, W.; Ossendorp, F.; Bouwstra, J. Hollow microneedle-mediated micro-injections of a liposomal HPV E7(43–63) synthetic long peptide vaccine for efficient induction of cytotoxic and T-helper responses. J. Control Release 2018, 269, 347–354. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, F.; Xiang, J.; Zhou, X.; Wu, B.; Fan, B.; Tang, H.; Liu, B.; Chen, L. Mesoporous Microneedles Enabled Localized Controllable Delivery of Stimulator of Interferon Gene Agonist Nanoexosomes for FLASH Radioimmunotherapy against Breast Cancer. ACS Appl. Mater. Interfaces 2024, 16, 58180–58190. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, W.; Zhang, L.; He, L.; Wang, S.; Wang, J.; Xiang, M.; Yuan, X.; Gou, M. Intradermal Delivery of Cell Vaccine via Ice Microneedles for Cancer Treatment. Adv. Healthc. Mater. 2025, 14, e2400678. [Google Scholar] [CrossRef]
- Chang, H.; Wen, X.; Li, Z.; Ling, Z.; Zheng, Y.; Xu, C. Co-delivery of dendritic cell vaccine and anti-PD-1 antibody with cryomicroneedles for combinational immunotherapy. Bioeng. Transl. Med. 2023, 8, e10457. [Google Scholar] [CrossRef]
- Yang, D.; Chen, M.; Sun, Y.; Shi, C.; Wang, W.; Zhao, W.; Wen, T.; Liu, T.; Fu, J.; Lu, C.; et al. Microneedle-assisted vaccination combined with autophagy regulation for antitumor immunotherapy. J. Control Release 2023, 357, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Cole, G.; Ali, A.A.; McCrudden, C.M.; McBride, J.W.; McCaffrey, J.; Robson, T.; Kett, V.L.; Dunne, N.J.; Donnelly, R.F.; McCarthy, H.O. DNA vaccination for cervical cancer: Strategic optimisation of RALA mediated gene delivery from a biodegradable microneedle system. Eur. J. Pharm. Biopharm. 2018, 127, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, Z.; Zeng, W.; Li, Y.; Zhu, C. A hyaluronic acid-based dual-functional hydrogel microneedle system for sequential melanoma ablation and skin regeneration. Int. J. Biol. Macromol. 2024, 283, 138039. [Google Scholar] [CrossRef]
- Yao, W.D.; Zhou, J.N.; Tang, C.; Zhang, J.L.; Chen, Z.Y.; Li, Y.; Gong, X.J.; Qu, M.Y.; Zeng, Q.; Jia, Y.L.; et al. Hydrogel Microneedle Patches Loaded with Stem Cell Mitochondria-Enriched Microvesicles Boost the Chronic Wound Healing. ACS Nano 2024, 18, 26733–26750. [Google Scholar] [CrossRef]
- Yuan, M.; Liu, K.; Jiang, T.; Li, S.; Chen, J.; Wu, Z.; Li, W.; Tan, R.; Wei, W.; Yang, X.; et al. GelMA/PEGDA microneedles patch loaded with HUVECs-derived exosomes and Tazarotene promote diabetic wound healing. J. Nanobiotechnol. 2022, 20, 147. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Yang, H.; Lu, X.; Li, Y.; Liu, Z.; Li, F.; Shang, Z.; Wang, X.; Li, X.; Li, J.; et al. Three-Dimensional-Cultured MSC-Derived Exosome-Hydrogel Hybrid Microneedle Array Patch for Spinal Cord Repair. Nano Lett. 2022, 22, 6391–6401. [Google Scholar] [CrossRef]
- Han, L.; Peng, K.; Qiu, L.Y.; Li, M.; Ruan, J.H.; He, L.L.; Yuan, Z.X. Hitchhiking on Controlled-Release Drug Delivery Systems: Opportunities and Challenges for Cancer Vaccines. Front. Pharmacol. 2021, 12, 679602. [Google Scholar] [CrossRef]
- Nguyen, H.X.; Nguyen, C.N. Microneedle-Mediated Transdermal Delivery of Biopharmaceuticals. Pharmaceutics 2023, 15, 277. [Google Scholar] [CrossRef]
- Rai, C.I.; Kuo, T.H.; Chen, Y.C. Novel Administration Routes, Delivery Vectors, and Application of Vaccines Based on Biotechnologies: A Review. Vaccines 2024, 12, 1002. [Google Scholar] [CrossRef]
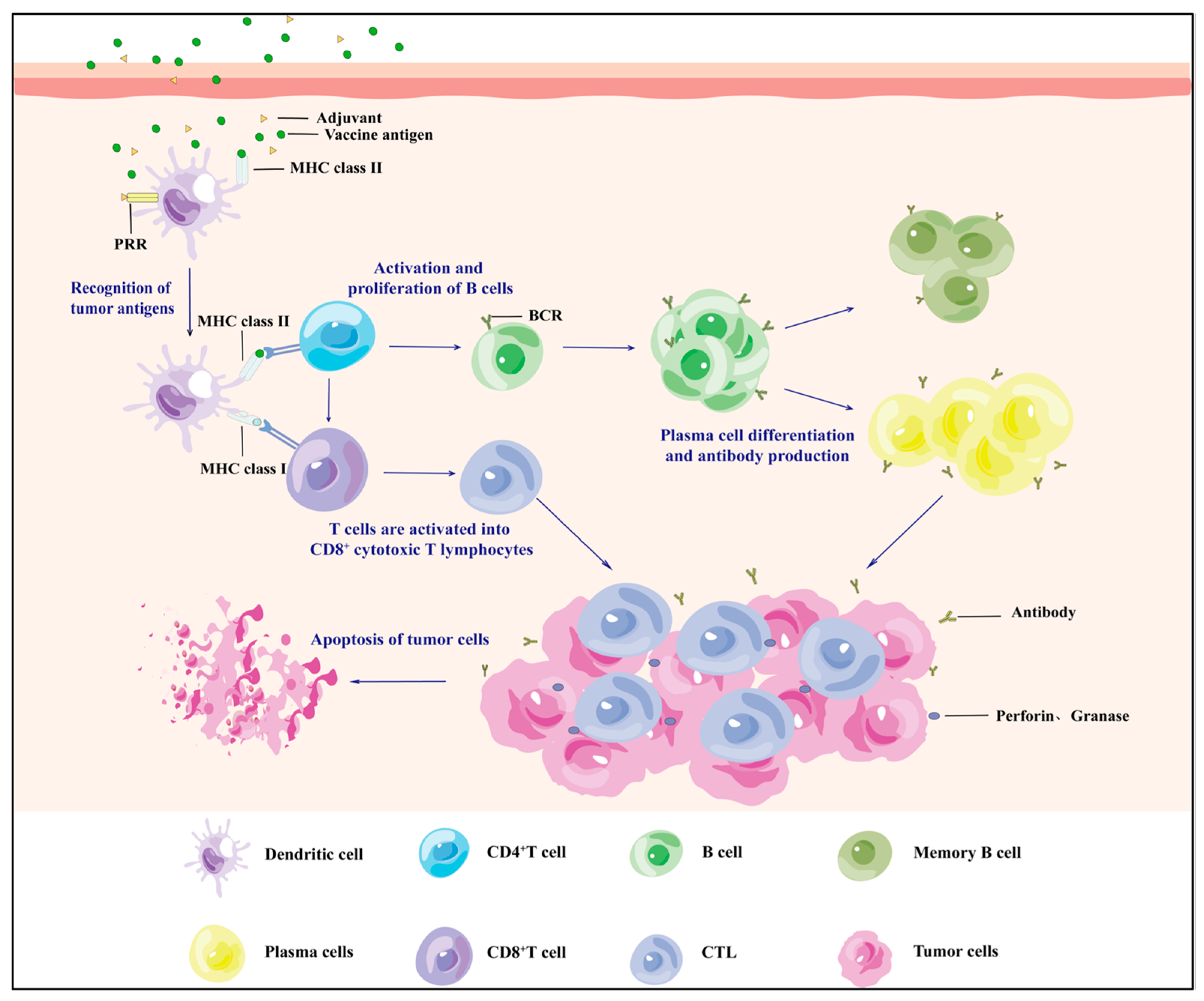
| Type of Inoculation | Dominance | Shortage | Drug Delivery | Effect | Application | Published | |
|---|---|---|---|---|---|---|---|
| Nasal spray | —— | Non-invasive, good patient compliance, faster accumulation from nose to lungs | May cause asthma | SARS-CoV-2 live attenuated vaccine | High safety and efficacy of dNS1-RBD against COVID-19 | Clinical trial: ChiCTR2100051391 | 2023 [38] |
| Live attenuated influenza vaccine | Influenza prevention | Data collection and questionnaires | 2021 [39] | ||||
| Inhalation | —— | The vaccine is nebulised so that it is inhaled through the mouth and accumulates in the lungs | Not suitable for people with respiratory problems | Negatively charged CAS-LNP formulations | Cancer prevention and treatment vaccine development | Validation in metastatic lung cancer mouse model | 2024 [40] |
| Dual-target mRNA NPs with cationic lipids and hyaluronic acid | Treatment of lung cancer and pneumonia | Data aggregation and analysis/basic experiments | 2024 [41], 2023 [17] | ||||
| Oral method | —— | Vaccines are easy to store and patient compliance is good | Not for those with GI dysfunction; hepatic first pass may reduce vaccine efficacy | βGlus/mRNA@LNPs vaccine | Effective anti-tumour response | Validation on a hormonal mouse model | 2024 [20] |
| Whole glucan particle WGP-OVA vaccine | Inhibition of tumour growth | Validated in melanoma and LLC mouse tumour models | 2022 [42] | ||||
| Injection | Intramuscular injection | Accurate and wide range of vaccination doses | Not suitable for people with localised skin infections or muscular disorders | LNP-delivered mRNA | Elicited an effective cellular immune response | Validated in a melanoma vaccine model | 2023 [27] |
| Hypodermic injection | The effects of the vaccine are relatively stable and long-lasting | Limitations on the number of doses of injected vaccines | Neoantigen-coated serum exosomes with PD-1 antibodies | Relieved tumour growth | Melanoma and colon cancer models | 2023 [30] | |
| RBC vaccine | Improvement of chemo-immunotherapy | Colon cancer models | 2022 [31] | ||||
| Intradermal injection | Good results can be achieved with very small doses | Significant pain and need for professional inoculation | Peptide vaccine rindopepimut | Effective for glioblastoma treatment | Analysis/Clinical Trial: NCT01480479 | 2022 [43], 2017 [44] | |
| LMP2-DCs vaccine | Improving NPC patients’ immunotherapy | Patients were followed up | 2020 [32] | ||||
| Intravenous injection | Accurate dosing enables full vaccine effect | High medical staff skill required, low patient compliance | Pembrolizumab | Treatment of local advanced head–neck SCC | Data Summary and Analysis/Clinical Trial: NCT02641093 | 2024 [45], 2019 [46] | |
| mRNA cancer vaccine | Treatment of pancreatic ductal adenocarcinoma | Analysis/clinical trial: NCT04161755 | 2023 [47], 2023 [48] | ||||
| Intratumoural injection | Precise vaccine delivery site reduces drug side effects | Operational errors can cause serious complications | PC7A nanovaccine | Anti-tumour immune response | Studies in TC-1 and B16-OVA cancer models | 2022 [37] | |
| Type of Inoculation | Merits | Shortage | Drug Delivery | Application | Effect | Published |
|---|---|---|---|---|---|---|
| Novel needle-free thermal release-driven jet Injector | Allows precise needle-free drug delivery | Discomfort | OVA expression plasmid DNA | Basic studies using OVA as a model antigen and selection of transplantable tumours expressing OVA E.G7-OVA | Strong anti-tumour immune response | 2023 [50] |
| DNA vaccine | pOVA as a model antigen and assessment of initial gene expression in the intradermal region | PJI efficiently delivers plasmid DNA to the nucleus in the dermal zone and induces efficient gene expression | 2019 [51] | |||
| Tattooing | Slow release can be achieved and compliance is good | Immature tech; high R&D and production costs | DNA vaccine (pDERMATT) | Clinical Trial: NCT05309421 | New possibilities for cancer vaccine development | 2024 [52] |
| HPV-16 E6/E7 DNA vaccine | Clinical trial: NTR4607 | Good results for uVIN patients | 2021 [53] | |||
| Nanogel | Immature tech; high R&D and production costs | Harsh storage conditions | Carrageenan gel | Trial registration: ISRCTN96104919 | Reduced risk of HPV infection | 2021 [54] |
| TLR7/8 agonist Resiquimod gel | Clinical Trial: NCT00821652 | Effective for resected high-risk melanoma patients | 2015 [55] | |||
| Microneedle | Easy to operate and no cross-contamination | High cost and lot-to-lot variation | Measles and rubella vaccine | Clinical trial: PACTR202008836432905 | May help eliminate measles and rubella | 2024 [56] |
| Bionic nanovaccine (HAP@Vac) | Validated on a B16 cell-loaded model in C57BL/6 mice | Postoperative treatment of malignant melanoma | 2024 [57] |
| Microneedle Type | Manufacturing Method | Specifications | Matrix Material | Loaded Drug | Application | Published |
|---|---|---|---|---|---|---|
| Solid microneedles | Micromoulding | 800 μm height, 200 μm base, and 500 μm pitch | 10% PVP and 10% sucrose (20% total concentration) | Adenovirus (PeptiCRAd) | Antitumour effect validation for melanoma and pulmonary carcinoma | 2024 [75] |
| Hollow microneedles | 3D printing | Single needle height of 800 μm, bottom diameter of 500 μm | PDMS | —— | Detection of glucose and lactate expression levels in ISF | 2023 [76] |
| Etching | 50 μm inner diameter | Sapphire | Cationic liposome HPV E743-63 SLP vaccine | CNC-controlled hollow microneedle injection system for tumour vaccine delivery | 2018 [77] | |
| Porous microneedles | Micromoulding | Tip size 250 μm × 250 μm, height 700 μm, MN patch size 1 cm × 1 cm | GelMa | Nano-exosome (EXO) | Optimising the results of FLASH radiotherapy | 2024 [78] |
| Frozen microneedles | Micromoulding | 13 × 13 array, 1 mm tip-to-tip distance, tips -900 μm high and 400 μm wide | Culture medium | Live tumour cell vaccine (TCV) | Inhibition of melanoma growth and recurrence | 2025 [79] |
| Low-temperature micromoulding | Height 950 μm, base width 400 μm | 2.5% (v/v) DMSO and 100 mM sucrose in phosphate-buffered saline (PBS) | Co-delivery of DC vaccine and aPD1 | Prevention and treatment of melanoma | 2022 [80] | |
| Dissolvable microneedles | Micromoulding | Quadrilateral pyramid-shaped needles, 800 μm high, 300 μm base diameter, in 12 × 12 uniform array, 750 μm tip spacing | Hyaluronic acid (HA) and PVP K90 | Antigen-undefined whole tumour cell vaccine (TCV) | Effectively inhibits melanoma invasion and regresses existing malignancies | 2023 [81] |
| Micromoulding | 300 μm diameter and 600 μm length, spaced 50 μm apart | PVA solution | DNA vaccine | Superior to standard intramuscular injection in preclinical cervical cancer models | 2018 [82] | |
| Hydrogel microneedles | Two-step process | —— | HA | Photothermal agent CCa@TF/Ce6 | Combined melanoma treatment and skin regeneration | 2024 [83] |
| Micromoulding | MNP needle tip spacing is 700 μm in diameter, 500 μm in height, and 270 μm in base | DAM and HAMA | Stem-cell-derived mitochondria-rich EV | Promotes chronic wound healing | 2024 [84] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, R.; Li, X.; Zhu, J.; He, J.; Na, J. Clinical Translation Challenges and Strategies for Tumour Vaccines Considering Multiple Delivery Routes. Vaccines 2025, 13, 469. https://doi.org/10.3390/vaccines13050469
Song R, Li X, Zhu J, He J, Na J. Clinical Translation Challenges and Strategies for Tumour Vaccines Considering Multiple Delivery Routes. Vaccines. 2025; 13(5):469. https://doi.org/10.3390/vaccines13050469
Chicago/Turabian StyleSong, Ruiyun, Xiao Li, Junsong Zhu, Jian He, and Jintong Na. 2025. "Clinical Translation Challenges and Strategies for Tumour Vaccines Considering Multiple Delivery Routes" Vaccines 13, no. 5: 469. https://doi.org/10.3390/vaccines13050469
APA StyleSong, R., Li, X., Zhu, J., He, J., & Na, J. (2025). Clinical Translation Challenges and Strategies for Tumour Vaccines Considering Multiple Delivery Routes. Vaccines, 13(5), 469. https://doi.org/10.3390/vaccines13050469







