A Marker-Free Bordetella bronchiseptica aroA/bscN Double Deleted Mutant Confers Protection against Lethal Challenge
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Plasmids, Primers, and Culture Conditions
2.2. Plasmid Construction
2.3. Plasmid Conjugation and Mutant Screening
2.4. Animals and Ethic Statement
2.5. Virulence Studies in Mice
2.6. Safety Test in Piglets
2.7. Pig Immunization and Challenge
2.8. ELISA Assays
2.9. Statistics Analysis
3. Results
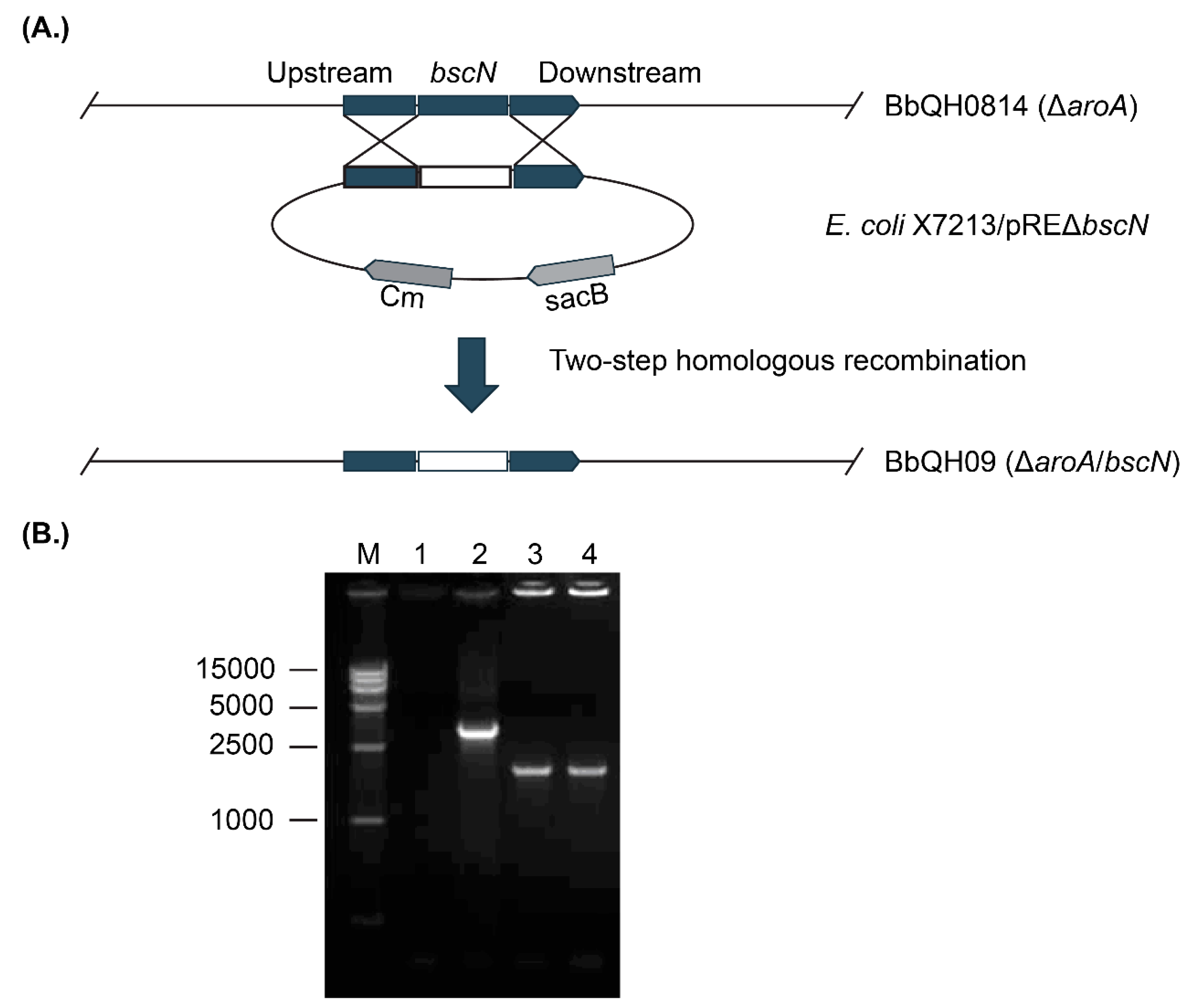
3.1. Construction of the Marker-Free aroA/bscN Double Deletion Strain QH09
3.2. QH09 Showed Attenuated Virulence and Capacity of Colonization in Mice
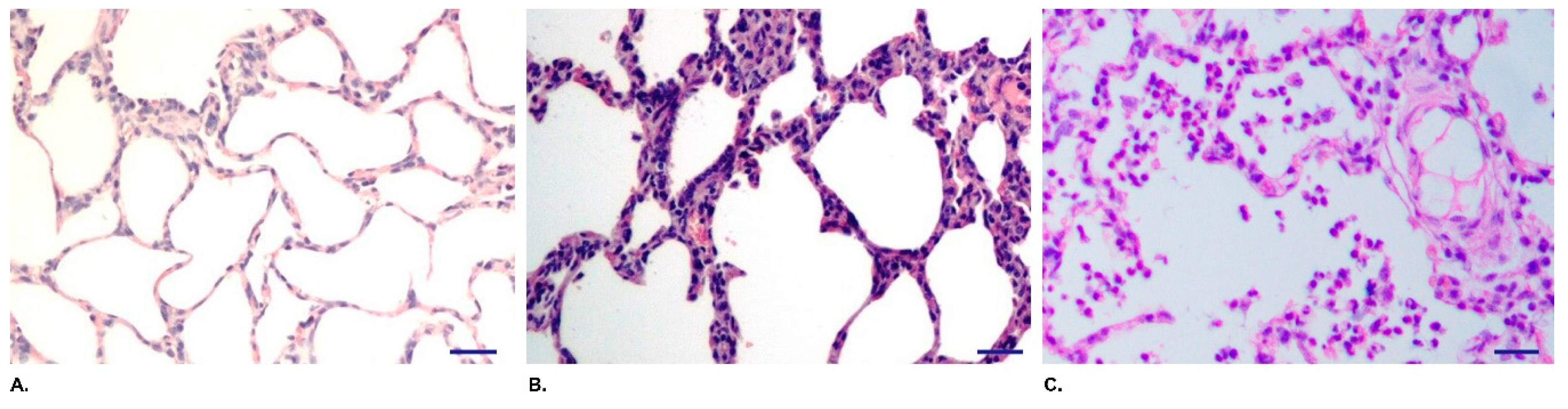
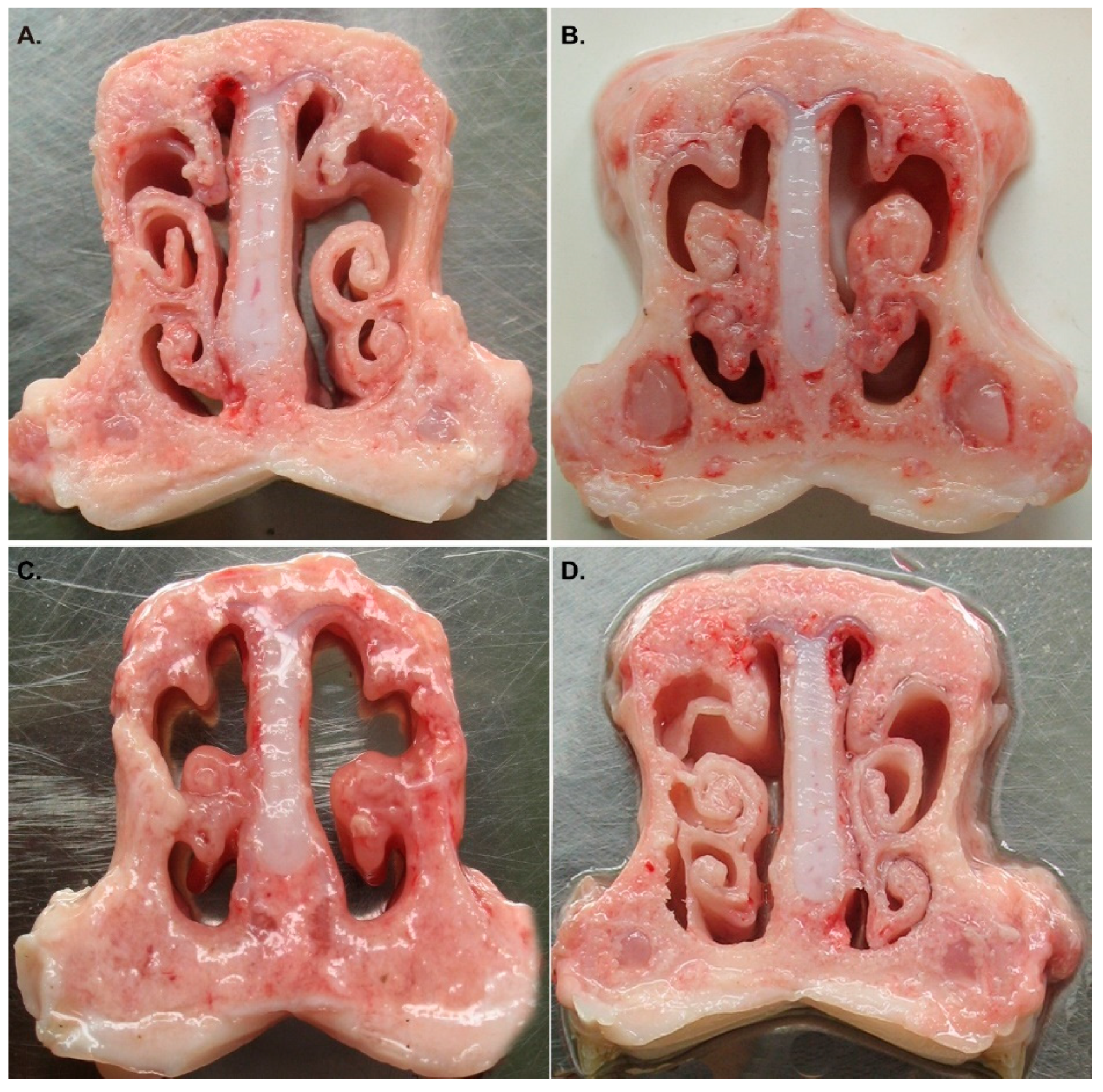
3.3. QH09 Was Safe to Piglets
3.4. QH09 Induced High Levels of Antibodies and Conferred Protection Against Lethal Challenge
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goodnow, R.A. Biology of Bordetella bronchiseptica. Microbiol. Rev. 1980, 44, 722–738. [Google Scholar] [PubMed]
- Cheong, Y.; Oh, C.; Lee, K.; Cho, K.H. Survey of porcine respiratory disease complex-associated pathogens among commercial pig farms in Korea via oral fluid method. J. Vet. Sci. 2017, 18, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Matherne, C.M.; Steffen, E.K.; Wagner, J.E. Efficacy of commercial vaccines for protecting guinea pigs against Bordetella bronchiseptica pneumonia. Lab. Anim. Sci. 1987, 37, 191–194. [Google Scholar] [PubMed]
- Neutra, M.R.; Kozlowski, P.A. Mucosal vaccines: The promise and the challenge. Nat. Rev. Immunol. 2006, 6, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Boyaka, P.N. Inducing Mucosal IgA: A Challenge for Vaccine Adjuvants and Delivery Systems. J. Immunol. 2017, 199, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hu, R.; Hu, J.; He, H.; Tang, X.; Jin, M.; Chen, H.; Wu, B. aroA deleted Bordetella bronchiseptica inspiring robust mucosal immune response and provide full protection against intranasal challenge. Res. Vet. Sci. 2013, 94, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Bernasconi, V.; Norling, K.; Bally, M.; Hook, F.; Lycke, N.Y. Mucosal Vaccine Development Based on Liposome Technology. J. Immunol. Res. 2016, 2016, 5482087. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Xue, Y.; Wu, B.; Tang, X.; Hu, R.; Xu, Y.; Guo, A.; Chen, H. Subcutaneous vaccination with attenuated Salmonella enterica serovar Choleraesuis C500 expressing recombinant filamentous hemagglutinin and pertactin antigens protects mice against fatal infections with both S. enterica serovar Choleraesuis and Bordetella bronchiseptica. Infect. Immun. 2008, 76, 2157–2163. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R.A.; Keller, L.H.; Schifferli, D.M. Improved allelic exchange vectors and their use to analyze 987P fimbria gene expression. Gene 1998, 207, 149–157. [Google Scholar] [CrossRef]
- Zhao, Z.; Xue, Y.; Tang, X.; Wu, B.; Cheng, X.; He, Q.; Zhang, C.; Guo, A.; Jin, M.; Chen, H. Immunogenicity of recombinant protective antigen and efficacy against intranasal challenge with Bordetella bronchiseptica. Vaccine 2009, 27, 2523–2528. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, D.N.; Kirimanjeswara, G.S.; Goebel, E.M.; Harvill, E.T. Comparative role of immunoglobulin A in protective immunity against the Bordetellae. Infect. Immun. 2007, 75, 4416–4422. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.; Laris, R.; Gray, A.W.; Jacobs, A.A. Studies of the efficacy of a novel intranasal vaccine against feline bordetellosis. Vet. Rec. 2002, 150, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Mann, P.; Goebel, E.; Barbarich, J.; Pilione, M.; Kennett, M.; Harvill, E. Use of a genetically defined double mutant strain of Bordetella bronchiseptica lacking adenylate cyclase and type III secretion as a live vaccine. Infect. Immun. 2007, 75, 3665–3672. [Google Scholar] [CrossRef] [PubMed]
- Yuk, M.H.; Harvill, E.T.; Miller, J.F. The BvgAS virulence control system regulates type III secretion in Bordetella bronchiseptica. Mol. Microbiol. 1998, 28, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, H.; Xiong, B.; Fan, G.; Cao, Z. Immunogenicity of recombinant outer membrane porin protein and protective efficacy against lethal challenge with Bordetella bronchiseptica in rabbits. J. Appl. Microbiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wang, C.; Xue, Y.; Ding, K.; Zhang, C.; Cheng, X.; Li, Y.; Liu, Y.; Wu, T. Immunogenicity and protective efficacy of pertactin recombinants against Bordetella bronchiseptica challenge. Wei Sheng Wu Xue Bao 2010, 50, 1239–1245. [Google Scholar] [PubMed]
- Yevsa, T.; Ebensen, T.; Fuchs, B.; Zygmunt, B.; Libanova, R.; Gross, R.; Schulze, K.; Guzman, C.A. Development and characterization of attenuated metabolic mutants of Bordetella bronchiseptica for applications in vaccinology. Environ. Microbiol. 2013, 15, 64–76. [Google Scholar] [CrossRef] [PubMed]
- McArthur, J.D.; West, N.P.; Cole, J.N.; Jungnitz, H.; Guzman, C.A.; Chin, J.; Lehrbach, P.R.; Djordjevic, S.P.; Walker, M.J. An aromatic amino acid auxotrophic mutant of Bordetella bronchiseptica is attenuated and immunogenic in a mouse model of infection. FEMS Microbiol. Lett. 2003, 221, 7–16. [Google Scholar] [CrossRef]
- Wang, C.; Liu, L.; Zhang, Z.; Yan, Z.; Yu, C.; Shao, M.; Jiang, X.; Chi, S.; Wei, K.; Zhu, R. Immunological and protective effects of Bordetella bronchiseptica subunit vaccines based on the recombinant N-terminal domain of dermonecrotic toxin. Int. Immunopharmacol. 2015, 28, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Corthesy, B. Multi-faceted functions of secretory IgA at mucosal surfaces. Front. Immunol. 2013, 4, 185. [Google Scholar] [CrossRef] [PubMed]

| Bacterial Strains | Characteristics | Source |
|---|---|---|
| B. bronchiseptica HH0809 | Wild type isolate from a pig with atrophic rhinitis | [7,8] |
| B. bronchiseptica QH0814 | Unmarked aroA deletion of HH0809 | [6] |
| B. bronchiseptica QH09 | Unmarked bscN deletion of QH0814 | This study |
| E. coli X7213 | Thi-1 thr-1 leuB6 fhuA21 lacY1 glnV44 △asdA4 recA1 RP4 2-Tc::Mu[λpir] Kmr | [9] |
| Plasmids | ||
| pBluescript II KS/SK (+) | oriColE1 oriF1(+) bla lacZa | Our collection |
| pRE112 (CmR) | oriT oriV sacB1, CmR, counterselectable suicide plasmid | [9] |
| pSKΔbscN | bscN upstream and downstream fragment amplified from QH0814 ligated into pBluescript II KS/SK (+) | This study |
| pREΔbscN | bscN upstream and downstream fragment from pSKΔbscN ligated into pRE112 | This study |
| Primers | ||
| F1 | 5′- CCCCCGCACATTTCCGAACTTC -3′ | Part of flagellin (237 bp) |
| F2 | 5′- AGGCTCCCAAGAGAGAAAGGCTT -3′ | |
| B1 | 5′- CGGGAATTCATGCGTCAGTACCACTACATCAC -3′ (EcoR I) | Part of bscN (1334 bp) |
| B2 | 5′- GCTAAGCTTTAGGATTCGGGTCCGATGATTTCAG -3′ (Hind III) | |
| B3 | 5′- TAA GGTACC GCGCTTGCGCTGGTGCTGTC -3′ (Kpn I) | bscN upstream flank (1148 bp) |
| B4 | 5′- TCA GAATTC ACGCGCACCCCCAGCTCC -3′ (EcoR I) | |
| B5 | 5′- TGTGAATTC GACGGCCACATCGTGCTCT -3′ (EcoR I) | bscN downstream flank (980 bp) |
| B6 | 5′- GGAGAGCTCCCTTCGCTTTCTCCTGTTCCAT -3′ (Sac I) | |
| Cm1 | 5′- TAAATACCTGTGACGGAAGAT -3′ | Cm resistant gene (700 bp) |
| Cm2 | 5′- TATCACTTATTCAGGCGTAGC -3′ |
| Group | Piglets ID | 24 hpc | 48 hpc | First Week | |||
|---|---|---|---|---|---|---|---|
| Appetite a | Mood a | Cough a | Appetite a | Spirit a | Cough a | ||
| QH09 (3 × 1011 CFU) | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| 2 | 1 | 1 | 0 | 0 | 0 | 0 | |
| 3 | 1 | 1 | 0 | 0 | 0 | 0 | |
| QH09 (1 × 1011 CFU) | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| 2 | 1 | 1 | 0 | 0 | 0 | 0 | |
| 3 | 1 | 1 | 0 | 0 | 0 | 0 | |
| HH0809 (3 × 1011 CFU) | 1 | 3 | 3 | 3 | 3 | 3 | 3 |
| 2 | 3 | 3 | 3 | 3 | 3 | 3 | |
| 3 | 3 | 3 | 3 | 3 | 3 | 3 | |
| HH0809 (1 × 1011 CFU) | 1 | 3 | 3 | 3 | 3 | 3 | 3 |
| 2 | 3 | 3 | 3 | 3 | 2 | 2 | |
| 3 | 3 | 3 | 3 | 3 | 2 | 2 | |
| PBS | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Mean | QH09 (3 × 1011 CFU) | 1.0 ± 0.00 | 1.0 ± 0.00 | 0 | 0 | 0 | 0 |
| QH09 (1 × 1011 CFU) | 1.0 ± 0.00 | 1.0 ± 0.00 | 0 | 0 | 0 | 0 | |
| HH0809 (3 × 1011 CFU) | 3.0 ± 0.00 | 3.0 ± 0.00 | 3.0 ± 0.00 | 3.0 ± 0.00 | 3.0 ± 0.00 | 3.0 ± 0.00 | |
| HH0809 (1 × 1011 CFU) | 3.0 ± 0.00 | 3.0 ± 0.00 | 3.0 ± 0.00 | 3.0 ± 0.00 | 2.3 ± 0.47 | 2.3 ± 0.47 | |
| PBS | 0 | 0 | 0 | 0 | 0 | 0 | |
| Antibodies (Log10) | Attenuated Live Vaccine (QH09) | Inactivated Vaccine (HH0809) | ||
|---|---|---|---|---|
| 14 Days (OD630) | 28 Days (OD630) | 14 Days (OD630) | 28 Days (OD630) | |
| IgG1 | 1.83 ± 0.17 | 2.20 ± 0.21 | 3.83 ± 0. 0.30 | 4.47 ± 0.17 |
| IgG2a | 2.60 ± 0.37 | 2.97 ± 0.17 | 3.13 ± 0.11 | 3.54 ± 0.15 |
| IgG1/IgG2a | 0.70 | 0.74 | 1.22 | 1.26 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ai, W.; Peng, Z.; Wang, F.; Zhang, Y.; Xie, S.; Liang, W.; Hua, L.; Wang, X.; Chen, H.; Wu, B. A Marker-Free Bordetella bronchiseptica aroA/bscN Double Deleted Mutant Confers Protection against Lethal Challenge. Vaccines 2019, 7, 176. https://doi.org/10.3390/vaccines7040176
Ai W, Peng Z, Wang F, Zhang Y, Xie S, Liang W, Hua L, Wang X, Chen H, Wu B. A Marker-Free Bordetella bronchiseptica aroA/bscN Double Deleted Mutant Confers Protection against Lethal Challenge. Vaccines. 2019; 7(4):176. https://doi.org/10.3390/vaccines7040176
Chicago/Turabian StyleAi, Weicheng, Zhong Peng, Fei Wang, Yue Zhang, Sisi Xie, Wan Liang, Lin Hua, Xiangru Wang, Huanchun Chen, and Bin Wu. 2019. "A Marker-Free Bordetella bronchiseptica aroA/bscN Double Deleted Mutant Confers Protection against Lethal Challenge" Vaccines 7, no. 4: 176. https://doi.org/10.3390/vaccines7040176





