Effective Activation of Human Antigen-Presenting Cells and Cytotoxic CD8+ T Cells by a Calcium Phosphate-Based Nanoparticle Vaccine Delivery System
Abstract
:1. Introduction
2. Materials and Methods
2.1. TLR-Ligands and Viral Peptides
2.2. Synthesis of Functionalized CaP Nanoparticles
2.3. Subjects
2.4. Isolation of PBMCs from Human Blood
2.5. Isolation of Primary DCs
2.6. Generation of Monocyte-Derived DCs
2.7. Uptake of Alexa488-Labeled CaP Nanoparticles by DCs
2.8. Stimulation of DCs with Functionalized CaP Nanoparticles
2.9. Isolation of CD8+ T Cells
2.10. In vitro Co-Culture of NP-Stimulated DCs and Virus-Specific CD8+ T Cells
2.11. Antibodies and Flow Cytometry
2.12. Cytokine Detection in Supernatants
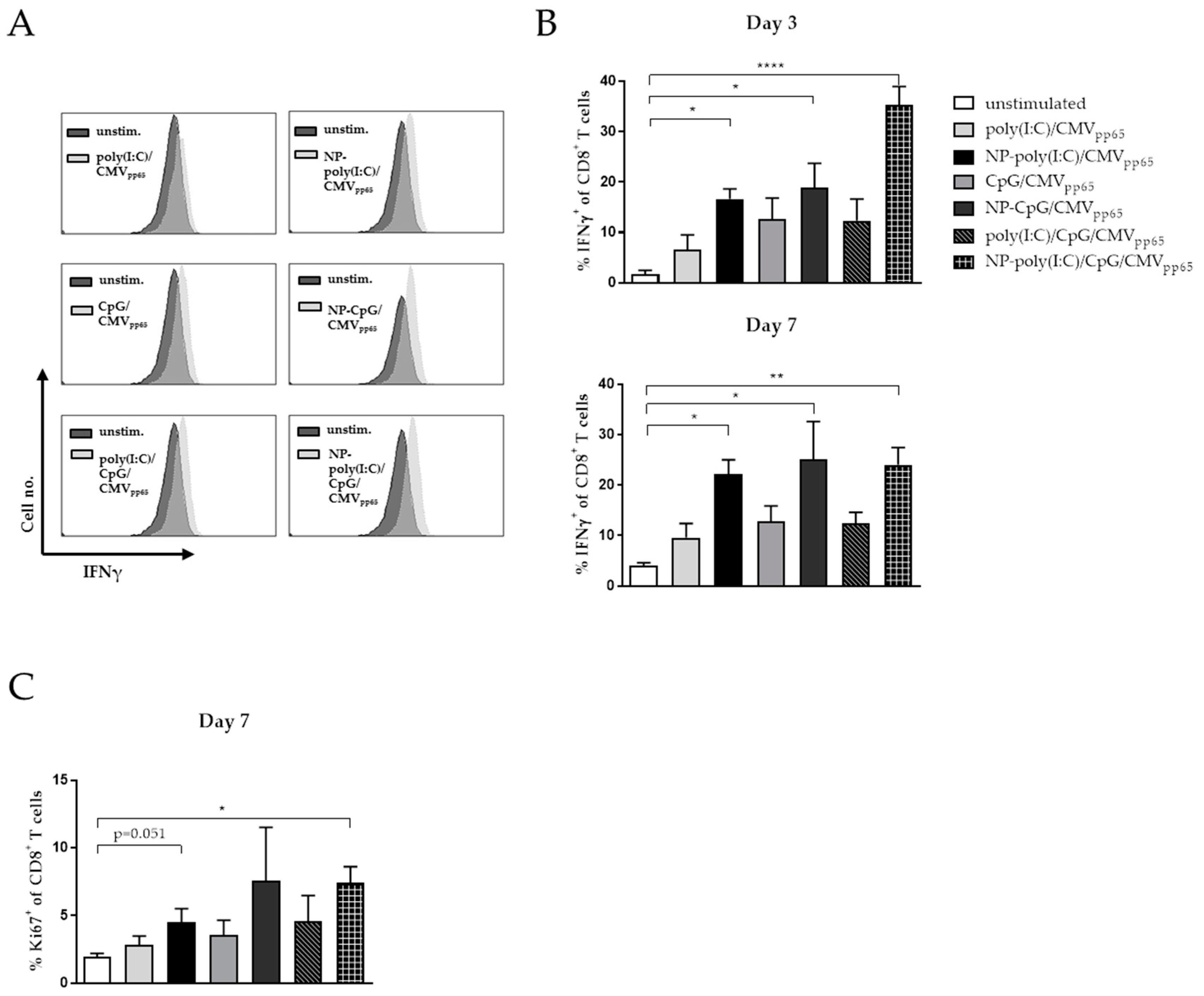
3. Results
3.1. Colloid-Chemical Characterization of Functionalized Calcium Phosphate Nanoparticles
3.2. Uptake of Functionalized CaP Nanoparticles by Human Antigen Presenting Cells
3.3. Maturation of Blood Derived DCs by Functionalized CaP Nanoparticles
3.4. Maturation of Monocyte Derived DCs by Functionalized CaP Nanoparticles
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wherry, E.J.; Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 2015, 15, 486–499. [Google Scholar] [CrossRef] [PubMed]
- Sau, S.; Alsaab, H.O.; Bhise, K.; Alzhrani, R.; Nabil, G.; Iyer, A.K. Multifunctional nanoparticles for cancer immunotherapy: A groundbreaking approach for reprogramming malfunctioned tumor environment. J. Control. Release 2018, 274, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Stead, S.O.; McInnes, S.J.P.; Kireta, S.; Rose, P.D.; Jesudason, S.; Rojas-Canales, D.; Warther, D.; Cunin, F.; Durand, J.O.; Drogemuller, C.J.; et al. Manipulating human dendritic cell phenotype and function with targeted porous silicon nanoparticles. Biomaterials 2018, 155, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Hubbell, J.A.; Thomas, S.N.; Swartz, M.A. Materials engineering for immunomodulation. Nature 2009, 462, 449–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cubas, R.; Zhang, S.; Kwon, S.; Sevick-Muraca, E.M.; Li, M.; Chen, C.; Yao, Q. Virus-like particle (VLP) lymphatic trafficking and immune response generation after immunization by different routes. J. Immunother. 2009, 32, 118–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hochweller, K.; Wabnitz, G.H.; Samstag, Y.; Suffner, J.; Hammerling, G.J.; Garbi, N. Dendritic cells control T cell tonic signaling required for responsiveness to foreign antigen. Proc. Natl. Acad. Sci. USA 2010, 107, 5931–5936. [Google Scholar] [CrossRef] [Green Version]
- Curtsinger, J.M.; Schmidt, C.S.; Mondino, A.; Lins, D.C.; Kedl, R.M.; Jenkins, M.K.; Mescher, M.F. Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. J. Immunol. 1999, 162, 3256–3262. [Google Scholar]
- Dzionek, A.; Fuchs, A.; Schmidt, P.; Cremer, S.; Zysk, M.; Miltenyi, S.; Buck, D.W.; Schmitz, J. BDCA-2, BDCA-3, and BDCA-4: Three markers for distinct subsets of dendritic cells in human peripheral blood. J. Immunol. 2000, 165, 6037–6046. [Google Scholar] [CrossRef] [Green Version]
- Collin, M.; Bigley, V. Human dendritic cell subsets: An update. Immunology 2018, 154, 3–20. [Google Scholar] [CrossRef]
- Hemont, C.; Neel, A.; Heslan, M.; Braudeau, C.; Josien, R. Human blood mDC subsets exhibit distinct TLR repertoire and responsiveness. J. Leukoc. Biol. 2013, 93, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Epple, M. Review of potential health risks associated with nanoscopic calcium phosphate. Acta Biomater. 2018, 77, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Habraken, W.; Habibovic, P.; Epple, M.; Bohner, M. Calcium phosphates in biomedical applications: Materials for the future? Mater. Today 2016, 19, 69–87. [Google Scholar] [CrossRef]
- Tadic, D.; Epple, M. A thorough physicochemical characterisation of 14 calcium phosphate-based bone substitution materials in comparison to natural bone. Biomaterials 2004, 25, 987–994. [Google Scholar] [CrossRef]
- Hannig, M.; Hannig, C. Nanomaterials in preventive dentistry. Nat. Nanotechnol. 2010, 5, 565–569. [Google Scholar] [CrossRef]
- Sokolova, V.; Kozlova, D.; Knuschke, T.; Buer, J.; Westendorf, A.M.; Epple, M. Mechanism of the uptake of cationic and anionic calcium phosphate nanoparticles by cells. Acta Biomater. 2013, 9, 7527–7535. [Google Scholar] [CrossRef]
- Knuschke, T.; Bayer, W.; Rotan, O.; Sokolova, V.; Wadwa, M.; Kirschning, C.J.; Hansen, W.; Dittmer, U.; Epple, M.; Buer, J.; et al. Prophylactic and therapeutic vaccination with a nanoparticle-based peptide vaccine induces efficient protective immunity during acute and chronic retroviral infection. Nanomedicine 2014, 10, 1787–1798. [Google Scholar] [CrossRef]
- Hesse, C.; Kollenda, S.; Rotan, O.; Pastille, E.; Adamczyk, A.; Wenzek, C.; Hansen, W.; Epple, M.; Buer, J.; Westendorf, A.M.; et al. A Tumor-Peptide-Based Nanoparticle Vaccine Elicits Efficient Tumor Growth Control in Antitumor Immunotherapy. Mol. Cancer Ther. 2019, 18, 1069–1080. [Google Scholar] [CrossRef] [Green Version]
- Knuschke, T.; Rotan, O.; Bayer, W.; Kollenda, S.; Dickow, J.; Sutter, K.; Hansen, W.; Dittmer, U.; Lang, K.S.; Epple, M.; et al. Induction of Type I Interferons by Therapeutic Nanoparticle-Based Vaccination Is Indispensable to Reinforce Cytotoxic CD8(+) T Cell Responses During Chronic Retroviral Infection. Front. Immunol. 2018, 9, 614. [Google Scholar] [CrossRef]
- Sokolova, V.; Knuschke, T.; Kovtun, A.; Buer, J.; Epple, M.; Westendorf, A.M. The use of calcium phosphate nanoparticles encapsulating Toll-like receptor ligands and the antigen hemagglutinin to induce dendritic cell maturation and T cell activation. Biomaterials 2010, 31, 5627–5633. [Google Scholar] [CrossRef]
- Guermonprez, P.; Valladeau, J.; Zitvogel, L.; Thery, C.; Amigorena, S. Antigen presentation and T cell stimulation by dendritic cells. Annu. Rev. Immunol. 2002, 20, 621–667. [Google Scholar] [CrossRef]
- Medzhitov, R. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 2001, 1, 135–145. [Google Scholar] [CrossRef]
- Sallusto, F.; Lanzavecchia, A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 1994, 179, 1109–1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tacken, P.J.; Zeelenberg, I.S.; Cruz, L.J.; van Hout-Kuijer, M.A.; van de Glind, G.; Fokkink, R.G.; Lambeck, A.J.; Figdor, C.G. Targeted delivery of TLR ligands to human and mouse dendritic cells strongly enhances adjuvanticity. Blood 2011, 118, 6836–6844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kastenmuller, K.; Wille-Reece, U.; Lindsay, R.W.; Trager, L.R.; Darrah, P.A.; Flynn, B.J.; Becker, M.R.; Udey, M.C.; Clausen, B.E.; Igyarto, B.Z.; et al. Protective T cell immunity in mice following protein-TLR7/8 agonist-conjugate immunization requires aggregation, type I IFN, and multiple DC subsets. J. Clin. Investig. 2011, 121, 1782–1796. [Google Scholar] [CrossRef] [Green Version]
- Bachmann, M.F.; Jennings, G.T. Vaccine delivery: A matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 2010, 10, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Bode, C.; Zhao, G.; Steinhagen, F.; Kinjo, T.; Klinman, D.M. CpG DNA as a vaccine adjuvant. Expert Rev. Vaccines 2011, 10, 499–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barton, G.M.; Medzhitov, R. Control of adaptive immune responses by Toll-like receptors. Curr. Opin. Immunol. 2002, 14, 380–383. [Google Scholar] [CrossRef]
- Muzio, M.; Bosisio, D.; Polentarutti, N.; D’Amico, G.; Stoppacciaro, A.; Mancinelli, R.; van’t Veer, C.; Penton-Rol, G.; Ruco, L.P.; Allavena, P.; et al. Differential expression and regulation of toll-like receptors (TLR) in human leukocytes: Selective expression of TLR3 in dendritic cells. J. Immunol. 2000, 164, 5998–6004. [Google Scholar] [CrossRef] [Green Version]
- de Titta, A.; Ballester, M.; Julier, Z.; Nembrini, C.; Jeanbart, L.; van der Vlies, A.J.; Swartz, M.A.; Hubbell, J.A. Nanoparticle conjugation of CpG enhances adjuvancy for cellular immunity and memory recall at low dose. Proc. Natl. Acad. Sci. USA 2013, 110, 19902–19907. [Google Scholar] [CrossRef] [Green Version]
- Croft, M. The role of TNF superfamily members in T-cell function and diseases. Nat. Rev. Immunol. 2009, 9, 271–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trinchieri, G.; Pflanz, S.; Kastelein, R.A. The IL-12 family of heterodimeric cytokines: New players in the regulation of T cell responses. Immunity 2003, 19, 641–644. [Google Scholar] [CrossRef] [Green Version]
- Pipkin, M.E.; Sacks, J.A.; Cruz-Guilloty, F.; Lichtenheld, M.G.; Bevan, M.J.; Rao, A. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity 2010, 32, 79–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grohmann, U.; Bianchi, R.; Belladonna, M.L.; Vacca, C.; Silla, S.; Ayroldi, E.; Fioretti, M.C.; Puccetti, P. IL-12 acts selectively on CD8 alpha- dendritic cells to enhance presentation of a tumor peptide in vivo. J. Immunol. 1999, 163, 3100–3105. [Google Scholar]
- Hathcock, K.S.; Laszlo, G.; Pucillo, C.; Linsley, P.; Hodes, R.J. Comparative analysis of B7-1 and B7-2 costimulatory ligands: Expression and function. J. Exp. Med. 1994, 180, 631–640. [Google Scholar] [CrossRef]
- Takata, H.; Buranapraditkun, S.; Kessing, C.; Fletcher, J.L.; Muir, R.; Tardif, V.; Cartwright, P.; Vandergeeten, C.; Bakeman, W.; Nichols, C.N.; et al. Delayed differentiation of potent effector CD8(+) T cells reducing viremia and reservoir seeding in acute HIV infection. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef] [Green Version]
- Gutjahr, A.; Tiraby, G.; Perouzel, E.; Verrier, B.; Paul, S. Triggering Intracellular Receptors for Vaccine Adjuvantation. Trends Immunol. 2016, 37, 573–587. [Google Scholar] [CrossRef]
- Shen, H.; Ackerman, A.L.; Cody, V.; Giodini, A.; Hinson, E.R.; Cresswell, P.; Edelson, R.L.; Saltzman, W.M.; Hanlon, D.J. Enhanced and prolonged cross-presentation following endosomal escape of exogenous antigens encapsulated in biodegradable nanoparticles. Immunology 2006, 117, 78–88. [Google Scholar] [CrossRef]
- Song, C.; Noh, Y.W.; Lim, Y.T. Polymer nanoparticles for cross-presentation of exogenous antigens and enhanced cytotoxic T-lymphocyte immune response. Int. J. Nanomed. 2016, 11, 3753–3764. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Anderson, J.L.; Lewin, S.R. Getting the "Kill" into "Shock and Kill": Strategies to Eliminate Latent HIV. Cell Host Microbe 2018, 23, 14–26. [Google Scholar] [CrossRef]
- Casazza, J.P.; Bowman, K.A.; Adzaku, S.; Smith, E.C.; Enama, M.E.; Bailer, R.T.; Price, D.A.; Gostick, E.; Gordon, I.J.; Ambrozak, D.R.; et al. Therapeutic vaccination expands and improves the function of the HIV-specific memory T-cell repertoire. J. Infect. Dis. 2013, 207, 1829–1840. [Google Scholar] [CrossRef] [PubMed]
- Barouch, D.H.; Deeks, S.G. Immunologic strategies for HIV-1 remission and eradication. Science 2014, 345, 169–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murakoshi, H.; Zou, C.; Kuse, N.; Akahoshi, T.; Chikata, T.; Gatanaga, H.; Oka, S.; Hanke, T.; Takiguchi, M. CD8(+) T cells specific for conserved, cross-reactive Gag epitopes with strong ability to suppress HIV-1 replication. Retrovirology 2018, 15, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altfeld, M.; Allen, T.M. Hitting HIV where it hurts: An alternative approach to HIV vaccine design. Trends Immunol. 2006, 27, 504–510. [Google Scholar] [CrossRef]
- Neek, M.; Tucker, J.A.; Kim, T.I.; Molino, N.M.; Nelson, E.L.; Wang, S.W. Co-delivery of human cancer-testis antigens with adjuvant in protein nanoparticles induces higher cell-mediated immune responses. Biomaterials 2018, 156, 194–203. [Google Scholar] [CrossRef] [Green Version]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scheffel, F.; Knuschke, T.; Otto, L.; Kollenda, S.; Sokolova, V.; Cosmovici, C.; Buer, J.; Timm, J.; Epple, M.; Westendorf, A.M. Effective Activation of Human Antigen-Presenting Cells and Cytotoxic CD8+ T Cells by a Calcium Phosphate-Based Nanoparticle Vaccine Delivery System. Vaccines 2020, 8, 110. https://doi.org/10.3390/vaccines8010110
Scheffel F, Knuschke T, Otto L, Kollenda S, Sokolova V, Cosmovici C, Buer J, Timm J, Epple M, Westendorf AM. Effective Activation of Human Antigen-Presenting Cells and Cytotoxic CD8+ T Cells by a Calcium Phosphate-Based Nanoparticle Vaccine Delivery System. Vaccines. 2020; 8(1):110. https://doi.org/10.3390/vaccines8010110
Chicago/Turabian StyleScheffel, Florian, Torben Knuschke, Lucas Otto, Sebastian Kollenda, Viktoriya Sokolova, Christine Cosmovici, Jan Buer, Jörg Timm, Matthias Epple, and Astrid M. Westendorf. 2020. "Effective Activation of Human Antigen-Presenting Cells and Cytotoxic CD8+ T Cells by a Calcium Phosphate-Based Nanoparticle Vaccine Delivery System" Vaccines 8, no. 1: 110. https://doi.org/10.3390/vaccines8010110
APA StyleScheffel, F., Knuschke, T., Otto, L., Kollenda, S., Sokolova, V., Cosmovici, C., Buer, J., Timm, J., Epple, M., & Westendorf, A. M. (2020). Effective Activation of Human Antigen-Presenting Cells and Cytotoxic CD8+ T Cells by a Calcium Phosphate-Based Nanoparticle Vaccine Delivery System. Vaccines, 8(1), 110. https://doi.org/10.3390/vaccines8010110




