Rubella Seroprevalence Boost in the Pediatric and Adolescent Population of Florence (Italy) as a Preventive Strategy for Congenital Rubella Syndrome (CRS)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Enrollment and Conservation of Sera Sample
2.2. Confirmation of Anamnestic and Vaccination Status
2.3. Serological Analysis
- Anti-rubella/IgG negative ΔA < 0.100 (cut-off);
- Anti-rubella/IgG positive ΔA > 0.200;
- Anti-rubella/IgG equivocal 0.100 ≤ ΔA ≤ 0.200.
2.4. Statistical Analysis
3. Results
3.1. Rubella Seroprevalence Analysis
3.2. Rubella Notification, Vaccination Status and Seroprevalence Assessment
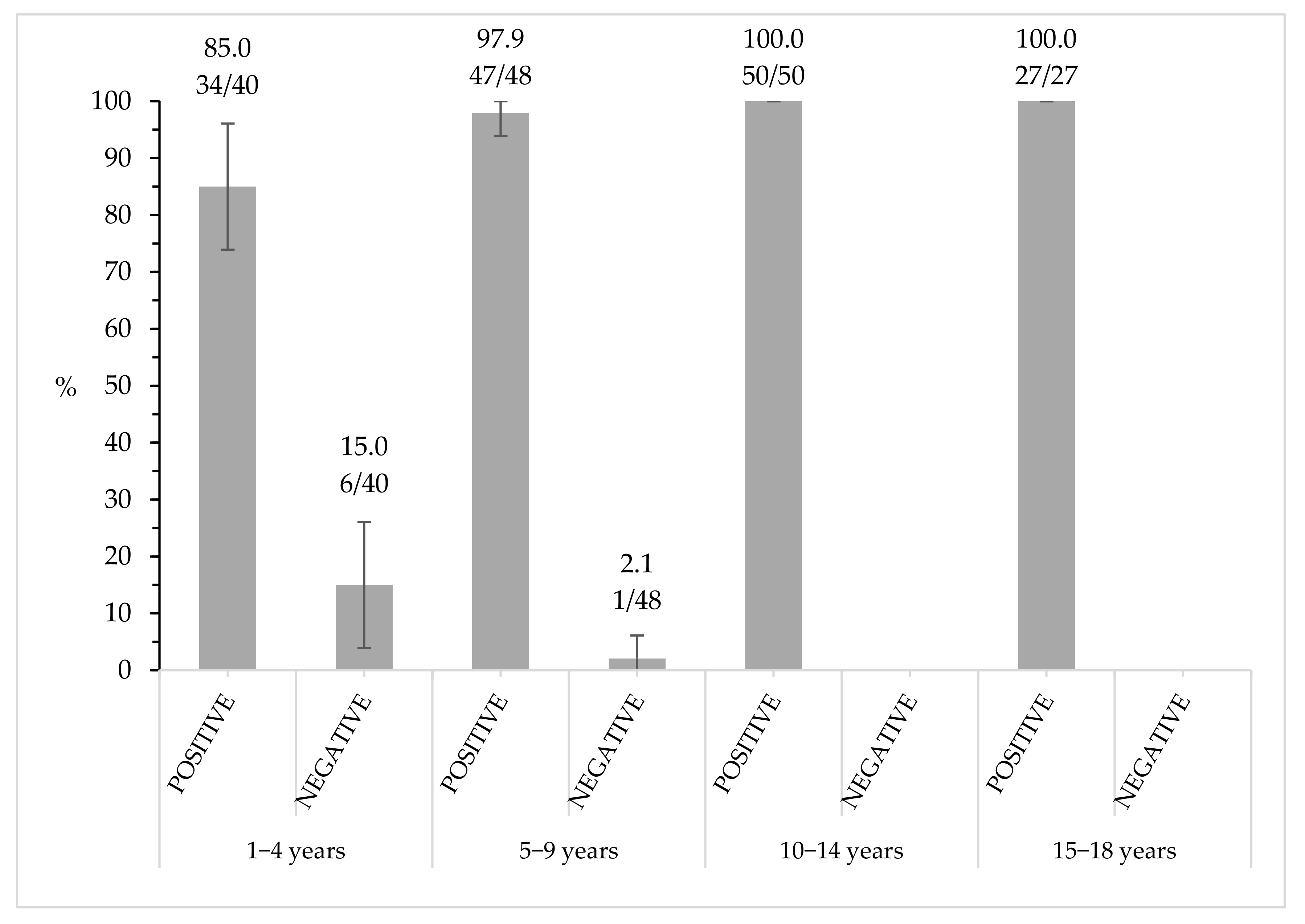
3.3. Seroprevalence Assessment by Number of Vaccine Doses and Time Elapsed since Last Vaccination
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smith, J. Rötheln (epidemic roseola-German measles-hybrid measles, etc.). Arch. Dermatol. 1875, 1, 1–13. [Google Scholar]
- Gregg, N.M. Congenital cataract following German measles in the mother. Trans. Ophthalmol. Soc. Aust. 1941, 3, 35–46. [Google Scholar]
- Lundstrom, L. Rubella during pregnancy: A follow-up study of children born after an epidemic of rubella in Sweden, 1951, with additional investigations on prophylaxis and treatment of maternal rubella. Acta Paediatr. Scand. 1962, 133, 1–110. [Google Scholar]
- Greenberg, M.; Pellitteri, O.; Barton, J. Frequency of defects in infants whose mothers had rubella during pregnancy. JAMA 1957, 165, 675–678. [Google Scholar] [CrossRef]
- Manson, M.; Logan, W.P.D.; Loy, R. Rubella and Other Virus Infections during Pregnancy (Report on Public Health and Mechanical Subjects, No. 101); HM Stationery Office: London, UK, 1960. [Google Scholar]
- Plotkin, S.A.; Oski, F.; Hartnett, E.; Al, E. Some recently recognized manifestations of the rubella syndrome. J. Pediatr. 1965, 67, 182–191. [Google Scholar] [CrossRef]
- Prinzie, A.; Huygelen, C.; Gold, J.; Farquhar, J.; McKee, J. Experimental live attenuated rubella virus vaccine: clinical evaluation of Cendehill strain. Am. J. Dis. Child. 1969, 118, 172–177. [Google Scholar] [CrossRef]
- Meyer, H.M.; Parkman, P.D.; Hobbins, T.E.; Larson, H.E.; Davis, W.J.; Simsarian, J.P.; Hopps, H.E. Attenuated rubella viruses: Laboratory and clinical characteristics. Am. J. Dis. Child. 1969, 118, 155–169. [Google Scholar] [CrossRef]
- Public Health Service Advisory Committee on Immunisation Practices. Rubella virus vaccine recommendation on immunisation practice: A pre-licensing statement. Ann. Intern. Med. 1969, 70, 1239–1241. [Google Scholar] [CrossRef]
- WHO. The Immunological Basis for Immunization Series - Module 11: Rubella. Immun. Vaccines Biol. 1983, 11, 1–24. [Google Scholar]
- Reef, S.E.; Plotkin, S.A. Rubella vaccines. In Plotkin’s Vaccines, 7th ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; Volume 53, pp. 970–1000. [Google Scholar]
- Grahame, R.; Armstrong, R.; Simmons, N.; Wilton, J.M.; Dyson, M.; Laurent, R.; Millis, R.; Mims, C.A. Chronic arthritis associated with the presence of intrasynovial rubella virus. Ann. Rheum. Dis. 1983, 42, 2–13. [Google Scholar] [CrossRef] [Green Version]
- Morse, E.; Zinkham, W.H.; Jackson, D. Thrombocytopenic purpura following rubella infection in children and adults. Arch. Intern. Med. 1966, 117, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Sherman, F.; Michaels, R.; Kenny, F. Acute encephalopathy (encephalitis) complicating rubella: Reports of cases with virologic studies, cortisol-production determinations and observations at autopsy. JAMA 1965, 192, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Lambert, N.; Strebel, P.; Orenstein, W.; Joseph, I.; Poland, G.A. Rubella. Lancet 2015, 385, 2297–2307. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Eighth Meeting of the European Regional Verification Commission for Measles and Rubella Elimination (Rvc); World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- World Health Organization. Eliminating Measles and Rubella; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- WHO Regional Office for Europe. Eliminating Measles and Rubella. Framework for the Verification Process in the WHO European Region; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Adamo, G.; Sturabotti, G.; D’Andrea, E.; Baccolini, V.; Romano, F.; Iannazzo, S.; Marzuillo, C.; Villari, P. The end of measles and congenital rubella: An achievable dream? Ann. Ig. 2017, 29, 1–26. [Google Scholar] [CrossRef]
- Ministero della Salute Piano nazionale per l’Eliminazione del Morbillo e della Rosolia Congenita 2010–2015. Available online: http://www.salute.gov.it/imgs/C_17_pubblicazioni_1519_allegato.pdf (accessed on 2 September 2020).
- Bechini, A.; Levi, M.; Boccalini, S.; Tiscione, E.; Panatto, D.; Amicizia, D.; Bonanni, P. Progress in the elimination of measles and congenital rubella in Central Italy. Hum. Vaccines Immunother. 2013, 9, 649–656. [Google Scholar] [CrossRef] [Green Version]
- Istituto Superiore di Sanità Morbillo e Rosolia News. Available online: https://www.epicentro.iss.it/morbillo/bollettino/RM_News_2019_59.pdf (accessed on 20 July 2020).
- ARS Toscana. La Sorveglianza Epidemiologica delle mMlattie Infettive in Toscana.Rapporto Novembre 2019. Available online: https://www.ars.toscana.it/images/pubblicazioni/Rapporti/2019/Rapporto_malattie_infettive.pdf (accessed on 20 July 2020).
- Zanella, B.; Boccalini, S.; Bonito, B.; Del Riccio, M.; Tiscione, E.; Bonanni, P.; Dhs, W.G.; Aoumeyer, W.G.; Ausltc, W.G.; Bechini, A.; et al. Increasing Measles Seroprevalence in a Sample of Pediatric and Adolescent Population of Tuscany (Italy): A Vaccination Campaign Success. Vaccines 2020, 8, 512. [Google Scholar] [CrossRef]
- Zanella, B.; Bechini, A.; Boccalini, S.; Sartor, G.; Tiscione, E.; Bonanni, P. Hepatitis B Seroprevalence in the Pediatric and Adolescent Population of Florence (Italy): An Update 27 Years after the Implementation of Universal Vaccination. Vaccines 2020, 8, 156. [Google Scholar] [CrossRef] [Green Version]
- Bechini, A.; Boccalini, S.; Tiscione, E.; Pesavento, G.; Mannelli, F.; Peruzzi, M.; Rapi, S.; Mercurio, S.; Bonanni, P. Progress towards measles and rubella elimination in Tuscany, Italy: The role of population seroepidemiological profile. Eur. J. Public Health 2010, 22, 133–139. [Google Scholar] [CrossRef] [Green Version]
- GeoDemo Istat. Popolazione Residente al 1° Gennaio 2017 nella Provincia di Firenze. Available online: http://demo.istat.it/pop2017/index.html (accessed on 20 July 2020).
- Epicentro. La sorveglianza Passi.I Dati per l’Italia-Vaccinazione Antirosolia. Available online: https://www.epicentro.iss.it/passi/dati/VaccinazioneAntirosolia (accessed on 20 July 2020).
- Istituto Nazionale di Statistica. Natalità e Fecondità della Popolazione Residente | Anno 2018 Natalità; Istituto Nazionale di Statistica: Rome, Italy, 2019; Volume 444. [Google Scholar]
- World Health Organization. European Regional Strategic Plan 2005–2010. In Eliminating Measles and Rubella and Preventing Congenital Rubella; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Tafuri, S.; Gallone, M.S.; Cappelli, M.G.; Martinelli, D.; Prato, R.; Germinario, C. Addressing the anti-vaccination movement and the role of HCWs. Vaccine 2014, 32, 4860–4865. [Google Scholar] [CrossRef]
- Ministero della Salute. Decreto Legge 7 giugno 2017; 2017. Available online: http://www.trovanorme.salute.gov.it/norme/dettaglioAtto?id=59548 (accessed on 23 July 2020).
- WHO. Vaccine-Preventable Diseases: Monitoring system. 2019 Global Summary Italy. Available online: https://apps.who.int/immunization_monitoring/globalsummary/countries?countrycriteria%5Bcountry%5D%5B%5D=ITA (accessed on 2 September 2020).
- WHO. Vaccine-Preventable Diseases: Monitoring System. 2019 Global Summary Senegal. Available online: https://apps.who.int/immunization_monitoring/globalsummary/countries?countrycriteria%5Bcountry%5D%5B%5D=SEN# (accessed on 2 September 2020).
- WHO. Vaccine-Preventable Diseases: Monitoring System. 2019 Global Summary Morocco. Available online: https://apps.who.int/immunization_monitoring/globalsummary/countries?countrycriteria%5Bcountry%5D%5B%5D=MAR (accessed on 2 September 2020).
- WHO. Vaccine-Preventable Diseases: Monitoring System. 2019 Global Summary Romania. Available online: https://apps.who.int/immunization_monitoring/globalsummary/countries?countrycriteria%5Bcountry%5D%5B%5D=ROU (accessed on 2 September 2020).
- WHO. Vaccine-Preventable Diseases: Monitoring System. 2019 Global Summary Philippines. Available online: https://apps.who.int/immunization_monitoring/globalsummary/countries?countrycriteria%5Bcountry%5D%5B%5D=PHL (accessed on 2 September 2020).
- Bechini, A.; Boccalini, S.; Alimenti, C.M.; Bonanni, P.; Galli, L.; Chiappini, E. Immunization status against measles, mumps, rubella and varicella in a large population of internationally adopted children referred to Meyer children’s university hospital from 2009 to 2018. Vaccines 2020, 8, 51. [Google Scholar] [CrossRef] [Green Version]
- Giambi, C.; Bella, A.; Filia, A.; Del Manso, M.; Nacca, G.; Declich, S.; Rota, M.C.; Di Giacomo, M.; Locuratolo, F.; Cauzillo, G.; et al. Underreporting of congenital rubella in Italy, 2010–2014. Eur. J. Pediatr. 2017, 176, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Pebody, R.G.; Edmunds, W.J.; Conyn-van Spaendonck, M.; Olin, P.; Berbers, G.; Rebiere, I.; Lecoeur, H.; Crovari, P.; Davidkin, I.; Gabutti, G.; et al. The seroepidemiology of rubella in Western Europe. Epidemiol. Infect. 2000, 125, 347–357. [Google Scholar] [CrossRef]
- Amicizia, D.; Domnich, A.; Gasparini, R.; Bragazzi, N.L.; Lai, P.L.; Panatto, D. An overview of current and potential use of information and communication technologies for immunization promotion among adolescents. Hum. Vaccines Immunother. 2013, 9, 2634–2642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dombkowski, K.J.; Cowan, A.E.; Reeves, S.L.; Foley, M.R.; Dempsey, A.F. The impacts of email reminder/recall on adolescent influenza vaccination. Vaccine 2017, 35, 3089–3095. [Google Scholar] [CrossRef] [PubMed]
- Ministero della Salute. Vaccinazioni dell’età Pediatrica e dell’Adolescenza–Coperture Vaccinali. Available online: http://www.salute.gov.it/portale/documentazione/p6_2_8_3_1.jsp?lingua=italiano&id=20 (accessed on 2 September 2020).

| Anti-Rubella Seroprevalence | ||
|---|---|---|
| Group | Positive % (n/N) | Negative % (n/N) |
| Overall | 95.8 (158/165) | 4.2 (7/165) |
| Male | 96.6 (84/87) | 3.4 (3/87) |
| Female | 94.9 (74/78) | 5.1 (4/78) |
| Italian | 96.0 (143/149) | 4.0 (6/149) |
| Not-Italian | 93.8 (15/16) | 6.2 (1/16) |
| Vaccination Status | Age Group (Years) | Positive % (n/N) | Negative % (n/N) | Total % (n/N) |
|---|---|---|---|---|
| Vaccinated | 98.7 (151/153) | 1.3 (2/153) | 92.7 (153/165) | |
| 1–4 | 94.4 (34/36) | 5.6 (2/36) | 23.5 (36/153) | |
| 5–9 | 100.0 (47/47) | 0.0 (0/47) | 30.7 (47/153) | |
| 10–14 | 100.0 (44/44) | 0.0 (0/44) | 28.8 (44/153) | |
| 15–18 | 100.0 (26/26) | 0.0 (0/26) | 17.0 (26/153) | |
| Unvaccinated | 58.3 (7/12) | 41.7 (5/12) | 7.3 (12/165) | |
| 1–4 | 0.0 (0/4) | 100.0 (4/4) | 33.3 (4/12) | |
| 5–9 | 0.0 (0/1) | 100.0 (1/1) | 8.3 (1/12) | |
| 10–14 | 100.0 (6/6) | 0.0 (0/6) | 50.0 (6/12) | |
| 15–18 | 100.0 (1/1) | 0.0 (0/1) | 8.3 (1/12) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanella, B.; Boccalini, S.; Bonito, B.; Del Riccio, M.; Manzi, F.; Tiscione, E.; Bonanni, P.; Working Group DHS; Working Group AOUMeyer; Working Group AUSLTC; et al. Rubella Seroprevalence Boost in the Pediatric and Adolescent Population of Florence (Italy) as a Preventive Strategy for Congenital Rubella Syndrome (CRS). Vaccines 2020, 8, 599. https://doi.org/10.3390/vaccines8040599
Zanella B, Boccalini S, Bonito B, Del Riccio M, Manzi F, Tiscione E, Bonanni P, Working Group DHS, Working Group AOUMeyer, Working Group AUSLTC, et al. Rubella Seroprevalence Boost in the Pediatric and Adolescent Population of Florence (Italy) as a Preventive Strategy for Congenital Rubella Syndrome (CRS). Vaccines. 2020; 8(4):599. https://doi.org/10.3390/vaccines8040599
Chicago/Turabian StyleZanella, Beatrice, Sara Boccalini, Benedetta Bonito, Marco Del Riccio, Federico Manzi, Emilia Tiscione, Paolo Bonanni, Working Group DHS, Working Group AOUMeyer, Working Group AUSLTC, and et al. 2020. "Rubella Seroprevalence Boost in the Pediatric and Adolescent Population of Florence (Italy) as a Preventive Strategy for Congenital Rubella Syndrome (CRS)" Vaccines 8, no. 4: 599. https://doi.org/10.3390/vaccines8040599
APA StyleZanella, B., Boccalini, S., Bonito, B., Del Riccio, M., Manzi, F., Tiscione, E., Bonanni, P., Working Group DHS, Working Group AOUMeyer, Working Group AUSLTC, & Bechini, A. (2020). Rubella Seroprevalence Boost in the Pediatric and Adolescent Population of Florence (Italy) as a Preventive Strategy for Congenital Rubella Syndrome (CRS). Vaccines, 8(4), 599. https://doi.org/10.3390/vaccines8040599







