A Homologous Bacterin Protects Sheep against Abortion Induced by a Hypervirulent Campylobacter jejuni Clone
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Vaccination
2.3. Intravenous Challenge and Necropsy
2.4. ELISA and Western Blotting
2.5. Statistical Analysis
3. Results
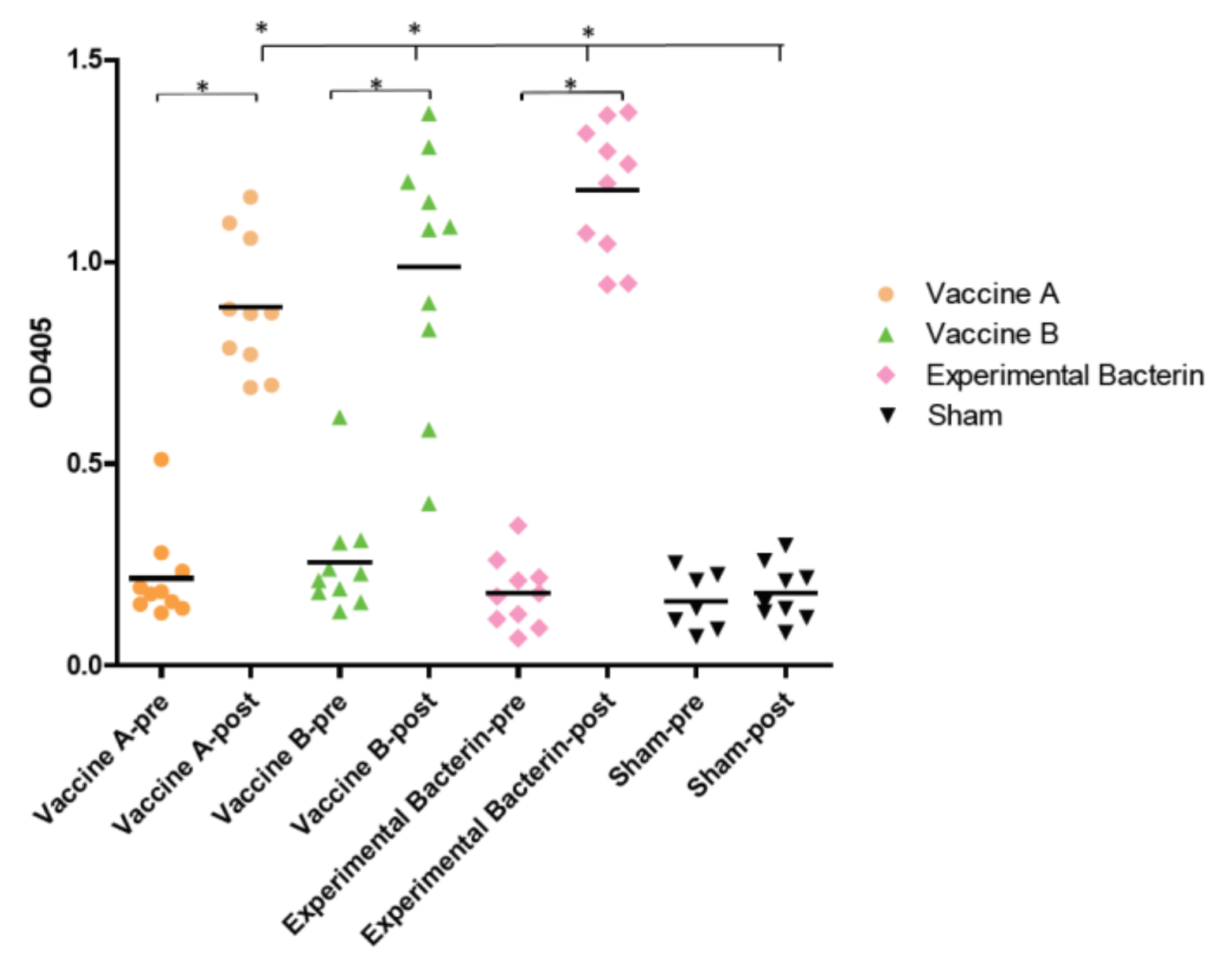
3.1. All Bacterin Vaccines Elicited Antibody Responses against C. jejuni Clone SA
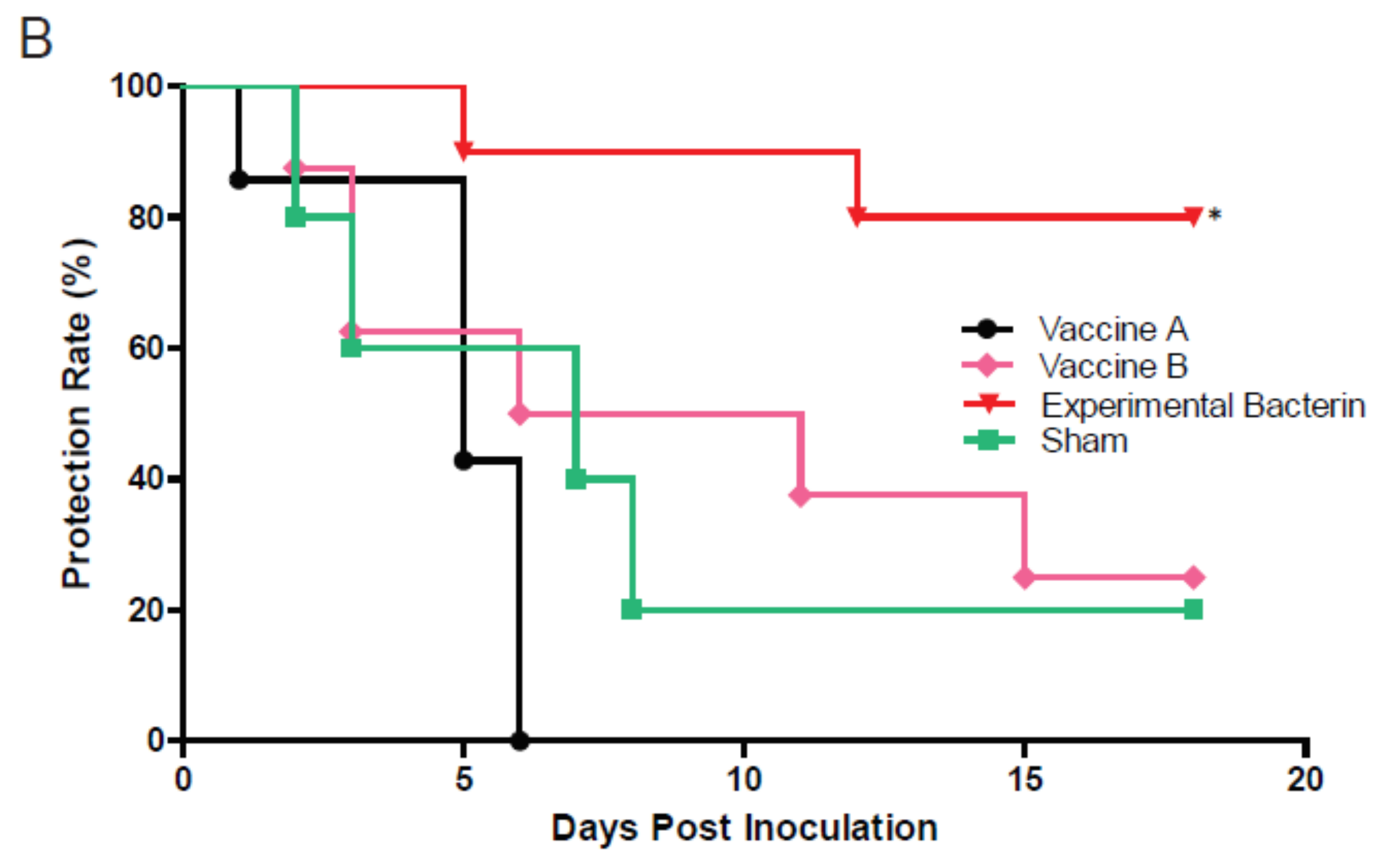
3.2. The Experimental Homologous Bacterin Elicited High Immune Protection against Clone SA-Induced Abortion
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Longbottom, D.; Entrican, G.; Wheelhouse, N.; Brough, H.; Milne, C. Evaluation of the impact and control of enzootic abortion of ewes. Vet. J. 2013, 195, 257–259. [Google Scholar] [CrossRef] [PubMed]
- Menzies, P.I. Control of Important Causes of Infectious Abortion in Sheep and Goats. Vet. Clin. N. Am. Food Anim. Pract. 2011, 27, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Sahin, O.; Yaeger, M.J.; Wu, Z.; Zhang, Q. Campylobacter-Associated Diseases in Animals. Annu. Rev. Anim. Biosci. 2017, 5, 21–42. [Google Scholar] [CrossRef] [PubMed]
- Skirrow, M.B. Diseases due to Campylobacter, Helicobacter and related bacteria. J. Comp. Pathol. 1994, 111, 113–149. [Google Scholar] [CrossRef]
- Lacasta, D.; Ferrer, L.M.; Ramos, J.J.; González, J.M.; Ortín, A.; Fthenakis, G.C. Vaccination schedules in small ruminant farms. Vet. Microbiol. 2015, 181, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Delong, W.J.; Jaworski, M.D.; Ward, A.C. Antigenic and restriction enzyme analysis of Campylobacter spp. associated with abortion in sheep. Am. J. Vet. Res. 1996, 57, 163–167. [Google Scholar] [PubMed]
- Sahin, O.; Plummer, P.J.; Jordan, D.M.; Sulaj, K.; Pereira, S.; Robbe-Austerman, S.; Wang, L.; Yaeger, M.J.; Hoffman, L.J.; Zhang, Q. Emergence of a Tetracycline-Resistant Campylobacter jejuni Clone Associated with Outbreaks of Ovine Abortion in the United States. J. Clin. Microbiol. 2008, 46, 1663–1671. [Google Scholar] [CrossRef]
- Tang, Y.; Meinersmann, R.J.; Sahin, O.; Wu, Z.; Dai, L.; Carlson, J.; Lawrence, J.P.; Genzlinger, L.; LeJeune, J.T.; Zhang, Q.J. Wide but Variable Distribution of a Hypervirulent Campylobacter jejuni Clone in Beef and Dairy Cattle in the United States. Appl. Environ. Microbiol. 2017, 83, e01425-17. [Google Scholar] [CrossRef]
- Hedstrom, O.R.; Sonn, R.J.; Lassen, E.D.; Hultgren, B.D.; Crisman, R.O.; Smith, B.B.; Snyder, S.P. Pathology of Campylobacter jejuni Abortion in Sheep. Vet. Pathol. 1987, 24, 419–426. [Google Scholar] [CrossRef]
- Miller, V.A.; Jensen, R. Experimental immunization against ovine vibriosis. I. The use of live and formalin-killed Vibrio fetus vaccines. Am. J. Vet. Res. 1961, 22, 43–46. [Google Scholar]
- Miller, V.A.; Jensen, R.; Ogg, J.E. Immunization of Sheep against Ovine Vibriosis with Bacterins Containing Serotype I and Serotype V in Mineral Oil. Am. J. Vet. Res. 1964, 25, 664–667. [Google Scholar]
- Storz, J.; Miner, M.L.; Marriott, M.E.; Olson, A.E. Prevention of ovine vibriosis by vaccination: Duration of protective immunity. Am. J. Vet. Res. 1966, 27, 110–114. [Google Scholar] [PubMed]
- Williams, C.E.; Renshaw, H.W.; Meinershagen, W.A.; Everson, D.O.; Chamberlain, R.K.; Hall, R.F.; Waldhalm, D.G. Ovine campylobacterosis: Preliminary studies of the efficacy of the in vitro serum bactericidal test as an assay for the potency of Campylobacter (Vibrio) fetus subsp intestinalis bacterins. Am. J. Vet. Res. 1976, 37, 409–415. [Google Scholar]
- Fenwick, S.G.; West, D.M.; Hunter, J.E.; Sargison, N.D.; Ahmed, F.; Lumsden, J.S.; Collett, M.G. Campylobacter fetus fetusabortions in vaccinated ewes. N. Z. Vet. J. 2000, 48, 155–157. [Google Scholar] [CrossRef]
- Burrough, E.R.; Sahin, O.; Plummer, P.J.; DiVerde, K.D.; Zhang, Q.; Yaeger, M.J. Comparison of two commercial ovine Campylobacter vaccines and an experimental bacterin in guinea pigs inoculated with Campylobacter jejuni. Am. J. Vet. Res. 2011, 72, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Sahin, O.; Dai, L.; Sippy, R.; Wu, Z.; Zhang, Q. Development of a Loop-Mediated Isothermal Amplification Assay for Rapid, Sensitive and Specific Detection of a Campylobacter jejuni Clone. J. Vet. Med. Sci. 2012, 74, 591–596. [Google Scholar] [CrossRef]
- Zhang, Q.J.; Meitzler, J.C.; Huang, S.X.; Morishita, T. Sequence Polymorphism, Predicted Secondary Structures, and Surface-Exposed Conformational Epitopes of Campylobacter Major Outer Membrane Protein. Infect. Immun. 2000, 68, 5679–5689. [Google Scholar] [CrossRef]
- Wu, Z.; Sahin, O.; Wang, F.; Zhang, Q. Proteomic identification of immunodominant membrane-related antigens in Campylobacter jejuni associated with sheep abortion. J. Proteom. 2014, 99, 111–122. [Google Scholar] [CrossRef]
- Burrough, E.R.; Sahin, O.; Plummer, P.J.; Zhang, Q.J.; Yaeger, M.J. Pathogenicity of an emergent, ovine abortifacient Campylobacter jejuni clone orally inoculated into pregnant guinea pigs. Am. J. Vet. Res. 2009, 70, 1269–1276. [Google Scholar] [CrossRef]
- Acik, M.N.; Cetinkaya, B. Heterogeneity of Campylobacter jejuni and Campylobacter coli strains from healthy sheep. Vet. Microbiol. 2006, 115, 370–375. [Google Scholar] [CrossRef]
- Hanlon, K.E.; Miller, M.F.; Guillen, L.M.; Echeverry, A.; Dormedy, E.; Cemo, B.; Branham, L.A.; Sanders, S.; Brashears, M.M. Presence of Salmonella and Escherichia coli O157 on the hide, and presence of Salmonella, Escherichia coli O157 and Campylobacter in feces from small-ruminant (goat and lamb) samples collected in the United States, Bahamas and Mexico. Meat Sci. 2018, 135, 1–5. [Google Scholar] [CrossRef]
- Kilár, A.; Dörnyei, Á.; Kocsis, B. Structural characterization of bacterial lipopolysaccharides with mass spectrometry and on- and off-line separation techniques. Mass Spectrom. Rev. 2013, 32, 90–117. [Google Scholar] [CrossRef] [PubMed]
- Opal, S.M. Endotoxins and Other Sepsis Triggers. Contrib. Nephrol. 2010, 167, 14–24. [Google Scholar] [CrossRef]
- Parker, C.T.; Gilbert, M.; Yuki, N.; Endtz, H.P.; Mandrell, R.E. Characterization of Lipooligosaccharide-Biosynthetic Loci of Campylobacter jejuni Reveals New Lipooligosaccharide Classes: Evidence of Mosaic Organizations. J. Bacteriol. 2008, 190, 5681–5689. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.; Karwaski, M.-F.; Bernatchez, S.; Young, N.M.; Taboada, E.; Michniewicz, J.; Cunningham, A.-M.; Wakarchuk, W.W. The Genetic Bases for the Variation in the Lipo-oligosaccharide of the Mucosal Pathogen, Campylobacter jejuni. Biosynthesis of sialylated ganglioside mimics in the core oligosaccharide. J. Biol. Chem. 2002, 277, 327–337. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Yaeger, M.J.; Sahin, O.; Xu, C.; Beyi, A.F.; Plummer, P.J.; Meral Ocal, M.; Zhang, Q. A Homologous Bacterin Protects Sheep against Abortion Induced by a Hypervirulent Campylobacter jejuni Clone. Vaccines 2020, 8, 662. https://doi.org/10.3390/vaccines8040662
Wu Z, Yaeger MJ, Sahin O, Xu C, Beyi AF, Plummer PJ, Meral Ocal M, Zhang Q. A Homologous Bacterin Protects Sheep against Abortion Induced by a Hypervirulent Campylobacter jejuni Clone. Vaccines. 2020; 8(4):662. https://doi.org/10.3390/vaccines8040662
Chicago/Turabian StyleWu, Zuowei, Michael J. Yaeger, Orhan Sahin, Changyun Xu, Ashenafi F. Beyi, Paul J. Plummer, Melda Meral Ocal, and Qijing Zhang. 2020. "A Homologous Bacterin Protects Sheep against Abortion Induced by a Hypervirulent Campylobacter jejuni Clone" Vaccines 8, no. 4: 662. https://doi.org/10.3390/vaccines8040662
APA StyleWu, Z., Yaeger, M. J., Sahin, O., Xu, C., Beyi, A. F., Plummer, P. J., Meral Ocal, M., & Zhang, Q. (2020). A Homologous Bacterin Protects Sheep against Abortion Induced by a Hypervirulent Campylobacter jejuni Clone. Vaccines, 8(4), 662. https://doi.org/10.3390/vaccines8040662





