The Effects of Imprinting and Repeated Seasonal Influenza Vaccination on Adaptive Immunity after Influenza Vaccination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Assays
2.2.1. Antibody-Secreting Cell ELISpot Assay
2.2.2. Hemagglutination Inhibition (HAI) Assay
2.2.3. Statistical Analysis
3. Results
3.1. Demographics
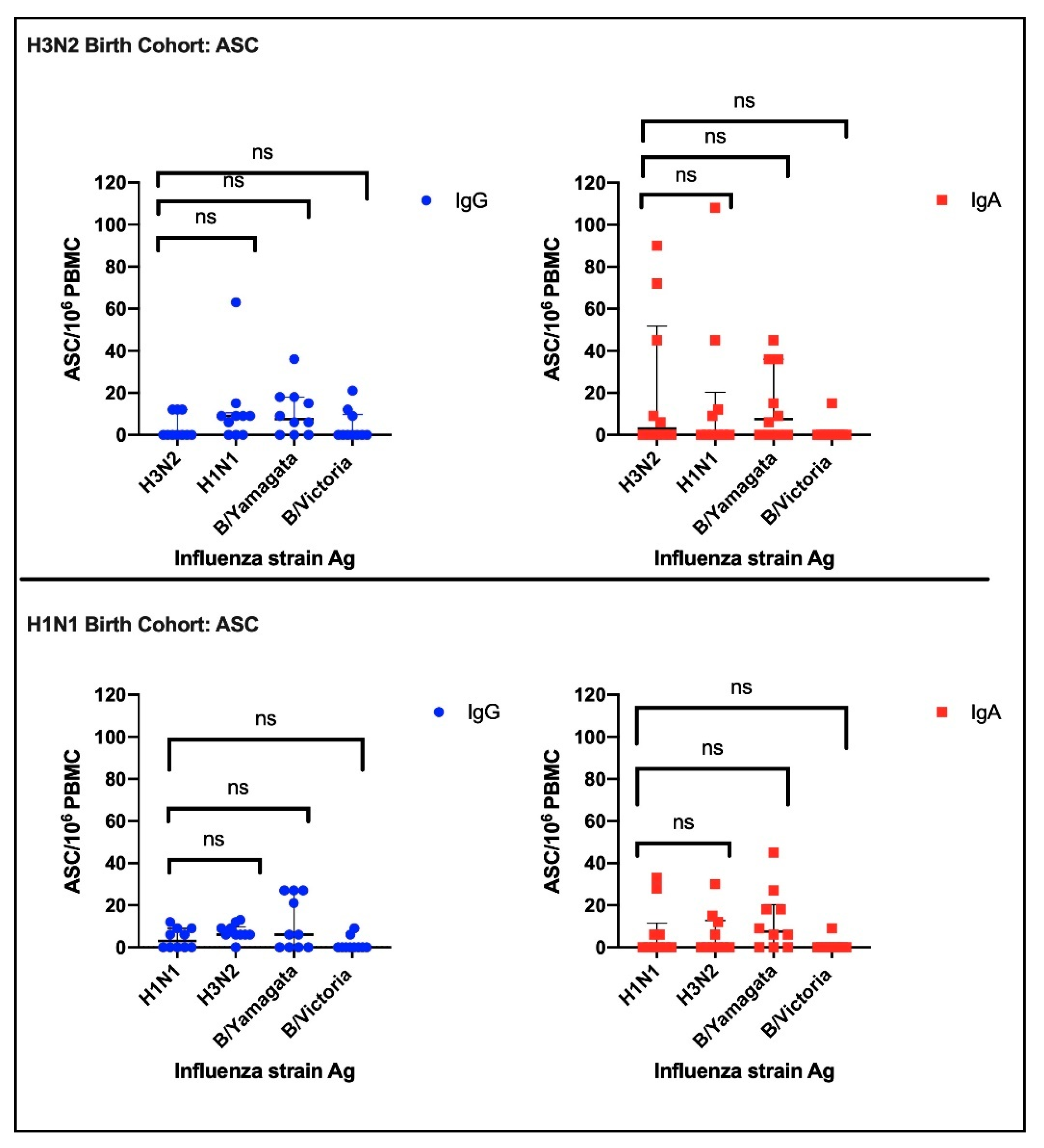
3.2. Imprinting
3.2.1. Antibody Secreting Cells (ASC)
3.2.2. HAI Results
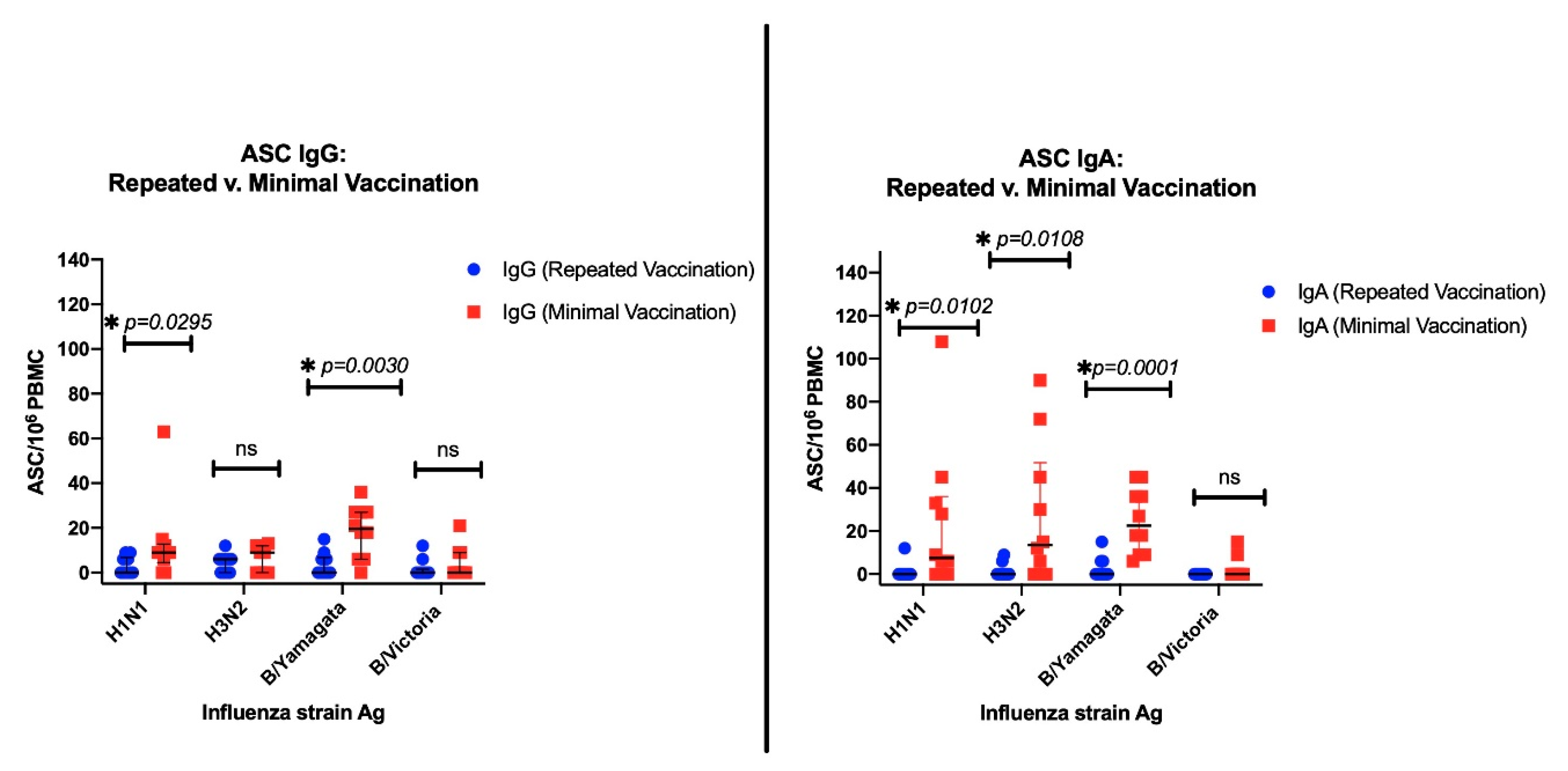
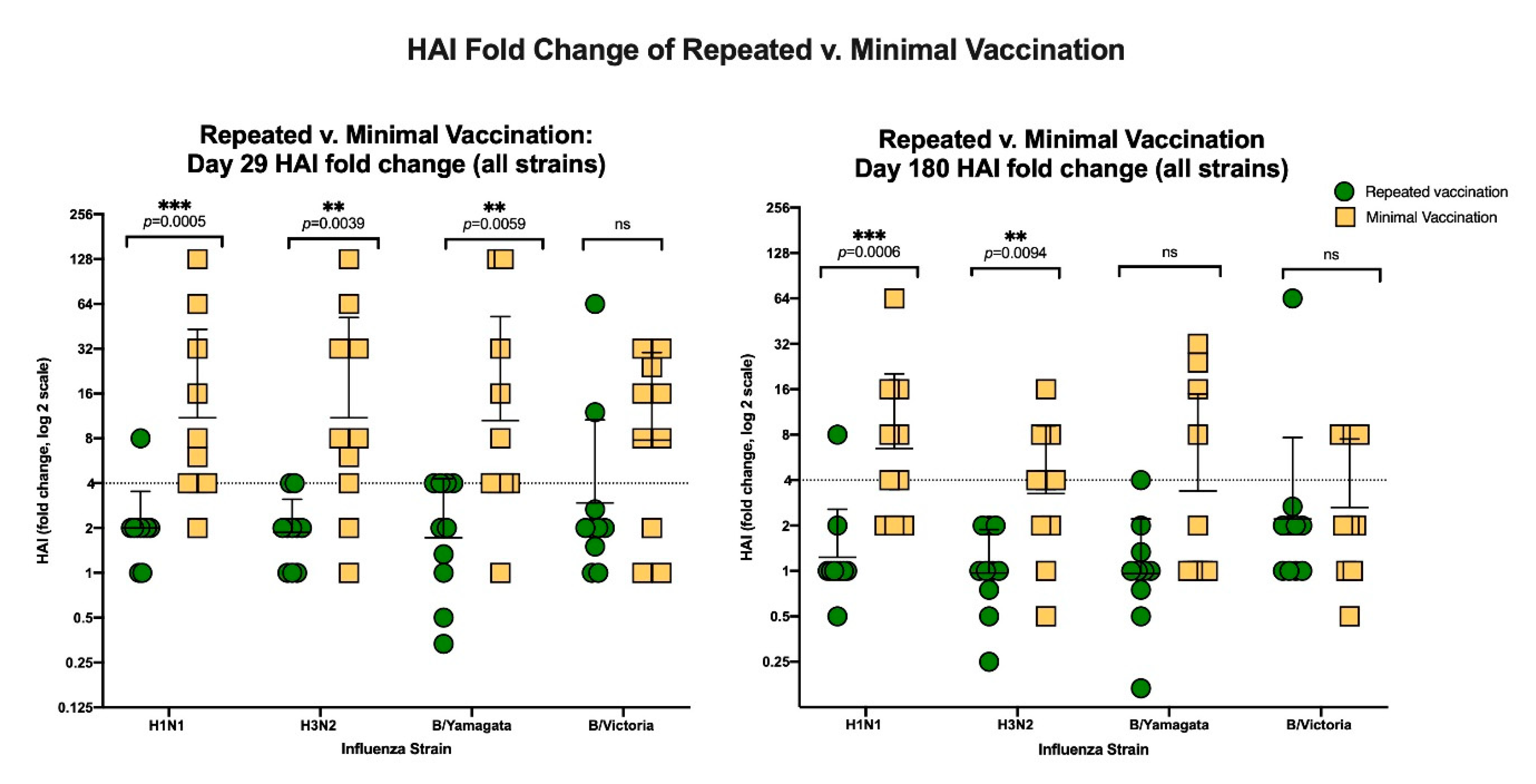
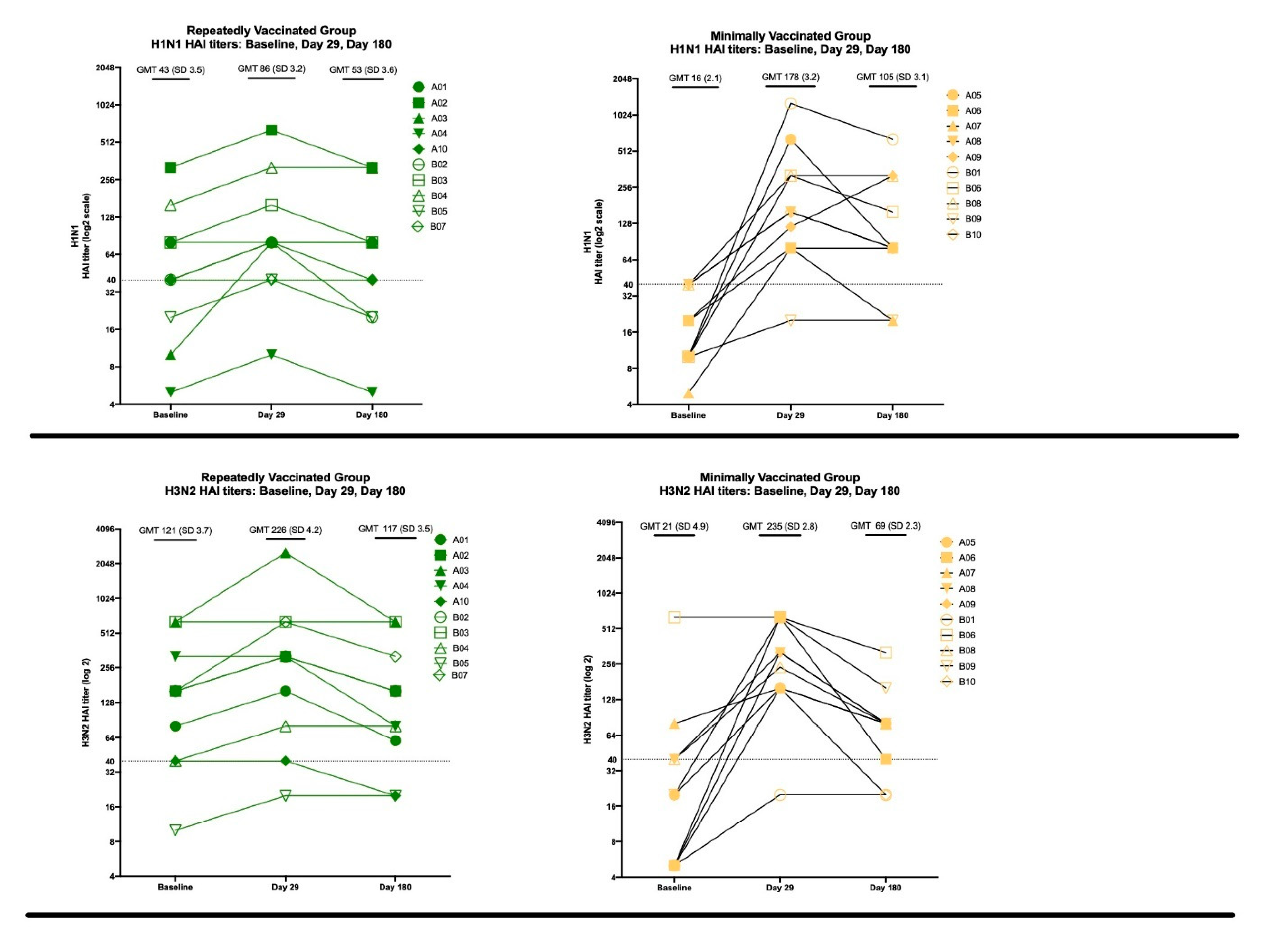
3.3. The Effects of Repeated Vaccination
3.3.1. ASC Results
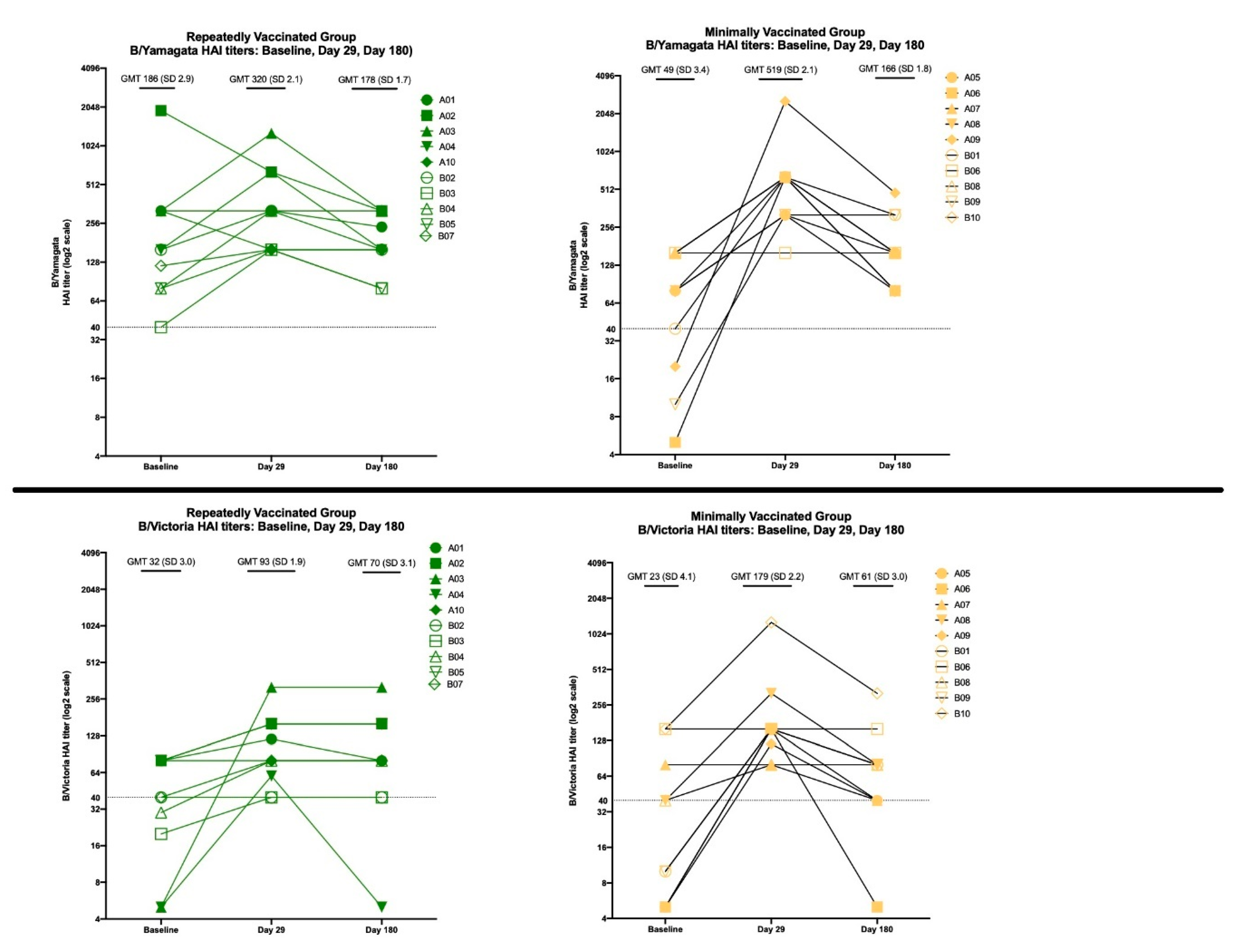
3.3.2. HAI Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- WHO. “WHO | Influenza.” World Health Organization. Available online: http://www.who.int/influenza/en/ (accessed on 25 August 2020).
- “Disease Burden of Influenza” | Seasonal Influenza (Flu) | CDC, National Center for Immunization and Respiratory Diseases (NCIRD). Available online: https://www.cdc.gov/flu/about/disease/burden.htm (accessed on 10 January 2020).
- Francis, T. On the Doctrine of Original Antigenic Sin. Proc. Am Philos Soc. 1960, 104, 572–578. [Google Scholar]
- Nachbagauer, R.; Choi, A.; Hirsh, A.; Margine, I.; Iida, S.; Barrera, A.; Ferres, M.; Albrecht, R.A.; García-Sastre, R.A.A.A.; Bouvier, N.M.; et al. Defining the Antibody Cross-Reactome against the Influenza Virus Surface Glycoproteins. Nat. Immunol. 2017, 18, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Abreu, R.B.; Kirchenbaum, G.A.; Clutter, E.F.; Sautto, G.A.; Ross, T.M. Preexisting Subtype Immunodominance Shapes Memory B Cell Recall Response to Influenza Vaccination. JCI Insight 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Gostic, K.M.; Ambrose, M.; Worobey, M.; Lloyd-Smith, J.O. Potent protection against H5N1 and H7N9 influenza via childhood hemagglutinin imprinting. Science 2016, 354, 722–726. [Google Scholar] [CrossRef] [Green Version]
- Arevalo, P.; McLean, H.Q.; Belongia, E.A.; Cobey, S. Earliest infections predict the age distribution of seasonal influenza A cases. ELife 2020, 9, 50060. [Google Scholar] [CrossRef]
- Gostic, K.M.; Bridge, R.; Brady, S.; Viboud, C.; Worobey, M.; Lloyd-Smith, J.O. Childhood immune imprinting to influenza A shapes birth year-specific risk during seasonal H1N1 and H3N2 epidemics. PLoS Pathog. 2019, 15, e1008109. [Google Scholar] [CrossRef] [Green Version]
- Fonville, J.M.; Wilks, S.H.; James, S.L.; Fox, A.; Ventresca, M.; Aban, M.; Xue, L.; Jones, T.C.; Le, N.M.H.; Pham, Q.T.; et al. Antibody landscapes after influenza virus infection or vaccination. Science 2014, 346, 996–1000. [Google Scholar] [CrossRef] [Green Version]
- Jhung, M.A.; Swerdlow, D.; Olsen, S.J.; Jernigan, D.; Biggerstaff, M.; Kamimoto, L.; Kniss, K.; Reed, C.; Fry, A.; Brammer, L.; et al. Epidemiology of 2009 Pandemic Influenza A (H1N1) in the United States. Clin. Infect. Dis. 2011, 52, S13–S26. [Google Scholar] [CrossRef]
- McCullers, J.A.; Van De Velde, L.-A.; Allison, K.J.; Branum, K.C.; Webby, R.J.; Flynn, P. Recipients of Vaccine against the 1976 “Swine Flu” Have Enhanced Neutralization Responses to the 2009 Novel H1N1 Influenza Virus. Clin. Infect. Dis. 2010, 50, 1487–1492. [Google Scholar] [CrossRef]
- Plant, E.P.; Eick-Cost, A.A.; Ezzeldin, H.; Sanchez, J.L.; Ye, Z.; Cooper, M.J. The Effects of Birth Year, Age and Sex on Hemagglutination Inhibition Antibody Responses to Influenza Vaccination. Vaccines 2018, 6, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Couch, R.B.; Atmar, R.L.; Franco, L.M.; Quarles, J.M.; Niño, D.; Wells, J.M.; Arden, N.; Cheung, S.; Belmont, J.W. Prior Infections With Seasonal Influenza A/H1N1 Virus Reduced the Illness Severity and Epidemic Intensity of Pandemic H1N1 Influenza in Healthy Adults. Clin. Infect. Dis. 2011, 54, 311–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flannery, B.; Smith, C.; Garten, R.; Levine, M.Z.; Chung, J.R.; Jackson, M.L.; Jackson, L.A.; Monto, A.S.; Martin, E.T.; Belongia, E.A.; et al. Influence of Birth Cohort on Effectiveness of 2015–2016 Influenza Vaccine Against Medically Attended Illness Due to 2009 Pandemic Influenza A(H1N1) Virus in the United States. J. Infect. Dis. 2018, 218, 189–196. [Google Scholar] [CrossRef] [Green Version]
- Skowronski, D.M.; Chambers, C.; Sabaiduc, S.; De Serres, G.; Winter, A.-L.; Dickinson, J.A.; Gubbay, J.B.; Drews, S.J.; Martineau, C.; Charest, H.; et al. Beyond Antigenic Match: Possible Agent-Host and Immuno-epidemiological Influences on Influenza Vaccine Effectiveness During the 2015–2016 Season in Canada. J. Infect. Dis. 2017, 216, 1487–1500. [Google Scholar] [CrossRef] [Green Version]
- Gagnon, A.; Acosta, E.; Hallman, S.; Bourbeau, R.; Dillon, L.Y.; Ouellette, N.; Earn, D.J.D.; Herring, D.A.; Inwood, K.; Madrenas, J.; et al. Pandemic Paradox: Early Life H2N2 Pandemic Influenza Infection Enhanced Susceptibility to Death during the 2009 H1N1 Pandemic. MBio 2018, 9, e02091-17. [Google Scholar] [CrossRef] [Green Version]
- Hoskins, T.; Davies, J.; Smith, A.; Miller, C.; Allchin, A. Assessment of Inactivated Influenza-A Vaccine after Three Outbreaks of Influenza a at Christ’s Hospital. Lancet 1979, 313, 33–35. [Google Scholar] [CrossRef]
- McLean, H.Q.; Thompson, M.G.; Sundaram, M.E.; Meece, J.K.; McClure, D.L.; Friedrich, T.C.; Belongia, E.A. Impact of Repeated Vaccination on Vaccine Effectiveness Against Influenza A(H3N2) and B During 8 Seasons. Clin. Infect. Dis. 2014, 59, 1375–1385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skowronski, D.M.; Chambers, C.; Sabaiduc, S.; De Serres, G.; Winter, A.-L.; Dickinson, J.A.; Krajden, M.; Gubbay, J.B.; Drews, S.J.; Martineau, C.; et al. A Perfect Storm: Impact of Genomic Variation and Serial Vaccination on Low Influenza Vaccine Effectiveness During the 2014–2015 Season. Clin. Infect. Dis. 2016, 63, 21–32. [Google Scholar] [CrossRef]
- Ohmit, S.E.; Thompson, M.G.; Petrie, J.G.; Thaker, S.N.; Jackson, M.L.; Belongia, E.A.; Zimmerman, R.K.; Gaglani, M.; Lamerato, L.; Spencer, S.M.; et al. Influenza Vaccine Effectiveness in the 2011–2012 Season: Protection Against Each Circulating Virus and the Effect of Prior Vaccination on Estimates. Clin. Infect. Dis. 2013, 58, 319–327. [Google Scholar] [CrossRef]
- Petrie, J.G.; Malosh, R.E.; Cheng, C.K.; Ohmit, S.E.; Martin, E.T.; Johnson, E.; Truscon, R.; Eichelberger, M.C.; Gubareva, L.V.; Fry, A.M.; et al. The Household Influenza Vaccine Effectiveness Study: Lack of Antibody Response and Protection Following Receipt of 2014–2015 Influenza Vaccine. Clin. Infect. Dis. 2017, 65, 1644–1651. [Google Scholar]
- Ohmit, S.E.; Petrie, J.G.; Malosh, R.E.; Fry, A.M.; Thompson, M.G.; Monto, A.S. Influenza vaccine effectiveness in households with children during the 2012–2013 season: Assessments of prior vaccination and serologic susceptibility. J. Infect. Dis. 2015, 211, 1519–1528. [Google Scholar]
- Ohmit, S.E.; Petrie, J.G.; Malosh, R.E.; Johnson, E.; Truscon, R.; Aaron, B.; Martens, C.; Cheng, C.; Fry, A.M.; Monto, A.S. Substantial influenza vaccine effectiveness in households with children during the 2013–2014 influenza season, when 2009 pandemic influenza a(H1N1) virus predominated. J. Infect. Dis. 2016, 213, 1229–1236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keitel, W.A.; Cate, T.R.; Couch, R.B.; Huggins, L.L.; Hess, K.R. Efficacy of repeated annual immunization with inactivated influenza virus vaccines over a five year period. Vaccine 1997, 15, 1114–1122. [Google Scholar] [CrossRef]
- Ahmed, A.; Nicholson, K.; Nguyen-Van-Tam, J.; Nguyen-Van-Tam, J.S. Reduction in mortality associated with influenza vaccine during 1989-90 epidemic. Lancet 1995, 346, 591–595. [Google Scholar] [CrossRef]
- Bartoszko, J.J.; McNamara, I.F.; Aras, O.A.; Hylton, D.A.; Zhang, Y.B.; Malhotra, D.; Hyett, S.L.; Morassut, R.E.; Rudziak, P.; Loeb, M. Does consecutive influenza vaccination reduce protection against influenza: A systematic review and meta-analysis. Vaccine 2018, 36, 3434–3444. [Google Scholar] [CrossRef]
- Zelner, J.; Petrie, J.G.; Trangucci, R.; Martin, E.T.; Monto, A.S. Effects of Sequential Influenza A(H1N1)pdm09 Vaccination on Antibody Waning. J. Infect. Dis. 2019. [Google Scholar] [CrossRef]
- International Reagent Resource (IRR). Available online: https://www.internationalreagentresource.org/ (accessed on 7 November 2020).
- World Health Organization. Manual for the Laboratory Diagnosis and Virological Surveillance of Influenza; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Rouphael, N.G.; Paine, M.; Mosley, R.; Henry, S.; McAllister, D.V.; Kalluri, H.; Pewin, W.; Frew, P.M.; Yu, T.; Thornburg, N.J.; et al. The safety, immunogenicity, and acceptability of inactivated influenza vaccine delivered by microneedle patch (TIV-MNP 2015): A randomised, partly blinded, placebo-controlled, phase 1 trial. Lancet 2017, 390, 649–658. [Google Scholar] [CrossRef]
- Linderman, S.L.; Hensley, S.E. Antibodies with ‘Original Antigenic Sin’ Properties Are Valuable Components of Secondary Immune Responses to Influenza Viruses. PLoS Pathog. 2016, 12, e1005806. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Davis, W.G.; Sambhara, S.; Jacob, J. Strategies to alleviate original antigenic sin responses to influenza viruses. Proc. Natl. Acad. Sci. USA 2012, 109, 13751–13756. [Google Scholar] [CrossRef] [Green Version]
- Immunogenicity, Efficacy, and Effectiveness of Influenza Vaccines. Available online: https://www.cdc.gov/flu/professionals/acip/immunogenicity.htm (accessed on 13 September 2019).
- Nelson, S.A.; Sant, A.J. Imprinting and Editing of the Human CD4 T Cell Response to Influenza Virus. Front. Immunol. 2019, 10, 932. [Google Scholar] [CrossRef] [Green Version]
- Petrie, J.G.; Ohmit, S.E.; Johnson, E.; Cross, R.T.; Monto, A.S. Efficacy Studies of Influenza Vaccines: Effect of End Points Used and Characteristics of Vaccine Failures. J. Infect. Dis. 2011, 203, 1309–1315. [Google Scholar] [CrossRef] [PubMed]
- Ellebedy, A.H. Immunizing the Immune: Can We Overcome Influenza’s Most Formidable Challenge? Vaccines 2018, 6, 68. [Google Scholar] [CrossRef] [Green Version]
- Khurana, S.; Hahn, M.; Coyle, E.M.; King, L.R.; Lin, T.-L.; Treanor, J.; Sant, A.; Golding, H. Repeat vaccination reduces antibody affinity maturation across different influenza vaccine platforms in humans. Nat. Commun. 2019, 10, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanyal, M.; Holmes, T.H.; Maecker, H.T.; Albrecht, R.A.; Dekker, C.L.; He, X.-S.; Greenberg, H.B. Diminished B-Cell Response After Repeat Influenza Vaccination. J. Infect. Dis. 2019, 219, 1586–1595. [Google Scholar] [CrossRef]
- Kräutler, N.J.; Suan, D.; Butt, D.; Bourne, K.; Hermes, J.R.; Chan, T.D.; Sundling, C.; Kaplan, W.; Schofield, P.; Jackson, J.; et al. Differentiation of germinal center B cells into plasma cells is initiated by high-affinity antigen and completed by Tfh cells. J. Exp. Med. 2017, 214, 1259–1267. [Google Scholar] [CrossRef] [Green Version]

| Influenza A(H1N1) | Influenza A(H3N2) | Influenza B (Victoria Lineage) | Influenza B (Yamagata Lineage) |
|---|---|---|---|
| A/Michigan/45/2015-like virus | A/Singapore/INFIMH-16-0019/2016-like virus | B/Colorado/06/2017-like virus | B/Phuket/3073/2013-like virus |
| H3N2 Birth Cohort, Born between 1968 and 1977 (n = 10) | H1N1 Birth Cohort, Born between 1948 and 1957 (n = 10) | |
|---|---|---|
| Mean Age (SD) | 43.3 (2.3) | 65.5 (2.1) |
| Mean Birth Year (range) | 1974 (1968–1977) | 1953 (1949–1955) |
| Female sex—no. (%) | 3 (30) | 6 (60) |
| Minimally Vaccinated—no. (%) | 5 (50) | 5 (50) |
| Repeatedly Vaccinated—no. (%) | 5 (50) | 5 (50) |
| Race or Ethnic Group—no. (%) | ||
| White | 5 (50) | 6 (60) |
| Black or African American | 1 (10) | 3 (30) |
| Asian | 2 (20) | 0 (0) |
| More than one race | 1 (10) | 0 (0) |
| Choose not to report | 1 (10) | 1 (10) |
| Hispanic | 0 (0) | 0 (0) |
| Mean BMI (SD) | 26.53 (4.4) | 25.97 (5.2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sherman, A.C.; Lai, L.; Bower, M.; Natrajan, M.S.; Huerta, C.; Karmali, V.; Kleinhenz, J.; Xu, Y.; Rouphael, N.; Mulligan, M.J. The Effects of Imprinting and Repeated Seasonal Influenza Vaccination on Adaptive Immunity after Influenza Vaccination. Vaccines 2020, 8, 663. https://doi.org/10.3390/vaccines8040663
Sherman AC, Lai L, Bower M, Natrajan MS, Huerta C, Karmali V, Kleinhenz J, Xu Y, Rouphael N, Mulligan MJ. The Effects of Imprinting and Repeated Seasonal Influenza Vaccination on Adaptive Immunity after Influenza Vaccination. Vaccines. 2020; 8(4):663. https://doi.org/10.3390/vaccines8040663
Chicago/Turabian StyleSherman, Amy C., Lilin Lai, Mary Bower, Muktha S. Natrajan, Christopher Huerta, Vinit Karmali, Jennifer Kleinhenz, Yongxian Xu, Nadine Rouphael, and Mark J. Mulligan. 2020. "The Effects of Imprinting and Repeated Seasonal Influenza Vaccination on Adaptive Immunity after Influenza Vaccination" Vaccines 8, no. 4: 663. https://doi.org/10.3390/vaccines8040663
APA StyleSherman, A. C., Lai, L., Bower, M., Natrajan, M. S., Huerta, C., Karmali, V., Kleinhenz, J., Xu, Y., Rouphael, N., & Mulligan, M. J. (2020). The Effects of Imprinting and Repeated Seasonal Influenza Vaccination on Adaptive Immunity after Influenza Vaccination. Vaccines, 8(4), 663. https://doi.org/10.3390/vaccines8040663





