Modulation Effects of Toxoplasma gondii Histone H2A1 on Murine Macrophages and Encapsulation with Polymer as a Vaccine Candidate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statements
2.2. Animals and Parasites
2.3. Cell Isolation and Culture
2.4. Construction of the Prokaryotic Expression Plasmid
2.5. Expression and Purification of TgH2A1 Recombinant Protein
2.6. Western Blot Analysis of rTgH2A1 and Native TgH2A1
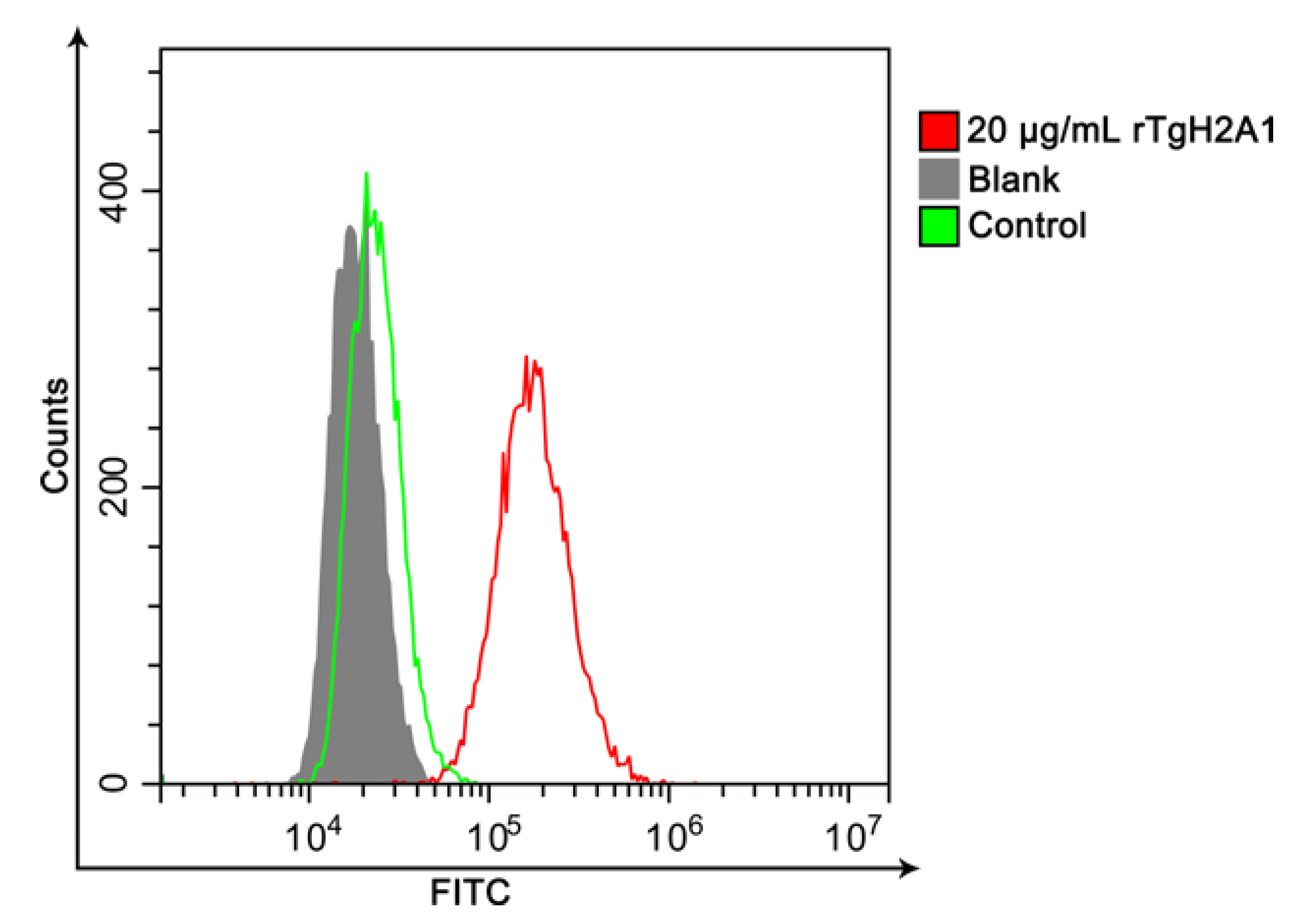
2.7. Verification of rTgH2A1 Binding to Murine Macrophages
2.8. Detection of Cell Proliferation
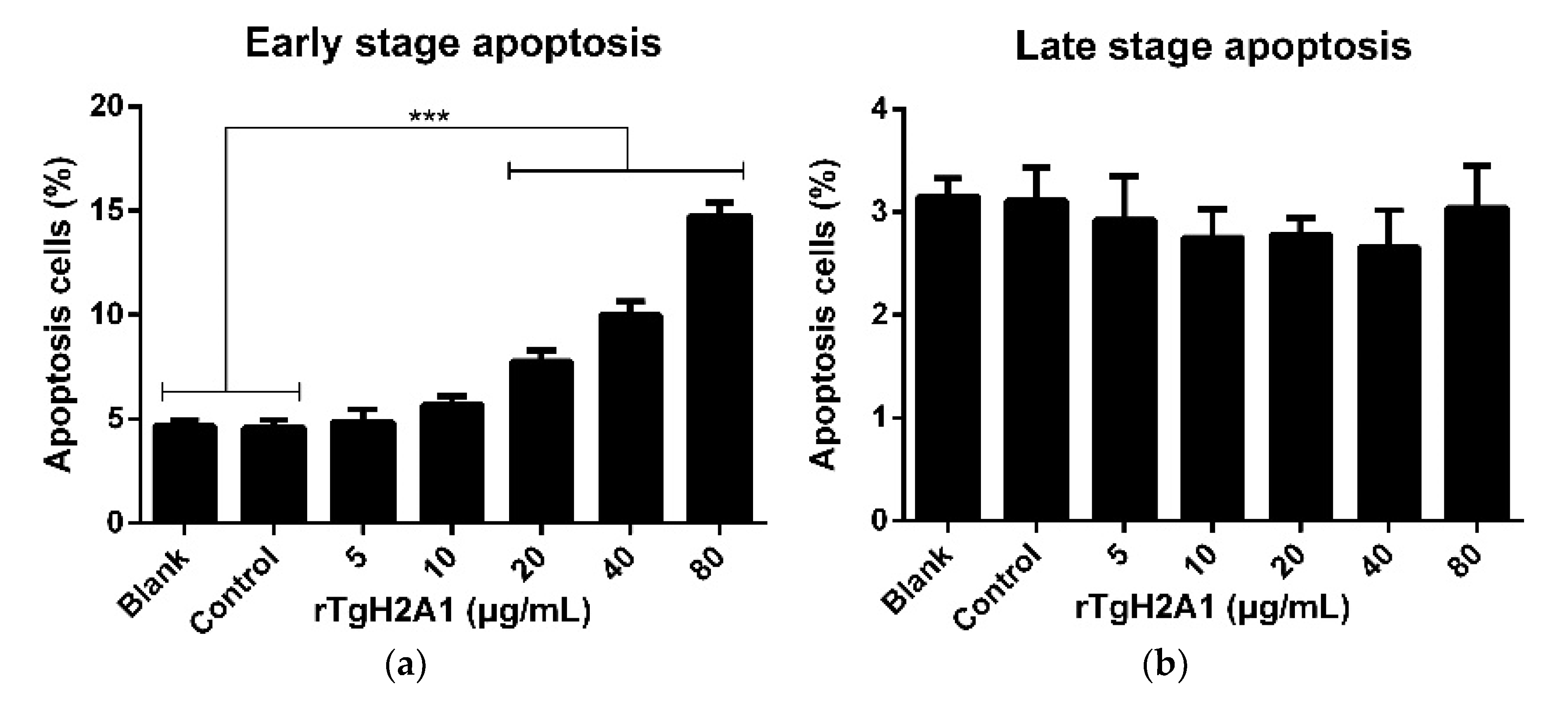
2.9. Cell Apoptosis Assay
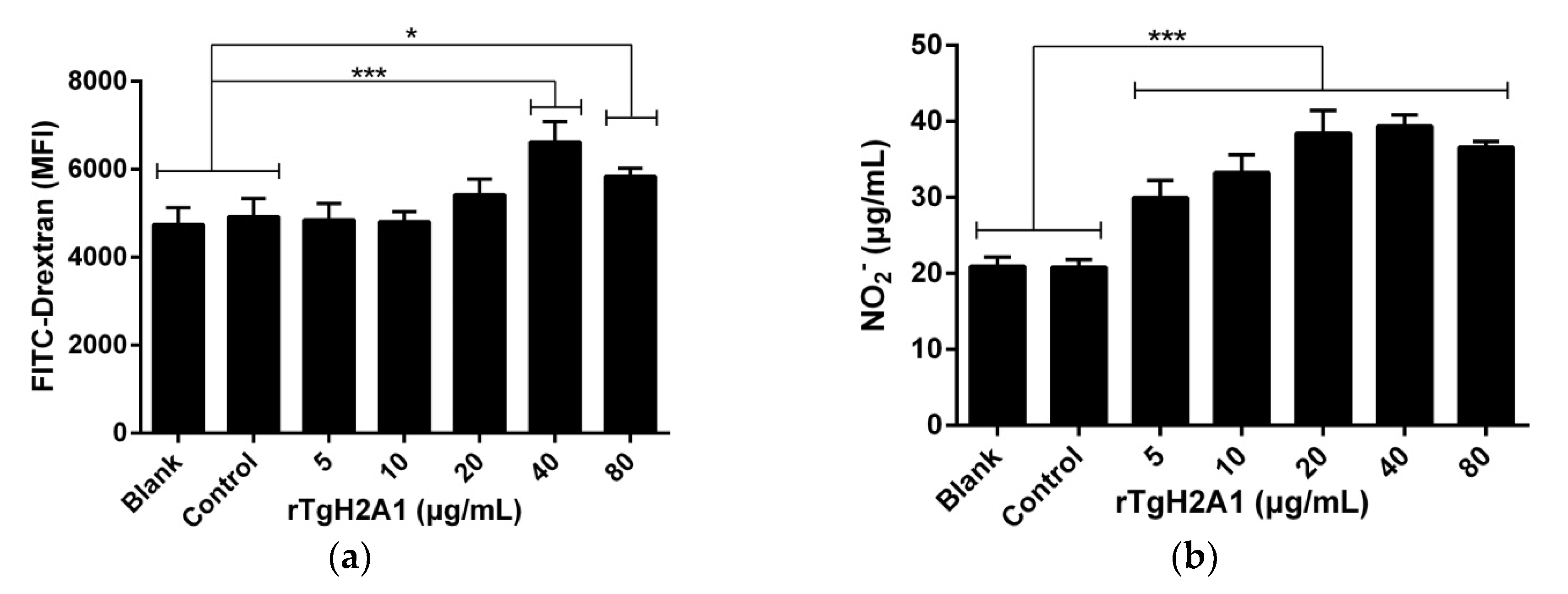
2.10. Internalization of FITC-Dextran
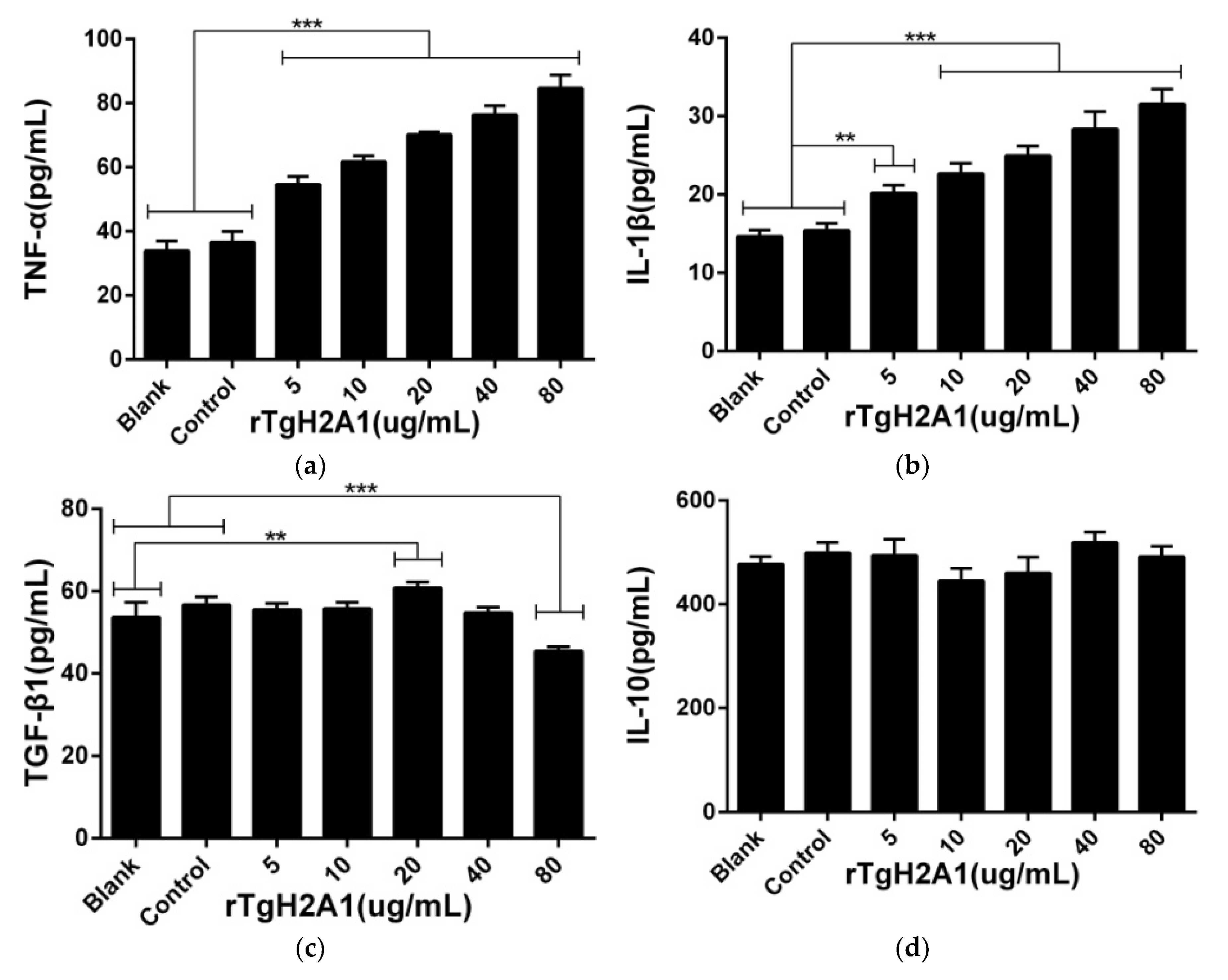
2.11. Detection of NO and Cytokines Concentration in Cell Supernatants
2.12. Fabrication of PLGA Nanoparticles
2.13. Preparation of Chitosan Microspheres
2.14. Encapsulation Efficiency and Physical Characterization of Nanoparticles
2.15. Mice Immunization and Challenge
2.16. Determination of Antibodies and Cytokines
2.17. Statistical Analysis
3. Results
3.1. Restriction Analysis of pET32a/H2A1
3.2. Western Blot Analysis of Purified rTgH2A1
3.3. Confirmation of the Combination of rTgH2A1 with Murine Macrophages
3.4. rTgH2A1 Promoted the Proliferation of Murine Macrophages and Induced Apoptosis
3.5. rTgH2A1 Increased Murine Macrophages in Phagocytosis and NO Secretion
3.6. rTgH2A1 Modulates the Cytokines of Murine Macrophages
3.7. Physical Characterization and Encapsulation Efficiency of Nanoparticles
3.8. Humoral Response and Cytokine Production
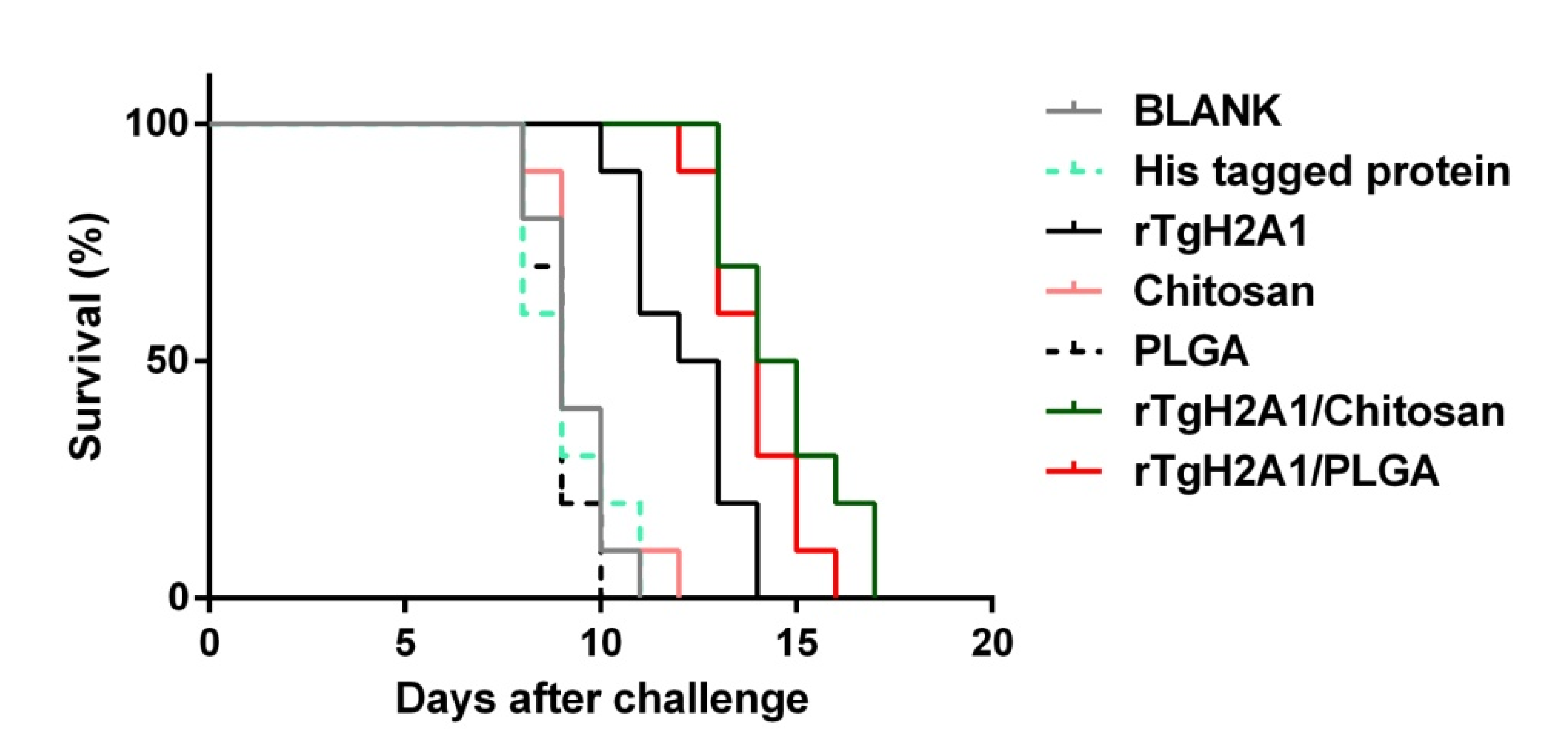
3.9. Microsphere Immunization against Acute Toxoplasmosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Halonen, S.K.; Weiss, L.M. Toxoplasmosis. Handb. Clin. Neurol. 2013, 114, 125–145. [Google Scholar] [CrossRef] [Green Version]
- Montoya, J.G.; Liesenfeld, O. Toxoplasmosis. Lancet 2004, 363, 1965–1976. [Google Scholar] [CrossRef]
- Lewis, J.M.; Clifford, S.; Nsutebu, E. Toxoplasmosis in immunosuppressed patients. Rheumatology (Oxford) 2015, 54, 1939–1940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barakat, A.M.A.; Ahmed, S.O.; Zaki, M.S.; El Fadaly, H.A.; Abd El-Razik, K.A.; El-Hariri, H.M.; Johar, D. New approach to differentiate primary from latent Toxoplasma gondii abortion through immunoglobulin and DNA interpretation. Microb. Pathog. 2018, 125, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Blader, I.J.; Coleman, B.I.; Chen, C.T.; Gubbels, M.J. Lytic Cycle of Toxoplasma gondii: 15 Years Later. Annu. Rev. Microbiol. 2015, 69, 463–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubey, J.P. The History of Toxoplasma gondii-The First 100 Years. J. Eukaryot. Microbiol. 2008, 55, 467–475. [Google Scholar] [CrossRef]
- Marchioro, A.A.; Tiyo, B.T.; Colli, C.M.; de Souza, C.Z.; Garcia, J.L.; Gomes, M.L.; Falavigna-Guilherme, A.L. First Detection of Toxoplasma gondii DNA in the Fresh Leafs of Vegetables in South America. Vector-Borne Zoonotic Dis. 2016, 16, 624–626. [Google Scholar] [CrossRef]
- Hernandez-Cortazar, I.B.; Acosta-Viana, K.Y.; Guzman-Marin, E.; Ortega-Pacheco, A.; Segura-Correa, J.C.; Jimenez-Coello, M. Presence of Toxoplasma gondii in Drinking Water from an Endemic Region in Southern Mexico. Foodborne Pathog. Dis. 2017, 14, 288–292. [Google Scholar] [CrossRef]
- Alday, P.H.; Doggett, J.S. Drugs in development for toxoplasmosis: Advances, challenges, and current status. Drug Des. Dev. Ther. 2017, 11, 273–293. [Google Scholar] [CrossRef] [Green Version]
- Henriquez, F.L.; Woods, S.; Cong, H.; McLeod, R.; Roberts, C.W. Immunogenetics of Toxoplasma gondii informs vaccine design. Trends Parasitol. 2010, 26, 550–555. [Google Scholar] [CrossRef]
- Yap, G.S.; Sher, A. Effector cells of both nonhemopoietic and hemopoietic origin are required for interferon (IFN)-gamma- and tumor necrosis factor (TNF)-alpha-dependent host resistance to the intracellular pathogen, Toxoplasma gondii. J. Exp. Med. 1999, 189, 1083–1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, A.G.; Abi Abdallah, D.S.; Butcher, B.A.; Denkers, E.Y. Toxoplasma gondii triggers phosphorylation and nuclear translocation of dendritic cell STAT1 while simultaneously blocking IFNγ-induced STAT1 transcriptional activity. PLoS ONE 2013, 8, e60215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, I.A.; Schwartzman, J.D.; Matsuura, T.; Kasper, L.H. A dichotomous role for nitric oxide during acute Toxoplasma gondii infection in mice. Proc. Natl. Acad. Sci. USA 1997, 94, 13955–13960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, L.B.; Hibbs, J.B., Jr.; Taintor, R.R.; Krahenbuhl, J.L. Microbiostatic effect of murine-activated macrophages for Toxoplasma gondii. Role for synthesis of inorganic nitrogen oxides from L-arginine. J. Immunol. 1990, 144, 2725–2729. [Google Scholar]
- Matta, S.K.; Patten, K.; Wang, Q.; Kim, B.H.; MacMicking, J.D.; Sibley, L.D. NADPH Oxidase and Guanylate Binding Protein 5 Restrict Survival of Avirulent Type III Strains of Toxoplasma gondii in Naive Macrophages. Mbio 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Tenter, A.M.; Heckeroth, A.R.; Weiss, L.M. Toxoplasma gondii: From animals to humans. Int. J. Parasitol. 2000, 30, 1217–1258. [Google Scholar] [CrossRef] [Green Version]
- Aliberti, J.; Bafica, A. Anti-inflammatory pathways as a host evasion mechanism for pathogens. Prostaglandins Leukot. Essent. Fat. Acids 2005, 73, 283–288. [Google Scholar] [CrossRef]
- Stafford, J.L.; Neumann, N.F.; Belosevic, M. Macrophage-mediated innate host defense against protozoan parasites. Crit. Rev. Microbiol. 2002, 28, 187–248. [Google Scholar] [CrossRef]
- Labonte, A.C.; Tosello-Trampont, A.C.; Hahn, Y.S. The Role of Macrophage Polarization in Infectious and Inflammatory Diseases. Mol. Cells 2014, 37, 275–285. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.C.; Zou, X.B.; Chai, Y.F.; Yao, Y.M. Macrophage polarization in inflammatory diseases. Int. J. Biol. Sci. 2014, 10, 520–529. [Google Scholar] [CrossRef]
- Terrazas, C.A.; Juarez, I.; Terrazas, L.I.; Saavedra, R.; Calleja, E.A.; Rodriguez-Sosa, M. Toxoplasma gondii: Impaired maturation and pro-inflammatory response of dendritic cells in MIF-deficient mice favors susceptibility to infection. Exp. Parasitol. 2010, 126, 348–358. [Google Scholar] [CrossRef]
- Matowicka-Karna, J.; Dymicka-Piekarska, V.; Kemona, H. Does Toxoplasma gondii infection affect the levels of IgE and cytokines (IL-5, IL-6, IL-10, IL-12, and TNF-alpha)? Clin. Dev. Immunol. 2009, 2009, 374696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambarus, C.A.; Noordenbos, T.; de Hair, M.J.; Tak, P.P.; Baeten, D.L. Intimal lining layer macrophages but not synovial sublining macrophages display an IL-10 polarized-like phenotype in chronic synovitis. Arthritis Res. Ther. 2012, 14, R74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schraufstatter, I.U.; Zhao, M.; Khaldoyanidi, S.K.; DiScipio, R.G. The chemokine CCL18 causes maturation of cultured monocytes to macrophages in the M2 spectrum. Immunology 2012, 135, 287–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarique, A.A.; Logan, J.; Thomas, E.; Holt, P.G.; Sly, P.D.; Fantino, E. Phenotypic, Functional, and Plasticity Features of Classical and Alternatively Activated Human Macrophages. Am. J. Respir. Cell Mol. 2015, 53, 676–688. [Google Scholar] [CrossRef]
- Vogel, D.Y.S.; Glim, J.E.; Stavenuiter, A.W.D.; Breur, M.; Heijnen, P.; Amor, S.; Dijkstra, C.D.; Beelen, R.H.J. Human macrophage polarization in vitro: Maturation and activation methods compared. Immunobiology 2014, 219, 695–703. [Google Scholar] [CrossRef]
- Grencis, R.K.; Humphreys, N.E.; Bancroft, A.J. Immunity to gastrointestinal nematodes: Mechanisms and myths. Immunol. Rev. 2014, 260, 183–205. [Google Scholar] [CrossRef] [Green Version]
- Lebman, D.A.; Edmiston, J.S. The role of TGF-beta in growth, differentiation, and maturation of B lymphocytes. Microbes Infect. 1999, 1, 1297–1304. [Google Scholar] [CrossRef]
- Khorasanizadeh, S. The nucleosome: From genomic organization to genomic regulation. Cell 2004, 116, 259–272. [Google Scholar] [CrossRef] [Green Version]
- Angelov, D.; Lenouvel, F.; Hans, F.; Muller, C.W.; Bouvet, P.; Bednar, J.; Moudrianakis, E.N.; Cadet, J.; Dimitrov, S. The histone octamer is invisible when NF-kappaB binds to the nucleosome. J. Biol. Chem. 2004, 279, 42374–42382. [Google Scholar] [CrossRef] [Green Version]
- Vanagas, L.; Jeffers, V.; Bogado, S.S.; Dalmasso, M.C.; Sullivan, W.J.; Angel, S.O. Toxoplasma histone acetylation remodelers as novel drug targets. Expert Rev. Anti-Infect. Ther. 2012, 10, 1189–1201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalmasso, M.C.; Onyango, D.O.; Naguleswaran, A.; Sullivan, W.J.; Angel, S.O. Toxoplasma H2A Variants Reveal Novel Insights into Nucleosome Composition and Functions for this Histone Family. J. Mol. Biol. 2009, 392, 33–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Downs, J.A.; Lowndes, N.F.; Jackson, S.P. A role for Saccharomyces cerevisiae histone H2A in DNA repair. Nature 2000, 408, 1001–1004. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.W.; Shen, B.; Xie, Q.; Xu, L.X.; Yan, R.F.; Song, X.K.; Hassan Ibrahim, A.; Li, X.R. Isolation and Molecular Characterization of Toxoplasma gondii from Chickens in China. J. Integr. Agric. 2012, 11, 1347–1353. [Google Scholar] [CrossRef]
- Blasi, E.; Mathieson, B.J.; Varesio, L.; Cleveland, J.L.; Borchert, P.A.; Rapp, U.R. Selective immortalization of murine macrophages from fresh bone marrow by a raf/myc recombinant murine retrovirus. Nature 1985, 318, 667–670. [Google Scholar] [CrossRef]
- Hassan, I.A.; Wang, S.; Xu, L.X.; Yan, R.F.; Song, X.K.; Li, X.R. Immunoglobulin and cytokine changes induced following immunization with a DNA vaccine encoding Toxoplasma gondii selenium-dependent glutathione reductase protein. Exp. Parasitol. 2014, 146, 1–10. [Google Scholar] [CrossRef]
- Liu, X.C.; Li, X.Y.; Wang, Q.Q.; Sun, X.N.; Lu, M.M.; Ehsan, M.; Xu, L.X.; Yan, R.F.; Song, X.K.; Li, X.R. Toxoplasma gondii Histone 4 Affects Some Functions of Murine Ana-1 Macrophages In Vitro. J. Eukaryot. Microbiol. 2018, 65, 860–869. [Google Scholar] [CrossRef] [Green Version]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- McCall, R.L.; Sirianni, R.W. PLGA nanoparticles formed by single- or double-emulsion with vitamin E-TPGS. J. Vis. Exp. 2013, 51015. [Google Scholar] [CrossRef] [Green Version]
- Fan, W.; Yan, W.; Xu, Z.S.; Ni, H. Formation mechanism of monodisperse, low molecular weight chitosan nanoparticles by ionic gelation technique. Colloid Surface B 2012, 90, 21–27. [Google Scholar] [CrossRef]
- Tang, X.M.; Yin, G.W.; Qin, M.; Tao, G.R.; Suo, J.X.; Liu, X.Y.; Suo, X. Transgenic Eimeria tenella as a vaccine vehicle: Expressing TgSAG1 elicits protective immunity against Toxoplasma gondii infections in chickens and mice. Sci. Rep. UK 2016, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syn, G.; Anderson, D.; Blackwell, J.M.; Jamieson, S.E. Epigenetic dysregulation of host gene expression in Toxoplasma infection with specific reference to dopamine and amyloid pathways. Infect. Genet. Evol. 2018, 65, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.; Malik, I.; Hameed, M.; Kuthu, Z.H.; Zhou, J.L. Modifications of histones in parasites as drug targets. Vet. Parasitol. 2020, 278. [Google Scholar] [CrossRef] [PubMed]
- Batugedara, G.; Lu, X.M.; Bunnik, E.M.; Le Roch, K.G. The Role of Chromatin Structure in Gene Regulation of the Human Malaria Parasite. Trends Parasitol. 2017, 33, 364–377. [Google Scholar] [CrossRef] [Green Version]
- Vilcinskas, A. The role of epigenetics in host-parasite coevolution: Lessons from the model host insects Galleria mellonella and Tribolium castaneum. Zoology (Jena) 2016, 119, 273–280. [Google Scholar] [CrossRef]
- Park, J.; Hunter, C.A. The role of macrophages in protective and pathological responses to Toxoplasma gondii. Parasite Immunol. 2020, e12712. [Google Scholar] [CrossRef]
- Biswas, S.K.; Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Z.; Wang, Y.; Gadahi, J.A.; Xie, Q.; Xu, L.; Yan, R.; Song, X.; Li, X. Toxoplasma gondii excretory/secretory antigens (TgESAs) suppress pro-inflammatory cytokine secretion by inhibiting TLR-induced NF-kappaB activation in LPS-stimulated murine macrophages. Oncotarget 2017, 8, 88351–88359. [Google Scholar] [CrossRef] [Green Version]
- Malathi, K.; Dong, B.; Gale, M., Jr.; Silverman, R.H. Small self-RNA generated by RNase L amplifies antiviral innate immunity. Nature 2007, 448, 816–819. [Google Scholar] [CrossRef] [Green Version]
- Stuart, L.M.; Ezekowitz, R.A. Phagocytosis: Elegant complexity. Immunity 2005, 22, 539–550. [Google Scholar] [CrossRef] [Green Version]
- Upham, J.P.; Pickett, D.; Irimura, T.; Anders, E.M.; Reading, P.C. Macrophage receptors for influenza A virus: Role of the macrophage galactose-type lectin and mannose receptor in viral entry. J. Virol. 2010, 84, 3730–3737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peiser, L.; Gough, P.J.; Kodama, T.; Gordon, S. Macrophage class A scavenger receptor-mediated phagocytosis of Escherichia coli: Role of cell heterogeneity, microbial strain, and culture conditions in vitro. Infect. Immun. 2000, 68, 1953–1963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittal, R.; Sukumaran, S.K.; Selvaraj, S.K.; Wooster, D.G.; Babu, M.M.; Schreiber, A.D.; Verbeek, J.S.; Prasadarao, N.V. Fcγ receptor I alpha chain (CD64) expression in macrophages is critical for the onset of meningitis by Escherichia coli K1. PLoS Pathog. 2010, 6, e1001203. [Google Scholar] [CrossRef] [Green Version]
- Atri, C.; Guerfali, F.Z.; Laouini, D. Role of Human Macrophage Polarization in Inflammation during Infectious Diseases. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wandurska-Nowak, E. The role of nitric oxide (NO) in parasitic infections. Wiadomosci Parazytologiczne 2004, 50, 665–678. [Google Scholar] [PubMed]
- Egan, C.E.; Craven, M.D.; Leng, J.; Mack, M.; Simpson, K.W.; Denkers, E.Y. CCR2-dependent intraepithelial lymphocytes mediate inflammatory gut pathology during Toxoplasma gondii infection. Mucosal Immunol. 2009, 2, 527–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterson, P.K.; Gekker, G.; Hu, S.; Chao, C.C. Human astrocytes inhibit intracellular multiplication of Toxoplasma gondii by a nitric oxide-mediated mechanism. J. Infect. Dis. 1995, 171, 516–518. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.R.; Grau, G.E.; Pechère, J.C. Role of TNF and IL-1 in infections with Toxoplasma gondii. Immunology 1990, 69, 33–37. [Google Scholar]
- Roberts, C.W.; Ferguson, D.J.; Jebbari, H.; Satoskar, A.; Bluethmann, H.; Alexander, J. Different roles for interleukin-4 during the course of Toxoplasma gondii infection. Infect. Immun. 1996, 64, 897–904. [Google Scholar] [CrossRef] [Green Version]
- Skwarczynski, M.; Toth, I. Peptide-based synthetic vaccines. Chem Sci 2016, 7, 842–854. [Google Scholar] [CrossRef] [Green Version]
- Reddy, S.T.; van der Vlies, A.J.; Simeoni, E.; Angeli, V.; Randolph, G.J.; O’Neill, C.P.; Lee, L.K.; Swartz, M.A.; Hubbell, J.A. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat. Biotechnol. 2007, 25, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Danesh-Bahreini, M.A.; Shokri, J.; Samiei, A.; Kamali-Sarvestani, E.; Barzegar-Jalali, M.; Mohammadi-Samani, S. Nanovaccine for leishmaniasis: Preparation of chitosan nanoparticles containing Leishmania superoxide dismutase and evaluation of its immunogenicity in BALB/c mice. Int. J. Nanomed. 2011, 6, 835–842. [Google Scholar] [CrossRef] [Green Version]
- Naeem, H.; Sana, M.; Islam, S.; Khan, M.; Riaz, F.; Zafar, Z.; Akbar, H.; Shehzad, W.; Rashid, I. Induction of Th1 type-oriented humoral response through intranasal immunization of mice with SAG1-Toxoplasma gondii polymeric nanospheres. Artif. Cell Nanomed. B 2018, 46, 1025–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.J.; Sun, X.H.; Yin, H.Q.; Wang, T.; Li, Y.; Zhou, C.X.; Zhou, H.Y.; He, S.Y.; Cong, H. Chitosan Microsphere Used as an Effective System to Deliver a Linked Antigenic Peptides Vaccine Protect Mice Against Acute and Chronic Toxoplasmosis. Front. Cell Infect. Microbiol. 2018, 8. [Google Scholar] [CrossRef]
- Pohlmann, A.R.; Fonseca, F.N.; Paese, K.; Detoni, C.B.; Coradini, K.; Beck, R.C.; Guterres, S.S. Poly(-caprolactone) microcapsules and nanocapsules in drug delivery. Expert Opin. Drug Deliv. 2013, 10, 623–638. [Google Scholar] [CrossRef]
- Waeckerle-Men, Y.; Groettrup, M. PLGA microspheres for improved antigen delivery to dendritic cells as cellular vaccines. Adv. Drug Delive. Rev. 2005, 57, 475–482. [Google Scholar] [CrossRef] [Green Version]
- Hamdy, S.; Haddadi, A.; Hung, R.W.; Lavasanifar, A. Targeting dendritic cells with nano-particulate PLGA cancer vaccine formulations. Adv. Drug Deliv. Rev. 2011, 63, 943–955. [Google Scholar] [CrossRef]
- Nabi, H.; Rashid, I.; Ahmad, N.; Durrani, A.; Akbar, H.; Islam, S.; Bajwa, A.A.; Shehzad, W.; Ashraf, K.; Imran, N. Induction of specific humoral immune response in mice immunized with ROP18 nanospheres from Toxoplasma gondii. Parasitol. Res. 2017, 116, 359–370. [Google Scholar] [CrossRef]
- Zhang, N.Z.; Xu, Y.; Wang, M.; Chen, J.; Huang, S.Y.; Gao, Q.; Zhu, X.Q. Vaccination with Toxoplasma gondii calcium-dependent protein kinase 6 and rhoptry protein 18 encapsulated in poly(lactide-co-glycolide) microspheres induces long-term protective immunity in mice. BMC Infect. Dis. 2016, 16, 168. [Google Scholar] [CrossRef] [Green Version]
- Chuang, S.C.; Ko, J.C.; Chen, C.P.; Du, J.T.; Yang, C.D. Induction of long-lasting protective immunity against Toxoplasma gondii in BALB/c mice by recombinant surface antigen 1 protein encapsulated in poly (lactide-co-glycolide) microparticles. Parasite Vector 2013, 6. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Xu, M.X.; Cheng, X.J.; Kong, M.; Liu, Y.; Feng, C.; Chen, X.G. Positive/negative surface charge of chitosan based nanogels and its potential influence on oral insulin delivery. Carbohydr. Polym. 2016, 136, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, N.; Shahbazi, M.A.; Araujo, F.; Zhang, H.B.; Makila, E.M.; Kauppila, J.; Sarmento, B.; Salonen, J.J.; Hirvonen, J.T.; Santos, H.A. Chitosan-modified porous silicon microparticles for enhanced permeability of insulin across intestinal cell monolayers. Biomaterials 2014, 35, 7172–7179. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Tsai, S.C.; Lai, C.H.; Lee, C.H.; He, Z.S.; Tseng, G.C. Genipin-cross-linked fucose-chitosan/heparin nanoparticles for the eradication of Helicobacter pylori. Biomaterials 2013, 34, 4466–4479. [Google Scholar] [CrossRef] [PubMed]
- Teimouri, A.; Azami, S.J.; Keshavarz, H.; Esmaeili, F.; Alimi, R.; Mavi, S.A.; Shojaee, S. Anti-Toxoplasma activity of various molecular weights and concentrations of chitosan nanoparticles on tachyzoites of RH strain. Int. J. Nanomed. 2018, 13, 1341–1351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carroll, E.C.; Jin, L.; Mori, A.; Munoz-Wolf, N.; Oleszycka, E.; Moran, H.B.T.; Mansouri, S.; McEntee, C.P.; Lambe, E.; Agger, E.M.; et al. The Vaccine Adjuvant Chitosan Promotes Cellular Immunity via DNA Sensor cGAS-STING-Dependent Induction of Type I Interferons. Immunity 2016, 44, 597–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riteau, N.; Sher, A. Chitosan: An Adjuvant with an Unanticipated STING. Immunity 2016, 44, 522–524. [Google Scholar] [CrossRef] [Green Version]
- Schlosser, P.M.; Bale, A.S.; Gibbons, C.F.; Wilkins, A.; Cooper, G.S. Human health effects of dichloromethane: Key findings and scientific issues. Environ. Health Perspect. 2015, 123, 114–119. [Google Scholar] [CrossRef] [Green Version]
- Correa, D.; Canedo-Solares, I.; Ortiz-Alegria, L.B.; Caballero-Ortega, H.; Rico-Torres, C.P. Congenital and acquired toxoplasmosis: Diversity and role of antibodies in different compartments of the host. Parasite Immunol. 2007, 29, 651–660. [Google Scholar] [CrossRef]
- Sayles, P.C.; Gibson, G.W.; Johnson, L.L. B cells are essential for vaccination-induced resistance to virulent Toxoplasma gondii. Infect. Immun. 2000, 68, 1026–1033. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.L.; Elsheikha, H.M.; Zhu, W.N.; Chen, K.; Li, T.T.; Yue, D.M.; Zhang, X.X.; Huang, S.Y.; Zhu, X.Q. Immunization with Toxoplasma gondii GRA17 Deletion Mutant Induces Partial Protection and Survival in Challenged Mice. Front. Immunol. 2017, 8, 730. [Google Scholar] [CrossRef] [Green Version]
- Feng, L.; Qi, X.R.; Zhou, X.J.; Maitani, Y.; Wang, S.C.; Jiang, Y.; Nagai, T. Pharmaceutical and immunological evaluation of a single-dose hepatitis B vaccine using PLGA microspheres. J. Control. Release 2006, 112, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Ching, X.T.; Fong, M.Y.; Lau, Y.L. Evaluation of Immunoprotection Conferred by the Subunit Vaccines of GRA2 and GRA5 against Acute Toxoplasmosis in BALB/c Mice. Front. Microbiol. 2016, 7, 609. [Google Scholar] [CrossRef] [PubMed]
- Gigley, J.P.; Fox, B.A.; Bzik, D.J. Cell-mediated immunity to Toxoplasma gondii develops primarily by local Th1 host immune responses in the absence of parasite replication. J. Immunol. 2009, 182, 1069–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimier-Poisson, I.; Carpentier, R.; N’Guyen, T.T.; Dahmani, F.; Ducournau, C.; Betbeder, D. Porous nanoparticles as delivery system of complex antigens for an effective vaccine against acute and chronic Toxoplasma gondii infection. Biomaterials 2015, 50, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, N.Z.; Wang, M.; Dong, H.; Feng, S.Y.; Guo, H.C.; Zhu, X.Q. A long-lasting protective immunity against chronic toxoplasmosis in mice induced by recombinant rhoptry proteins encapsulated in poly (lactide-co-glycolide) microparticles. Parasitol. Res. 2015, 114, 4195–4203. [Google Scholar] [CrossRef] [PubMed]
- Roozbehani, M.; Falak, R.; Mohammadi, M.; Hemphill, A.; Razmjou, E.; Meamar, A.R.; Masoori, L.; Khoshmirsafa, M.; Moradi, M.; Gharavi, M.J. Characterization of a multi-epitope peptide with selective MHC-binding capabilities encapsulated in PLGA nanoparticles as a novel vaccine candidate against Toxoplasma gondii infection. Vaccine 2018, 36, 6124–6132. [Google Scholar] [CrossRef]
- Bessieres, M.H.; Swierczynski, B.; Cassaing, S.; Miedouge, M.; Olle, P.; Seguela, J.P.; Pipy, B. Role of IFN-gamma, TNF-alpha, IL4 and IL10 in the regulation of experimental Toxoplasma gondii infection. J. Eukaryot. Microbiol. 1997, 44, 87s. [Google Scholar] [CrossRef]
- Pinzan, C.F.; Sardinha-Silva, A.; Almeida, F.; Lai, L.; Lopes, C.D.; Lourenco, E.V.; Panunto-Castelo, A.; Matthews, S.; Roque-Barreira, M.C. Vaccination with Recombinant Microneme Proteins Confers Protection against Experimental Toxoplasmosis in Mice. PLoS ONE 2015, 10, e0143087. [Google Scholar] [CrossRef] [Green Version]
- Moroda, M.; Takamoto, M.; Iwakura, Y.; Nakayama, J.; Aosai, F. Interleukin-17A-Deficient Mice Are Highly Susceptible to Toxoplasma gondii Infection Due to Excessively Induced T. gondii HSP70 and Interferon Gamma Production. Infect. Immun. 2017, 85. [Google Scholar] [CrossRef] [Green Version]
- Kelly, M.N.; Kolls, J.K.; Happel, K.; Schwartzman, J.D.; Schwarzenberger, P.; Combe, C.; Moretto, M.; Khan, I.A. Interleukin-17/interleukin-17 receptor-mediated signaling is important for generation of an optimal polymorphonuclear response against Toxoplasma gondii infection. Infect. Immun. 2005, 73, 617–621. [Google Scholar] [CrossRef] [Green Version]
- Guiton, R.; Vasseur, V.; Charron, S.; Arias, M.T.; Van Langendonck, N.; Buzoni-Gatel, D.; Ryffel, B.; Dimier-Poisson, I. Interleukin 17 receptor signaling is deleterious during Toxoplasma gondii infection in susceptible BL6 mice. J. Infect. Dis. 2010, 202, 427–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, B.; Ding, J.; Chen, X.; Yu, H.; Lou, D.; Tong, Q.; Kong, Q.; Lu, S. Immuno-Efficacy of a T. gondii Secreted Protein with an Altered Thrombospondin Repeat (TgSPATR) As a Novel DNA Vaccine Candidate against Acute Toxoplasmosis in BALB/c Mice. Front. Microbiol. 2017, 8, 216. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.L.; Dubey, J.P. Foodborne toxoplasmosis. Clin. Infect. Dis. 2012, 55, 845–851. [Google Scholar] [CrossRef]
- Melchor, S.J.; Ewald, S.E. Disease Tolerance in Toxoplasma Infection. Front. Cell Infect. Microbiol. 2019, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]



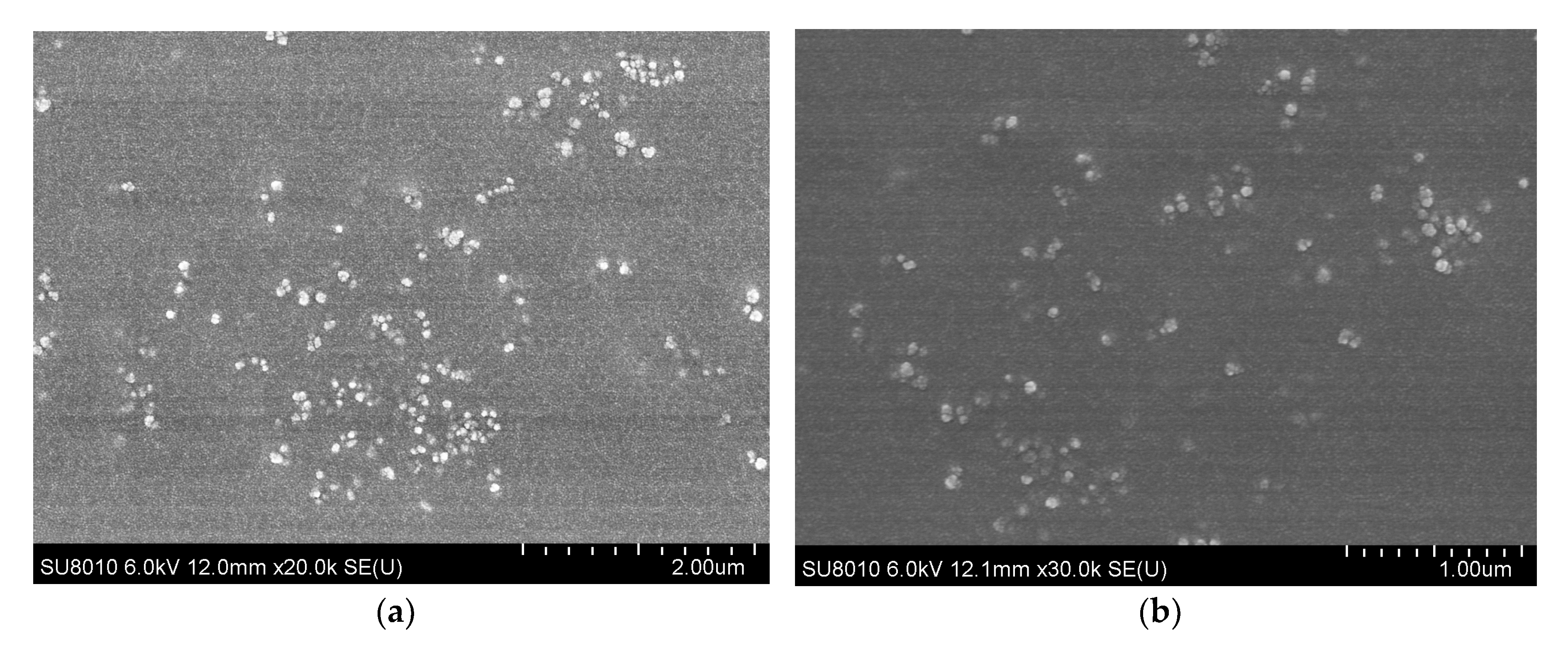

| Weeks | Group | Total IgG (OD Values) | p Value | p Value |
|---|---|---|---|---|
| 0 weeks | Blank | 0.605 ± 0.059 | - | 0.9965 b |
| His-tagged protein | 0.594 ± 0.037 | 0.9965 a | - | |
| rTgH2A1 | 0.605 ± 0.045 | >0.9999 a | 0.9978 b | |
| Chitosan | 0.611 ± 0.031 | 0.9997 a | 0.9811 b | |
| PLGA | 0.605 ± 0.045 | >0.9999 a | 0.9978 b | |
| rTgH2A1/Chitosan | 0.619 ± 0.015 | 0.9930 a | 0.8857 b | |
| rTgH2A1/PLGA | 0.626 ± 0.042 | 0.9542 a | 0.7542 b | |
| 2 weeks | Blank | 0.621 ± 0.033 | - | 0.7318 d |
| His-tagged protein | 0.671 ± 0.046 | 0.7318 c | - | |
| rTgH2A1 | 0.918 ± 0.030 | <0.0001 c | <0.0001 d | |
| Chitosan | 0.697 ± 0.081 | 0.3489 c | 0.9802 d | |
| PLGA | 0.633 ± 0.027 | 0.9996 c | 0.8878 d | |
| rTgH2A1/Chitosan | 1.112 ± 0.091 | <0.0001 c | <0.0001 d | |
| rTgH2A1/PLGA | 1.126 ± 0.085 | <0.0001 c | <0.0001 d | |
| 4 weeks | Blank | 0.648 ± 0.031 | - | 0.8815 f |
| His-tagged protein | 0.688 ± 0.037 | 0.8815 e | - | |
| rTgH2A1 | 0.998 ± 0.092 | <0.0001 e | <0.0001 f | |
| Chitosan | 0.693 ± 0.026 | 0.8187 e | 0.9999 f | |
| PLGA | 0.644 ± 0.049 | 0.9999 e | 0.8317 f | |
| rTgH2A1/Chitosan | 1.367 ± 0.087 | <0.0001 e | <0.0001 f | |
| rTgH2A1/PLGA | 1.489 ± 0.085 | <0.0001 e | <0.0001 f |
| Group | IgG1 (OD Values) | IgG1 Concentrations (ng/mL) | p Value a | p Value b | IgG2a (OD Values) | IgG2a Concentrations (ng/mL) | p Value a | p Value b |
|---|---|---|---|---|---|---|---|---|
| Blank | 0.389 ± 0.024 | 1876.942 ± 104.595 | - | 0.3985 | 0.649 ± 0.042 | 1543.657 ± 116.373 | - | 0.9164 |
| His-tagged protein | 0.425 ± 0.022 | 2031.688 ± 96.355 | 0.3985 | - | 0.678 ± 0.026 | 1623.244 ± 73.475 | 0.9164 | - |
| rTgH2A1 | 0.439 ± 0.021 | 2095.674 ± 93.427 | 0.1218 | 0.9612 | 0.900 ± 0.060 | 2260.896 ± 179.102 | <0.0001 | < 0.0001 |
| Chitosan | 0.400 ± 0.023 | 1924.944 ± 100.771 | 0.9906 | 0.7385 | 0.684 ± 0.030 | 1642.357 ± 83.402 | 0.8166 | 0.9997 |
| PLGA | 0.395 ± 0.039 | 1905.677 ± 168.024 | 0.9996 | 0.5971 | 0.643 ± 0.043 | 1527.466 ± 118.901 | 0.9997 | 0.8342 |
| rTgH2A1/Chitosan | 0.442 ± 0.030 | 2109.378 ± 133.144 | 0.0908 | 0.9121 | 1.120 ±0.022 | 2932.664 ± 70.216 | <0.0001 | <0.0001 |
| rTgH2A1/PLGA | 0.489 ± 0.046 | 2311.107 ± 201.347 | 0.0005 | 0.0304 | 1.206 ± 0.073 | 3213.309 ± 240.906 | <0.0001 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Z.; Zhou, T.; Luo, Y.; Dong, L.; Li, C.; Liu, J.; Luo, J.; Yan, R.; Xu, L.; Song, X.; et al. Modulation Effects of Toxoplasma gondii Histone H2A1 on Murine Macrophages and Encapsulation with Polymer as a Vaccine Candidate. Vaccines 2020, 8, 731. https://doi.org/10.3390/vaccines8040731
Yu Z, Zhou T, Luo Y, Dong L, Li C, Liu J, Luo J, Yan R, Xu L, Song X, et al. Modulation Effects of Toxoplasma gondii Histone H2A1 on Murine Macrophages and Encapsulation with Polymer as a Vaccine Candidate. Vaccines. 2020; 8(4):731. https://doi.org/10.3390/vaccines8040731
Chicago/Turabian StyleYu, Zhengqing, Tianyuan Zhou, Yanxin Luo, Lu Dong, Chunjing Li, Junlong Liu, Jianxun Luo, Ruofeng Yan, Lixin Xu, Xiaokai Song, and et al. 2020. "Modulation Effects of Toxoplasma gondii Histone H2A1 on Murine Macrophages and Encapsulation with Polymer as a Vaccine Candidate" Vaccines 8, no. 4: 731. https://doi.org/10.3390/vaccines8040731
APA StyleYu, Z., Zhou, T., Luo, Y., Dong, L., Li, C., Liu, J., Luo, J., Yan, R., Xu, L., Song, X., & Li, X. (2020). Modulation Effects of Toxoplasma gondii Histone H2A1 on Murine Macrophages and Encapsulation with Polymer as a Vaccine Candidate. Vaccines, 8(4), 731. https://doi.org/10.3390/vaccines8040731







