Efficacy of Whole Cell Inactivated Vibrio harveyi Vaccine against Vibriosis in a Marine Red Hybrid Tilapia (Oreochromis niloticus × O. mossambicus) Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Fish
2.2. Bacterial Strains and Culture
2.3. Preparation of Formalin-Killed Vibrio Harveyi (FKVh) Vaccine
2.4. Vaccine Safety Test
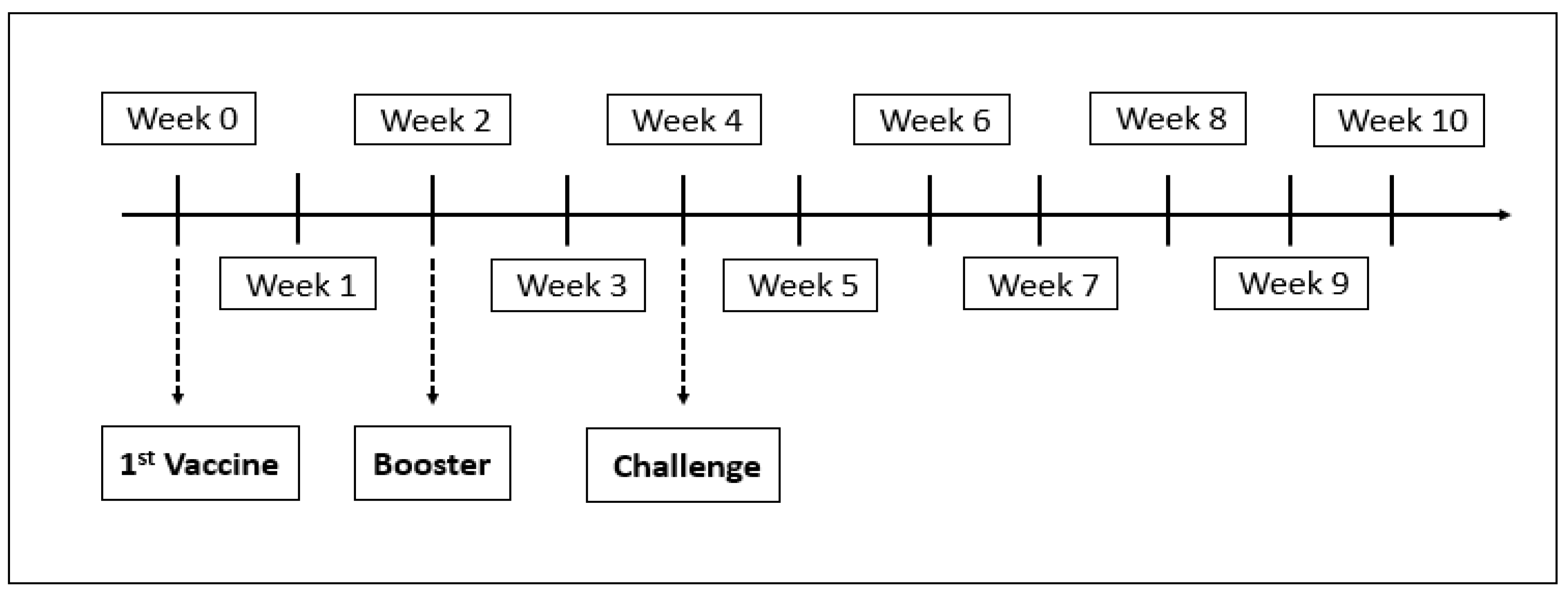
2.5. Experimental Design
2.5.1. Mucus
2.5.2. Serum
2.5.3. Gut Lavage
2.6. Sample Processing
2.6.1. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6.2. Lysozyme Assay
2.6.3. Histopathology Analysis
2.7. Data Analysis
2.8. Ethics Statement
3. Results
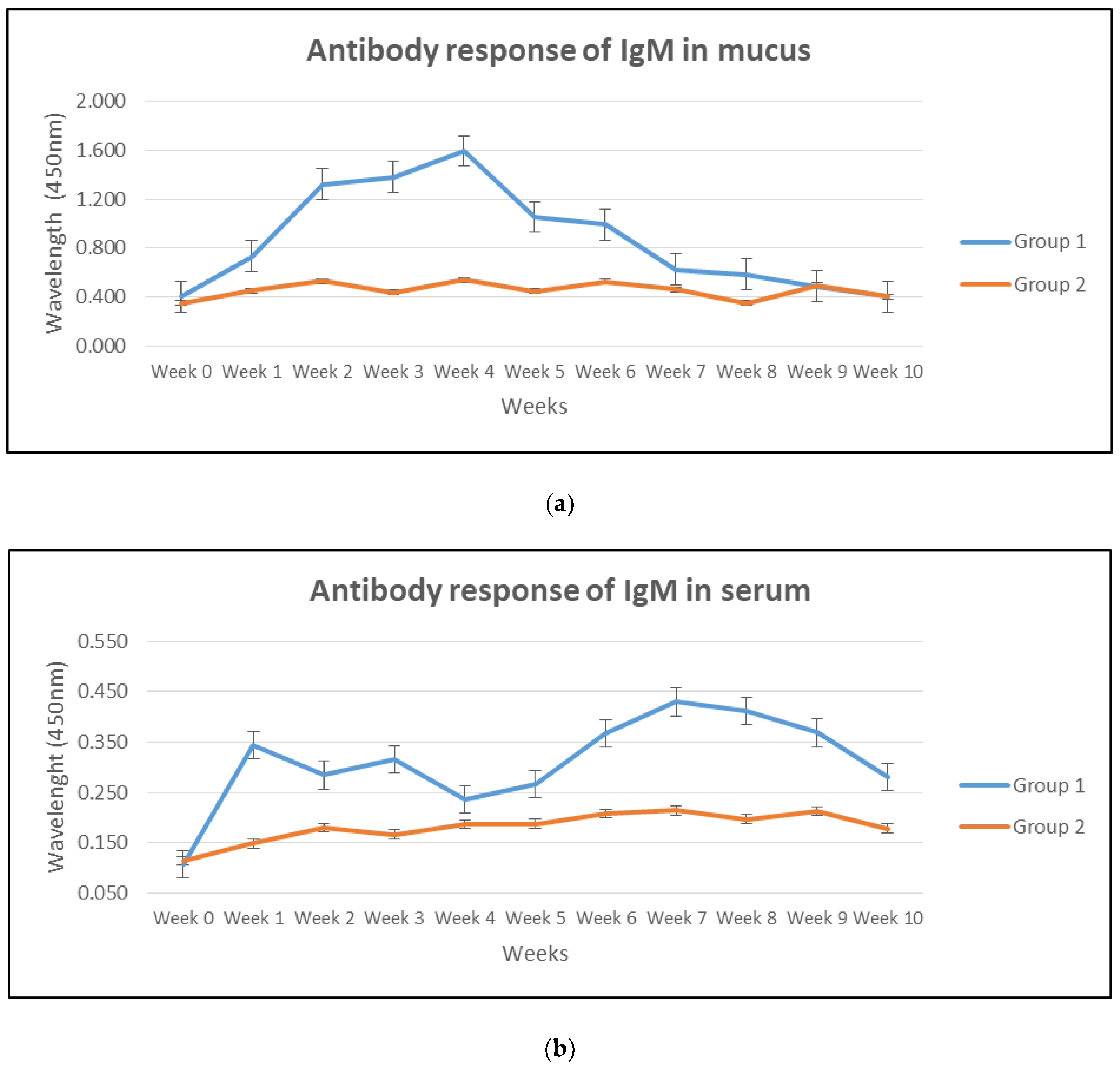
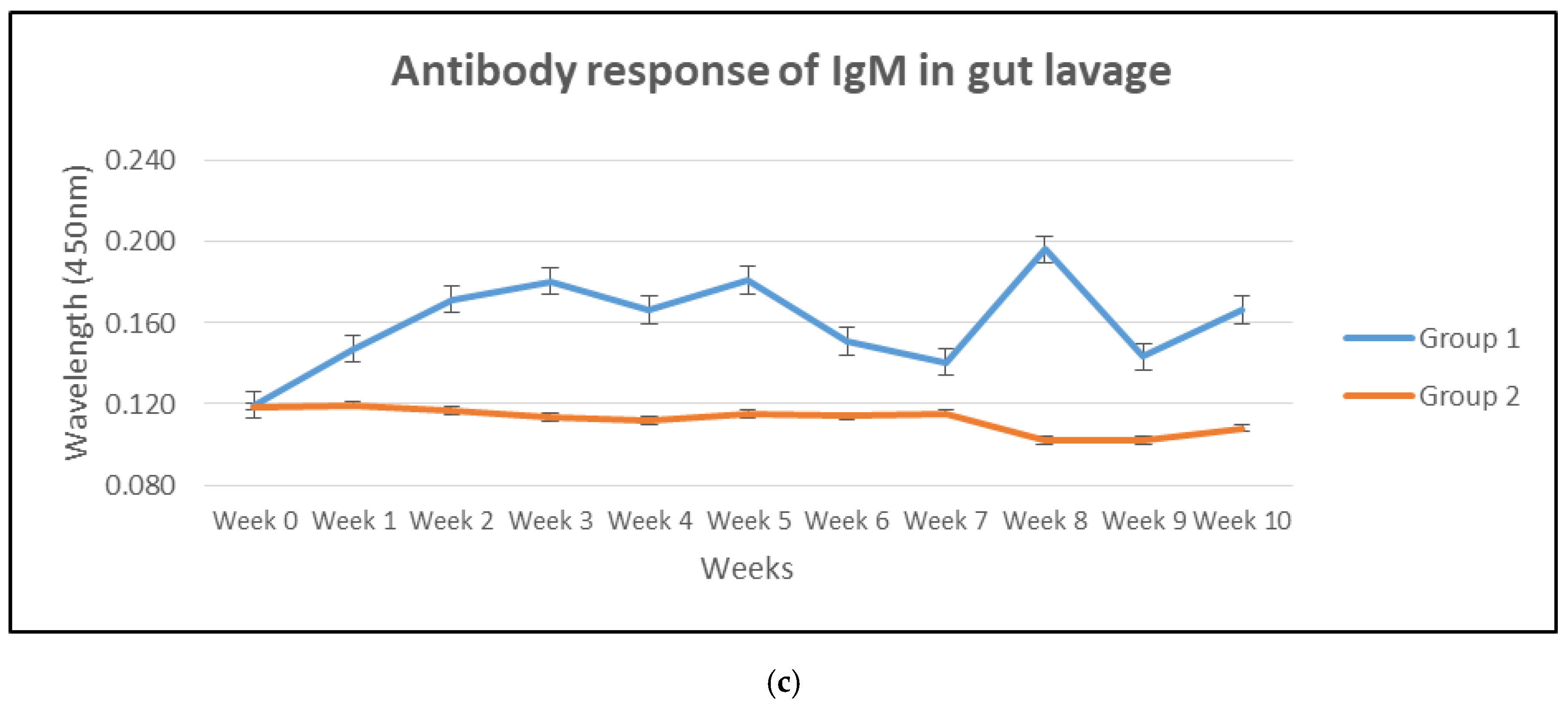
3.1. Antibody Response in Mucus, Serum and Gut Lavage
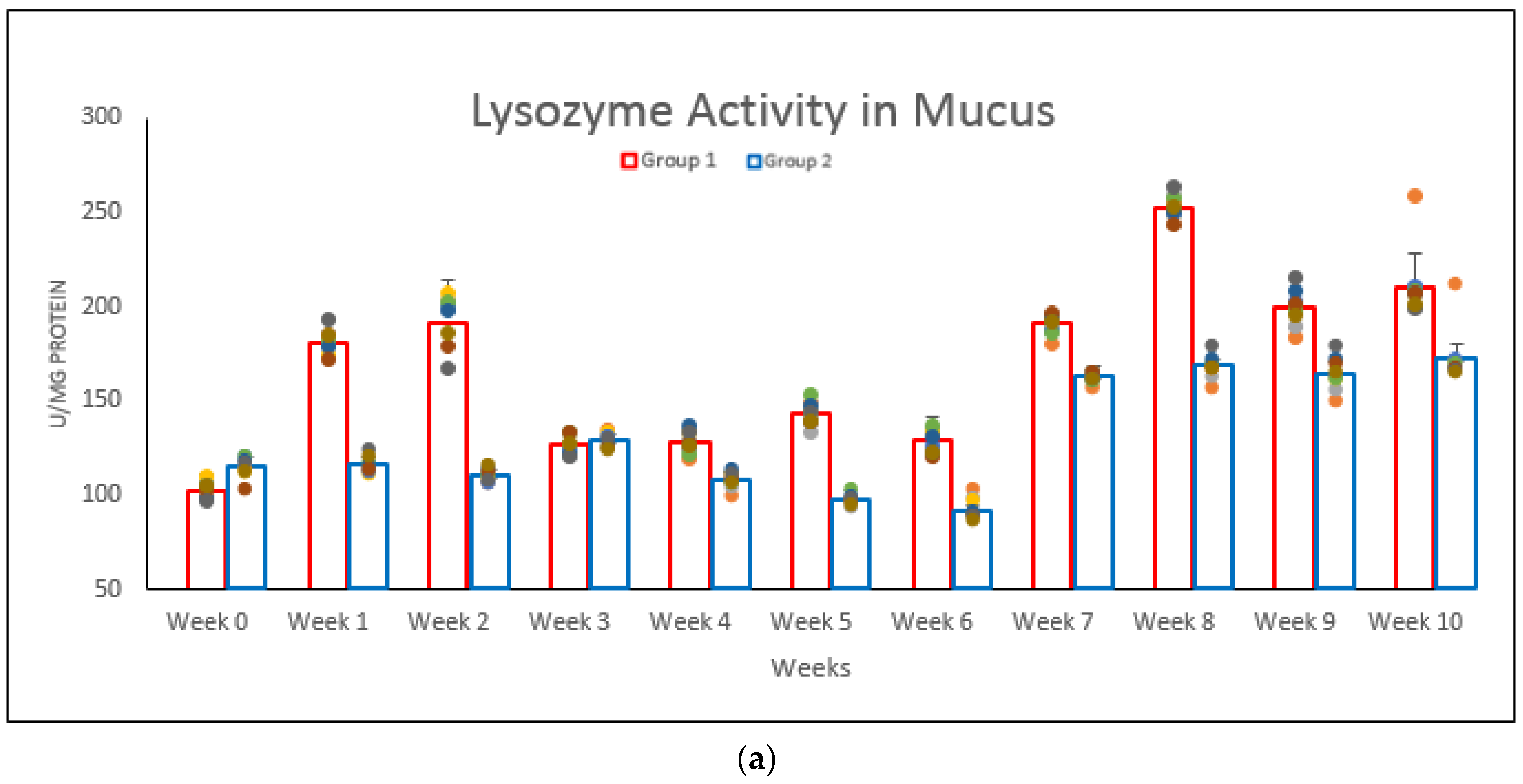
3.2. Lysozyme Activity in Body Mucus, Serum and Gut Lavage Fluid
3.3. Relative Percentage Survival and Clinical Signs
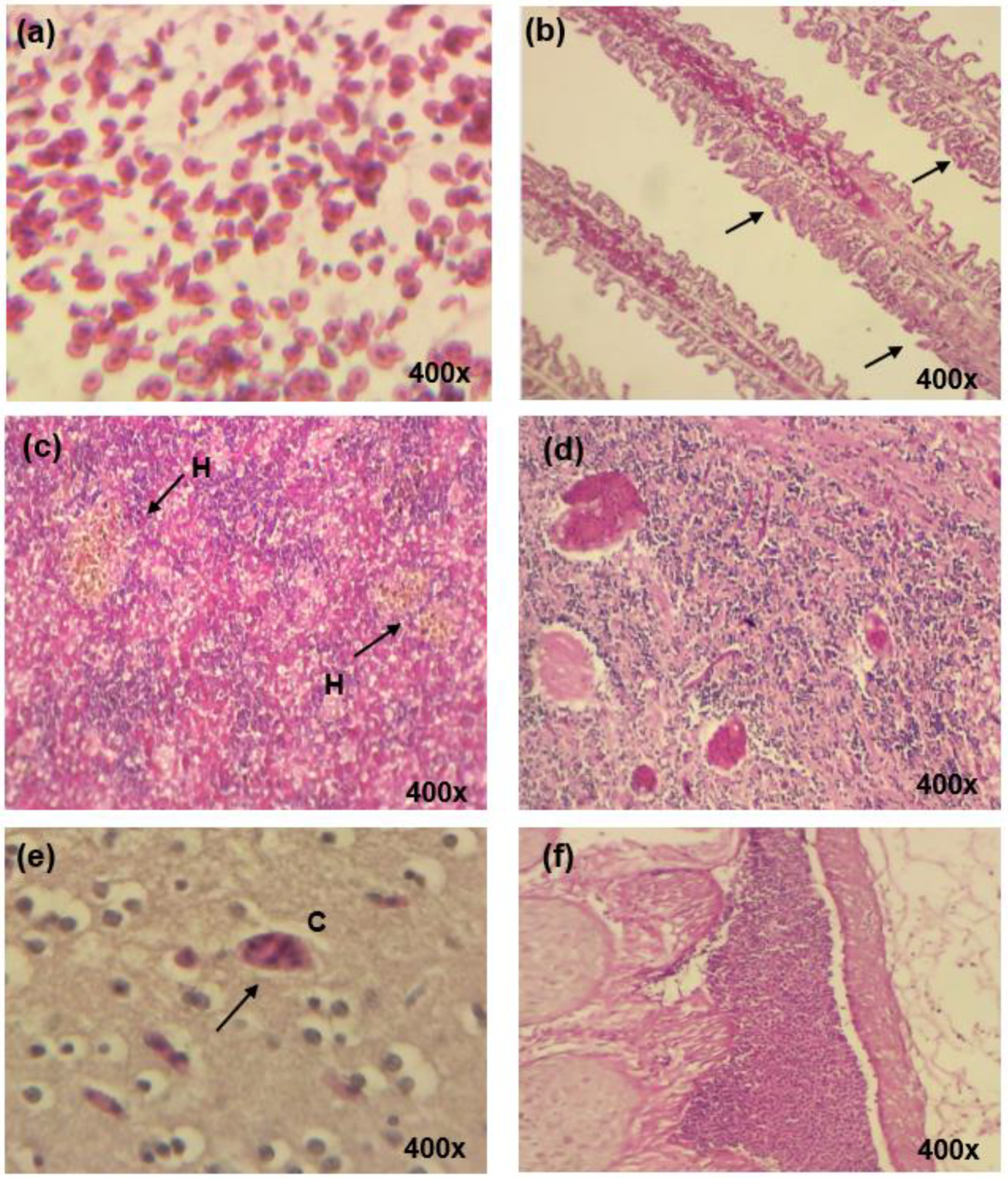
3.4. Pathological Changes in Organs
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ina-Salwany, Y.; Al-Saari, N.; Mohamad, A.; Mursidi, F.; Mohd-Aris, A.; Amal, M.N.A.; Kasai, H.; Mino, S.; Sawabe, T.; Zamri-Saad, M. Vibriosis in Fish: A Review on Disease Development and Prevention. J. Aquat. Anim. Health 2019, 31, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Canestrini, G. La mallattia dominante dellc angiiille. Atti 1st Veneto Sci. Lett. Arti Cl. Sci. Mat. Nat. 1893, 7, 809–814. (In Italian) [Google Scholar]
- Bergman, A.M. Die roten Beulenkrankheit des Aales. In Ber Bayerischen Biol Versuchs–Station 2; Ely: Stuttgart, Germany, 1909; pp. 10–54. (In German) [Google Scholar]
- Samuelsen, O.B.; Nerland, A.H.; Jørgensen, T.; Schrøder, M.B.; Svåsand, T.; Bergh, Ø. Viral and bacterial diseases of Atlantic cod Gadus morhua, their prophylaxis and treatment: A review. Dis. Aquat. Org. 2006, 71, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Sindermann, C.J. Disease in marine aquaculture. Helgol. Mar. Res. 1984, 37, 505–530. [Google Scholar] [CrossRef] [Green Version]
- Kusuda, R.; Salati, F. Major bacterial diseases affecting mariculture in Japan. Annu. Rev. Fish Dis. 1993, 3, 69–85. [Google Scholar] [CrossRef]
- Costa, A.A.; Leef, M.; Bridle, A.; Carson, J.; Nowak, B.F. Effect of vaccination against yersiniosis on the relative percent survival, bactericidal and lysozyme response of Atlantic salmon, Salmo salar. Aquaculture 2011, 315, 201–206. [Google Scholar] [CrossRef]
- Caipang, C.M.A.; Lucanas, J.B.; Lay-yag, C.M. Updates on the vaccination against bacterial diseases in tilapia, Oreochromis spp. and Asian seabass, Lates calcarifer. Aquac. Aquar. Conserv. Legis. 2014, 7, 184–193. [Google Scholar]
- Munang’Andu, H.M.; Paul, J.; Evensen, Ø. An Overview of Vaccination Strategies and Antigen Delivery Systems for Streptococcus agalactiae Vaccines in Nile Tilapia (Oreochromis niloticus). Vaccines 2016, 4, 48. [Google Scholar] [CrossRef] [Green Version]
- Ismail, M.S.; Siti-Zahrah, A.; Syafiq, M.R.M.; Amal, M.N.A.; Firdaus-Nawi, M.; Saad, M.Z. Feed-based vaccination regime against streptococcosis in red tilapia, Oreochromis niloticus x Oreochromis mossambicus. BMC Veter. Res. 2016, 12, 194. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, E.; He, Y.; Wang, K.; Yang, Q.; Wang, J.; Geng, Y.; Chen, D.; Huang, X.; Ouyang, P.; et al. Identification and screening of effective protective antigens for channel catfish against Streptococcus iniae. Oncotarget 2017, 8, 30793–30804. [Google Scholar] [CrossRef] [Green Version]
- Magnadottir, B. Immunological Control of Fish Diseases. Mar. Biotechnol. 2010, 12, 361–379. [Google Scholar] [CrossRef] [PubMed]
- Aris, A.M.; Saad, M.-Z.; Daud, H.M.; Yusof, M.T.; Ina-Salwany, Y. Vibrio harveyi protease deletion mutant as a live attenuated vaccine candidate against vibriosis and transcriptome profiling following vaccination for Epinephelus fuscoguttatus. Aquac. Int. 2018, 27, 125–140. [Google Scholar] [CrossRef]
- Saad, M.Z.; Amal, M.; Siti-Zahrah, A. Pathological Changes in Red Tilapias (Oreochromis spp.) Naturally Infected by Streptococcus agalactiae. J. Comp. Pathol. 2010, 143, 227–229. [Google Scholar] [CrossRef] [PubMed]
- Chin, Y.K.; Al-Saari, N.; Zulperi, Z.; Aris, A.M.; Salleh, A.; Silvaraj, S.; Mohamad, A.; Lee, J.; Zamri-Saad, M.; Ina-Salwany, Y. Efficacy of bath vaccination with a live attenuated Vibrio harveyi against vibriosis in Asian seabass fingerling, Lates calcarifer. Aquac. Res. 2019, 51, 389–399. [Google Scholar] [CrossRef]
- Pulpipat, T.; Maekawa, S.; Wang, P.-C.; Chen, S.-C. Immune Responses and Protective Efficacy of a Formalin-Killed Francisella Noatunensis Subsp. Orientalis Vaccine Evaluated through Intraperitoneal and Immersion Challenge Methods in Oreochromis Niloticus. Vaccines 2020, 8, 163. [Google Scholar] [CrossRef] [Green Version]
- Firdaus-Nawi, M.; Zamri-Saad, M. Major compenents of fish immunity: A review. Pertanika J. Trop. Agric. Sci. 2016, 39, 393–420. [Google Scholar]
- Firdaus-Nawi, M.; Sabri, M.Y.; Hanan, Y.; Siti-Zahrah, A.; Zamri-Saad, M. Efficacy of feed-based adjuvant vaccine against Streptococcus agalactiae in Oreochromis spp. in Malaysia. Aquac. Res. 2013, 45, 87–96. [Google Scholar] [CrossRef]
- Tukmechi, A.; Andani, H.R.R.; Manaffar, R.; Sheikhzadeh, N. Dietary administration of beta-mercapto-ethanol treated Saccharomyces cerevisiae enhanced the growth, innate immune response and disease resistance of the rainbow trout, Oncorhynchus mykiss. Fish Shellfish. Immunol. 2011, 30, 923–928. [Google Scholar] [CrossRef]
- Russo, R.; Mitchell, H.; Yanong, R.P. Characterization of Streptococcus inae isolated from ornamental cyprinid fishes and development of challenge model. Aquaculture 2006, 256, 105–110. [Google Scholar] [CrossRef]
- Toranzo, A.E.; Magariños, B.; Romalde, J.L. A review of the main bacterial fish diseases in mariculture systems. Aquaculture 2005, 246, 37–61. [Google Scholar] [CrossRef]
- Haldar, S.; Chatterjee, S. Vibrio Related Diseases in Aquaculture and Development of Rapid and Accurate Identification Methods. J. Mar. Sci. Res. Dev. 2012, 1, 1–7. [Google Scholar] [CrossRef]
- Mancuso, M.; Genovese, M.; Guerrera, M.C.; Casella, G.; Genovese, L.; Piccolo, G.; Maricchiolo, G. First episode of vibriosis in wild specimens of Pagellus bogaraveo (Brünnich, 1768) in the Mediterranean Sea. Cah. Biol. Mar. 2015, 56, 355–361. [Google Scholar]
- Mohamad, N.; Amal, M.N.A.; Yasin, I.S.M.; Saad, M.Z.; Nasruddin, N.S.; Al-Saari, N.; Mino, S.; Sawabe, T. Vibriosis in cultured marine fishes: A review. Aquaculture 2019, 512, 734289. [Google Scholar] [CrossRef]
- Elmahdi, S.; DaSilva, L.V.; Parveen, S. Antibiotic resistance of Vibrio parahaemolyticus and Vibrio vulnificus in various countries: A review. Food Microbiol. 2016, 57, 128–134. [Google Scholar] [CrossRef] [Green Version]
- Stalin, N.; Stalin, N. Molecular characterization of antibiotic resistant Vibrio harveyi isolated from shrimp aquaculture environment in the south east coast of India. Microb. Pathog. 2016, 97, 110–118. [Google Scholar] [CrossRef]
- Dehghani, S.; Akhlaghi, M.; Dehghani, M. Efficacy of formalin-killed, heat-killed and lipopolysaccharide vaccines against motile aeromonads infection in rainbow trout (Oncorhynchus mykiss). Glob. Vet. 2012, 9, 409–415. [Google Scholar]
- Bactol, I.D.C.; Padilla, L.V.; Hilario, A.L. Immune response of tilapia (Oreochromis niloticus) after vaccination with autoclave killed, heat-killed, and formalin-killed whole cell Aeromonas hydrophila vaccines as possible serotype-independent vaccines. Int. J. Agric. Biol. 2018, 20, 846–850. [Google Scholar]
- Cabello, F.C. Heavy use of prophylactic antibiotics in aquaculture: A growing problem for human and animal health and for the environment. Environ. Microbiol. 2006, 8, 1137–1144. [Google Scholar] [CrossRef]
- Xu-Dong, J.; Shuang, C.; Yong-Hua, H. Comparative study of the effects of aluminium adjuvants and Freund’s incomplete adjuvant on the immune response to an Edwardsiella tarda major antigen. Vaccine 2010, 28, 1832–1837. [Google Scholar]
- Saurabh, S.; Sahoo, P.K. Lysozyme: An important defence molecule of fish innate immune system. Aquac. Res. 2008, 39, 223–239. [Google Scholar] [CrossRef]
- Alexander, J.B.; Ingram, G.A. Noncellular nonspecific defence mechanisms of fish. Annu. Rev. Fish Dis. 1992, 2, 249–279. [Google Scholar] [CrossRef]
- Varsamos, S.; Nebel, C.; Charmantier, G. Ontogeny of osmoregulation in postembryonic fish: A review. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2005, 141, 401–429. [Google Scholar] [CrossRef] [PubMed]
- Esteban, M. An overview of the inmunological defenses in fish skin. ISRB Immunol. 2012, 2, 29. [Google Scholar]
- Liu, X.; Wu, H.; Chang, X.; Tang, Y.; Liu, Q.; Zhang, Y. Notable mucosal immune responses induced in the intestine of zebrafish (Danio rerio) bath-vaccinated with a live attenuated Vibrio anguillarum vaccine. Fish Shellfish. Immunol. 2014, 40, 99–108. [Google Scholar] [CrossRef]
- Secombes, C.J.; Wang, T. The innate and adaptive immune system of fish. In Infectious Disease in Aquaculture; Elsevier BV: Amsterdam, The Netherlands, 2012; pp. 3–68. [Google Scholar]
- Li, J.; Ma, S.; Woo, N.Y.S. Vaccination of Silver Sea Bream (Sparus sarba) against Vibrio alginolyticus: Protective Evaluation of Different Vaccinating Modalities. Int. J. Mol. Sci. 2015, 17, 40. [Google Scholar] [CrossRef] [Green Version]
- Munang’Andu, H.M. Intracellular Bacterial Infections: A Challenge for Developing Cellular Mediated Immunity Vaccines for Farmed Fish. Microorganisms 2018, 6, 33. [Google Scholar] [CrossRef] [Green Version]
- Shona, K.W. The innate immune response of finfish—A review of current knowledge. Fish Shellfish. Immunol. 2007, 23, 1127–1151. [Google Scholar]
- Chen, S.-L.; Li, J.; Deng, S.-P.; Tian, Y.-S.; Wang, Q.-Y.; Zhuang, Z.-M.; Sha, Z.-X.; Xu, J.-Y. Isolation of Female-Specific AFLP Markers and Molecular Identification of Genetic Sex in Half-Smooth Tongue Sole (Cynoglossus semilaevis). Mar. Biotechnol. 2007, 9, 273–280. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu Nor, N.; Zamri-Saad, M.; Md Yasin, I.-S.; Salleh, A.; Mustaffa-Kamal, F.; Matori, M.F.; Azmai, M.N.A. Efficacy of Whole Cell Inactivated Vibrio harveyi Vaccine against Vibriosis in a Marine Red Hybrid Tilapia (Oreochromis niloticus × O. mossambicus) Model. Vaccines 2020, 8, 734. https://doi.org/10.3390/vaccines8040734
Abu Nor N, Zamri-Saad M, Md Yasin I-S, Salleh A, Mustaffa-Kamal F, Matori MF, Azmai MNA. Efficacy of Whole Cell Inactivated Vibrio harveyi Vaccine against Vibriosis in a Marine Red Hybrid Tilapia (Oreochromis niloticus × O. mossambicus) Model. Vaccines. 2020; 8(4):734. https://doi.org/10.3390/vaccines8040734
Chicago/Turabian StyleAbu Nor, Nadirah, Mohd Zamri-Saad, Ina-Salwany Md Yasin, Annas Salleh, Farina Mustaffa-Kamal, Mohd Fuad Matori, and Mohd Noor Amal Azmai. 2020. "Efficacy of Whole Cell Inactivated Vibrio harveyi Vaccine against Vibriosis in a Marine Red Hybrid Tilapia (Oreochromis niloticus × O. mossambicus) Model" Vaccines 8, no. 4: 734. https://doi.org/10.3390/vaccines8040734






