Associations between Allelic Variants of the Human IgH 3? Regulatory Region 1 and the Immune Response to BNT162b2 mRNA Vaccine
Abstract
:1. Introduction
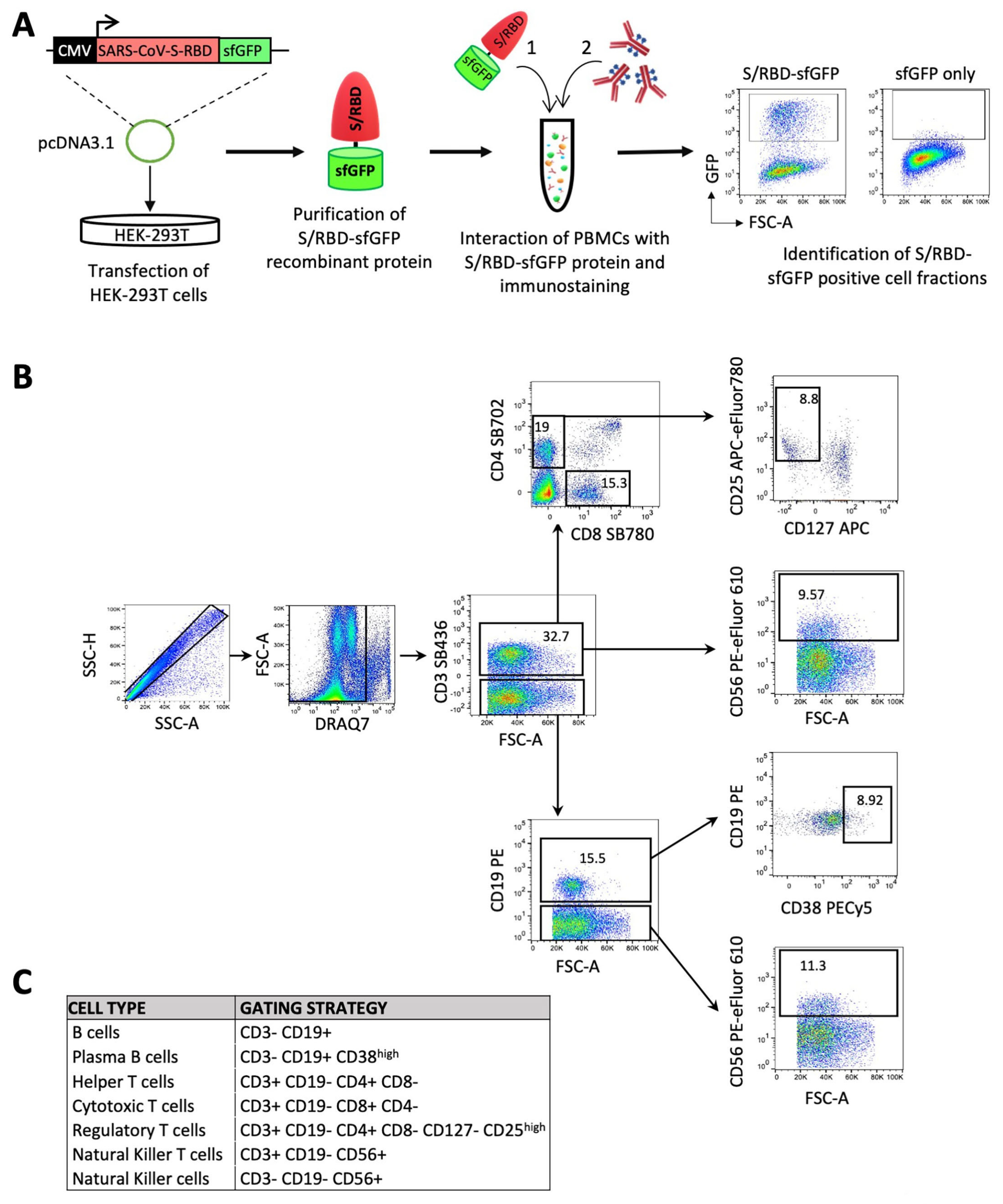
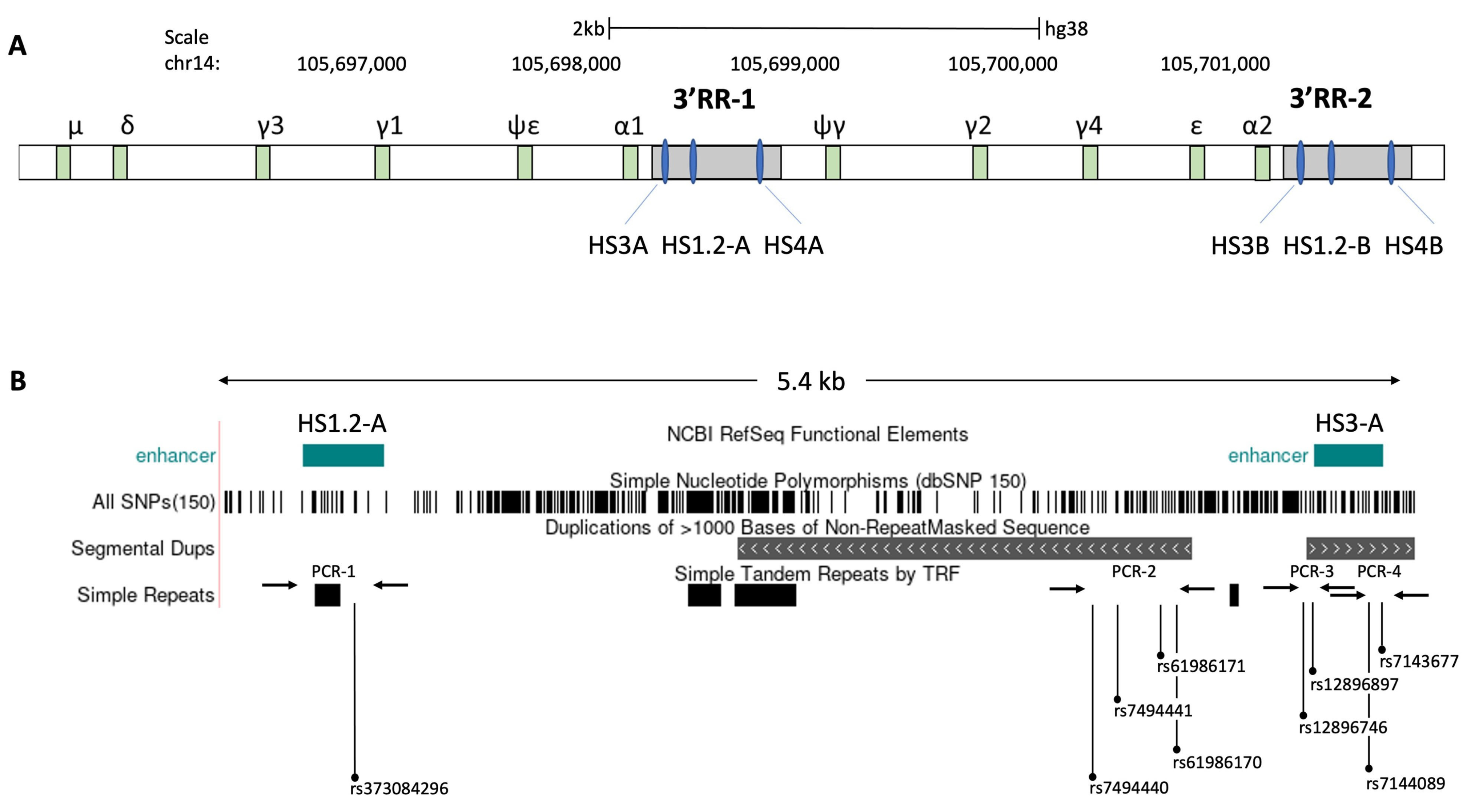
2. Materials and Methods
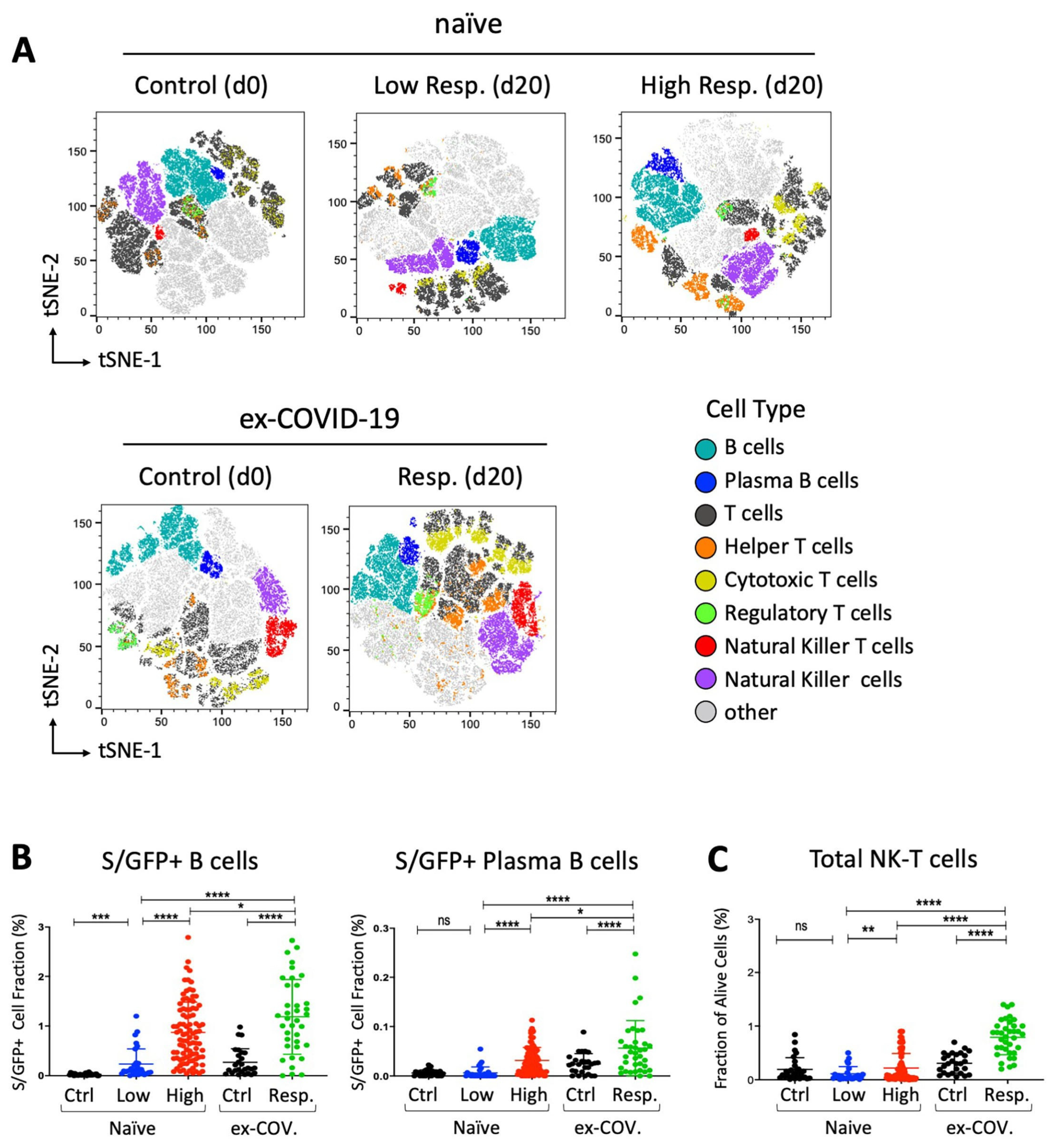
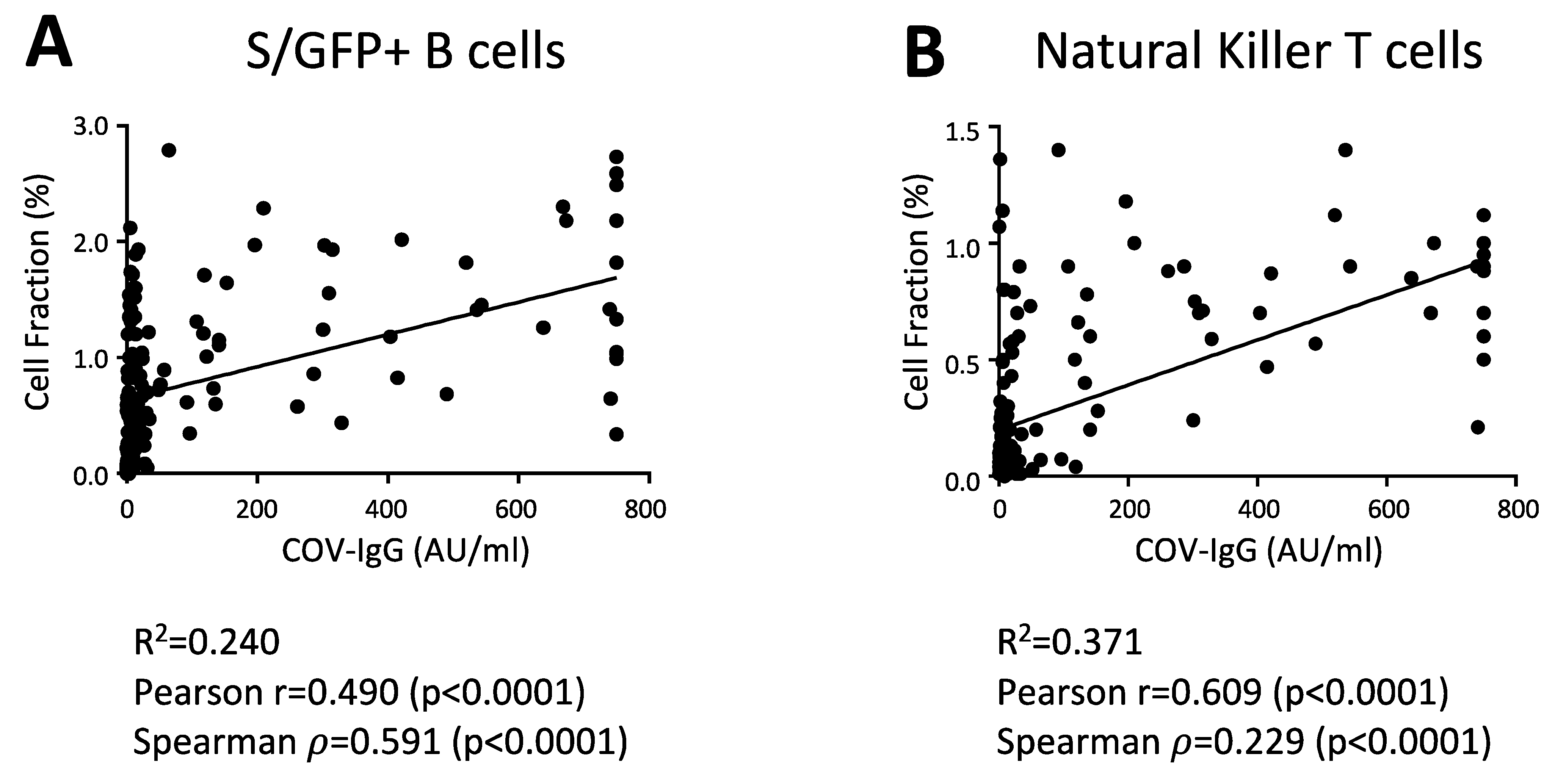
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- Walsh, E.E.; Frenck, R.W., Jr.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Jeyanathan, M.; Afkhami, S.; Smaill, F.; Miller, M.S.; Lichty, B.D.; Xing, Z. Immunological considerations for COVID-19 vaccine strategies. Nat. Rev. Immunol. 2020, 20, 615–632. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, A.; Bozzi, G.; Ungaro, R.; Villa, S.; Castelli, V.; Mangioni, D.; Muscatello, A.; Gori, A.; Bandera, A. Mini Review Immunological Consequences of Immunization With COVID-19 mRNA Vaccines: Preliminary Results. Front. Immunol. 2021, 12, 657711. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.K.; Dorosky, D.; Sharma, P.; Abbasi, S.A.; Dye, J.M.; Kranz, D.M.; Herbert, A.S.; Procko, E. Engineering human ACE2 to optimize binding to the spike protein of SARS coronavirus 2. Science 2020, 369, 1261–1265. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zou, F.; Yu, F.; Li, R.; Yuan, Y.; Zhang, Y.; Zhang, X.; Deng, J.; Chen, T.; Song, Z.; et al. Nanoparticle Vaccines Based on the Receptor Binding Domain (RBD) and Heptad Repeat (HR) of SARS-CoV-2 Elicit Robust Protective Immune Responses. Immunity 2020, 53, 1315–1330.e9. [Google Scholar] [CrossRef]
- Ebinger, J.E.; Fert-Bober, J.; Printsev, I.; Wu, M.; Sun, N.; Figueiredo, J.C.; Eyk, J.E.V.; Braun, J.G.; Cheng, S.; Sobhani, K. Prior COVID-19 Infection and Antibody Response to Single Versus Double Dose mRNA SARS-CoV-2 Vaccination. medRxiv 2021. [Google Scholar] [CrossRef]
- Ebinger, J.E.; Fert-Bober, J.; Printsev, I.; Wu, M.; Sun, N.; Prostko, J.C.; Frias, E.C.; Stewart, J.L.; Van Eyk, J.E.; Braun, J.G.; et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat. Med. 2021, 27, 981–984. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Xia, H.; Zhang, X.; Fontes-Garfias, C.R.; Swanson, K.A.; Cai, H.; Sarkar, R.; Chen, W.; Cutler, M.; et al. Neutralizing Activity of BNT162b2-Elicited Serum. N. Engl. J. Med. 2021, 384, 1466–1468. [Google Scholar] [CrossRef]
- Cocomazzi, G.; Piazzolla, V.; Squillante, M.M.; Antinucci, S.; Giambra, V.; Giuliani, F.; Maiorana, A.; Serra, N.; Mangia, A. Early Serological Response to BNT162b2 mRNA Vaccine in Healthcare Workers. Vaccines 2021, 9, 913. [Google Scholar] [CrossRef]
- Tauzin, A.; Nayrac, M.; Benlarbi, M.; Gong, S.Y.; Gasser, R.; Beaudoin-Bussieres, G.; Brassard, N.; Laumaea, A.; Vezina, D.; Prevost, J.; et al. A single BNT162b2 mRNA dose elicits antibodies with Fc-mediated effector functions and boost pre-existing humoral and T cell responses. bioRxiv 2021. [Google Scholar] [CrossRef]
- Li, J.; Hui, A.; Zhang, X.; Yang, Y.; Tang, R.; Ye, H.; Ji, R.; Lin, M.; Zhu, Z.; Tureci, O.; et al. Safety and immunogenicity of the SARS-CoV-2 BNT162b1 mRNA vaccine in younger and older Chinese adults: A randomized, placebo-controlled, double-blind phase 1 study. Nat. Med. 2021, 27, 1062–1070. [Google Scholar] [CrossRef]
- Fabricius, D.; Ludwig, C.; Scholz, J.; Rode, I.; Tsamadou, C.; Jacobsen, E.M.; Winkelmann, M.; Grempels, A.; Lotfi, R.; Janda, A.; et al. mRNA Vaccines Enhance Neutralizing Immunity against SARS-CoV-2 Variants in Convalescent and ChAdOx1-Primed Subjects. Vaccines 2021, 9, 918. [Google Scholar] [CrossRef] [PubMed]
- Poland, G.A.; Ovsyannikova, I.G.; Kennedy, R.B. Personalized vaccinology: A review. Vaccine 2018, 36, 5350–5357. [Google Scholar] [CrossRef]
- Poland, G.A.; Ovsyannikova, I.G.; Jacobson, R.M. Vaccine immunogenetics: Bedside to bench to population. Vaccine 2008, 26, 6183–6188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ovsyannikova, I.G.; Haralambieva, I.H.; Crooke, S.N.; Poland, G.A.; Kennedy, R.B. The role of host genetics in the immune response to SARS-CoV-2 and COVID-19 susceptibility and severity. Immunol. Rev. 2020, 296, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Rogers, C.H.; Mielczarek, O.; Corcoran, A.E. Dynamic 3D Locus Organization and Its Drivers Underpin Immunoglobulin Recombination. Front. Immunol. 2020, 11, 633705. [Google Scholar] [CrossRef]
- Xu, Z.; Zan, H.; Pone, E.J.; Mai, T.; Casali, P. Immunoglobulin class-switch DNA recombination: Induction, targeting and beyond. Nat. Rev. Immunol. 2012, 12, 517–531. [Google Scholar] [CrossRef] [Green Version]
- D’Addabbo, P.; Scascitelli, M.; Giambra, V.; Rocchi, M.; Frezza, D. Position and sequence conservation in Amniota of polymorphic enhancer HS1.2 within the palindrome of IgH 3’Regulatory Region. BMC Evol. Biol. 2011, 11, 71. [Google Scholar] [CrossRef] [Green Version]
- Le Noir, S.; Boyer, F.; Lecardeur, S.; Brousse, M.; Oruc, Z.; Cook-Moreau, J.; Denizot, Y.; Cogne, M. Functional anatomy of the immunoglobulin heavy chain 3 super-enhancer needs not only core enhancer elements but also their unique DNA context. Nucleic Acids Res. 2017, 45, 5829–5837. [Google Scholar] [CrossRef]
- D’Addabbo, P.; Serone, E.; Esposito, M.; Vaccari, G.; Gargioli, C.; Frezza, D.; Bianchi, L. Association between Psoriasis and haplotypes of the IgH 3’ Regulatory Region 1. Gene 2018, 669, 47–51. [Google Scholar] [CrossRef]
- Cianci, R.; Giambra, V.; Mattioli, C.; Esposito, M.; Cammarota, G.; Scibilia, G.; Magazzu, G.; Orlando, A.; Sandri, G.; Bianchi, L.; et al. Increased frequency of Ig heavy-chain HS1,2-A enhancer *2 allele in dermatitis herpetiformis, plaque psoriasis, and psoriatic arthritis. J. Investig. Dermatol. 2008, 128, 1920–1924. [Google Scholar] [CrossRef]
- Giambra, V.; Cianci, R.; Lolli, S.; Mattioli, C.; Tampella, G.; Cattalini, M.; Kilic, S.S.; Pandolfi, F.; Plebani, A.; Frezza, D. Allele *1 of HS1.2 enhancer associates with selective IgA deficiency and IgM concentration. J. Immunol. 2009, 183, 8280–8285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frezza, D.; Giambra, V.; Cianci, R.; Fruscalzo, A.; Giufre, M.; Cammarota, G.; Martinez-Labarga, C.; Rickards, O.; Scibilia, G.; Sferlazzas, C.; et al. Increased frequency of the immunoglobulin enhancer HS1,2 allele 2 in coeliac disease. Scand. J. Gastroenterol. 2004, 39, 1083–1087. [Google Scholar] [CrossRef] [Green Version]
- Cianci, R.; Lolli, S.; Pagliari, D.; Gambassi, G.; Frosali, S.; Marmo, R.; Melioli, G.; Orlando, A.; Newton, E.E.; Serone, E.; et al. The involvement of IgH enhancer HS1.2 in the pathogenesis of Crohn’s disease: How the immune system can influence a multifactorial disease. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 3618–3627. [Google Scholar] [PubMed]
- Chew, K.L.; Tan, S.S.; Saw, S.; Pajarillaga, A.; Zaine, S.; Khoo, C.; Wang, W.; Tambyah, P.; Jureen, R.; Sethi, S.K. Clinical evaluation of serological IgG antibody response on the Abbott Architect for established SARS-CoV-2 infection. Clin. Microbiol. Infect. 2020, 26, 1256.e9–1256.e11. [Google Scholar] [CrossRef] [PubMed]
- Giambra, V.; Gusscott, S.; Gracias, D.; Song, R.; Lam, S.H.; Panelli, P.; Tyshchenko, K.; Jenkins, C.E.; Hoofd, C.; Lorzadeh, A.; et al. Epigenetic Restoration of Fetal-like IGF1 Signaling Inhibits Leukemia Stem Cell Activity. Cell Stem Cell 2018, 23, 714–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Maaten, L.; Hinton, G. Visualizing data using t-SNE. J. Mach. Learn. Res. 2008, 9, 2579–2605. [Google Scholar]
- Lumley, S.F.; O’Donnell, D.; Stoesser, N.E.; Matthews, P.C.; Howarth, A.; Hatch, S.B.; Marsden, B.D.; Cox, S.; James, T.; Warren, F.; et al. Antibody Status and Incidence of SARS-CoV-2 Infection in Health Care Workers. N. Engl. J. Med. 2021, 384, 533–540. [Google Scholar] [CrossRef]
- Krammer, F.; Srivastava, K.; Alshammary, H.; Amoako, A.A.; Awawda, M.H.; Beach, K.F.; Bermudez-Gonzalez, M.C.; Bielak, D.A.; Carreno, J.M.; Chernet, R.L.; et al. Antibody Responses in Seropositive Persons after a Single Dose of SARS-CoV-2 mRNA Vaccine. N. Engl. J. Med. 2021, 384, 1372–1374. [Google Scholar] [CrossRef]
- Zhao, J.; Yuan, Q.; Wang, H.; Liu, W.; Liao, X.; Su, Y.; Wang, X.; Yuan, J.; Li, T.; Li, J.; et al. Antibody Responses to SARS-CoV-2 in Patients With Novel Coronavirus Disease 2019. Clin. Infect. Dis. 2020, 71, 2027–2034. [Google Scholar] [CrossRef]
- Manisty, C.; Otter, A.D.; Treibel, T.A.; McKnight, A.; Altmann, D.M.; Brooks, T.; Noursadeghi, M.; Boyton, R.J.; Semper, A.; Moon, J.C. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet 2021, 397, 1057–1058. [Google Scholar] [CrossRef]
- Leadbetter, E.A.; Brigl, M.; Illarionov, P.; Cohen, N.; Luteran, M.C.; Pillai, S.; Besra, G.S.; Brenner, M.B. NK T cells provide lipid antigen-specific cognate help for B cells. Proc. Natl. Acad. Sci. USA 2008, 105, 8339–8344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyasaka, T.; Aoyagi, T.; Uchiyama, B.; Oishi, K.; Nakayama, T.; Kinjo, Y.; Miyazaki, Y.; Kunishima, H.; Hirakata, Y.; Kaku, M.; et al. A possible relationship of natural killer T cells with humoral immune response to 23-valent pneumococcal polysaccharide vaccine in clinical settings. Vaccine 2012, 30, 3304–3310. [Google Scholar] [CrossRef] [PubMed]
- Davila, S.; Froeling, F.E.; Tan, A.; Bonnard, C.; Boland, G.J.; Snippe, H.; Hibberd, M.L.; Seielstad, M. New genetic associations detected in a host response study to hepatitis B vaccine. Genes Immun. 2010, 11, 232–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ovsyannikova, I.G.; Kennedy, R.B.; O’Byrne, M.; Jacobson, R.M.; Pankratz, V.S.; Poland, G.A. Genome-wide association study of antibody response to smallpox vaccine. Vaccine 2012, 30, 4182–4189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giambra, V.; Fruscalzo, A.; Giufre, M.; Martinez-Labarga, C.; Favaro, M.; Rocchi, M.; Frezza, D. Evolution of human IgH3’EC duplicated structures: Both enhancers HS1,2 are polymorphic with variation of transcription factor’s consensus sites. Gene 2005, 346, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Muik, A.; Wallisch, A.K.; Sanger, B.; Swanson, K.A.; Muhl, J.; Chen, W.; Cai, H.; Maurus, D.; Sarkar, R.; Tureci, O.; et al. Neutralization of SARS-CoV-2 lineage B.1.1.7 pseudovirus by BNT162b2 vaccine-elicited human sera. Science 2021, 371, 1152–1153. [Google Scholar] [CrossRef]
- Jones, N.K.; Rivett, L.; Seaman, S.; Samworth, R.J.; Warne, B.; Workman, C.; Ferris, M.; Wright, J.; Quinnell, N.; Shaw, A.; et al. Single-dose BNT162b2 vaccine protects against asymptomatic SARS-CoV-2 infection. eLife 2021, 10, e68808. [Google Scholar] [CrossRef]
- Pape, K.A.; Dileepan, T.; Kabage, A.J.; Kozysa, D.; Batres, R.; Evert, C.; Matson, M.; Lopez, S.; Krueger, P.D.; Graiziger, C.; et al. High-affinity memory B cells induced by SARS-CoV-2 infection produce more plasmablasts and atypical memory B cells than those primed by mRNA vaccines. Cell Rep. 2021, 37, 109823. [Google Scholar] [CrossRef] [PubMed]
- Sakharkar, M.; Rappazzo, C.G.; Wieland-Alter, W.F.; Hsieh, C.L.; Wrapp, D.; Esterman, E.S.; Kaku, C.I.; Wec, A.Z.; Geoghegan, J.C.; McLellan, J.S.; et al. Prolonged evolution of the human B cell response to SARS-CoV-2 infection. Sci. Immunol. 2021, 6. [Google Scholar] [CrossRef]
- DiMuzio, J.M.; Heimbach, B.C.; Howanski, R.J.; Dowling, J.P.; Patel, N.B.; Henriquez, N.; Nicolescu, C.; Nath, M.; Polley, A.; Bingaman, J.L.; et al. Unbiased interrogation of memory B cells from convalescent COVID-19 patients reveals a broad antiviral humoral response targeting SARS-CoV-2 antigens beyond the spike protein. Vaccine X 2021, 8, 100098. [Google Scholar] [CrossRef] [PubMed]
- Reyes, R.A.; Clarke, K.; Gonzales, S.J.; Cantwell, A.M.; Garza, R.; Catano, G.; Tragus, R.E.; Patterson, T.F.; Bol, S.; Bunnik, E.M. SARS-CoV-2 spike-specific memory B cells express markers of durable immunity after non-severe COVID-19 but not after severe disease. bioRxiv 2021. [Google Scholar] [CrossRef]
- Xie, J.; Ding, C.; He, J.; Zhang, Y.; Ni, S.; Zhang, X.; Chen, Q.; Wang, J.; Huang, L.; He, H.; et al. Novel Monoclonal Antibodies and Recombined Antibodies Against Variant SARS-CoV-2. Front. Immunol. 2021, 12, 715464. [Google Scholar] [CrossRef]
- Giambra, V.; Volpi, S.; Emelyanov, A.V.; Pflugh, D.; Bothwell, A.L.; Norio, P.; Fan, Y.; Ju, Z.; Skoultchi, A.I.; Hardy, R.R.; et al. Pax5 and linker histone H1 coordinate DNA methylation and histone modifications in the 3’ regulatory region of the immunoglobulin heavy chain locus. Mol. Cell Biol. 2008, 28, 6123–6133. [Google Scholar] [CrossRef] [Green Version]
- Puget, N.; Hirasawa, R.; Hu, N.S.; Laviolette-Malirat, N.; Feil, R.; Khamlichi, A.A. Insertion of an imprinted insulator into the IgH locus reveals developmentally regulated, transcription-dependent control of V(D)J recombination. Mol. Cell Biol. 2015, 35, 529–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Demographic and Clinical Characteristics of Partecipants | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sampling Distribution | COV-IgG | ||||||||||
| Counts | % of Tot. | M | F | Low Responsive (COV-IgG < 3.05 AU/mL) | High Responsive (COV-IgG ≥ 3.05 AU/mL) | Min (AU/mL) | Max (AU/mL) | Mean | SD | ||
| Individuals | Naive | 123 | 76.9% | 57 | 66 | 30 | 93 | 0 | >750 | 77.8 | 196.6 |
| ex-COVID-19 | 37 | 23.1% | 20 | 17 | 2 | 35 | 0.59 | >750 | 306.9 | 275.6 | |
| Tot. | 160 | 100% | 77 | 83 | 30 | 93 | 0 | >750 | 130.8 | 237.2 | |
| Counts | % of tot. | Average age | ex-COV.(%) | Low Responsive (COV-IgG < 3.05 AU/mL) | High Responsive (COV-IgG ≥ 3.05 AU/mL) | Min (AU/mL) | Max (AU/mL) | Mean | SD | ||
| Gender | M | 77 | 48.1% | 50 | 26% | 16 | 41 | 0 | >750 | 140.6 | 240.5 |
| F | 83 | 51.9% | 49 | 20.5% | 14 | 52 | 0 | >750 | 121.6 | 235 | |
| Counts | % of tot. | Gender | ex-COV.(%) | Low Responsive (COV-IgG < 3.05 AU/mL) | High Responsive (COV-IgG ≥ 3.05 AU/mL) | Min (AU/mL) | Max (AU/mL) | Mean | SD | ||
| Age Class | 21–30 | 16 | 10% | 9M–7F | 25% | 1 | 11 | 1.73 | >750 | 126.84 | 217.9 |
| 31–40 | 22 | 13.7% | 8M–14F | 27.3% | 3 | 13 | 0.28 | >750 | 136.36 | 251.9 | |
| 41–50 | 34 | 21.3% | 18M–16F | 20.6% | 5 | 22 | 0.53 | 673 | 136.98 | 238.2 | |
| 51–60 | 52 | 32.5% | 20M–32F | 21.2% | 10 | 31 | 0 | >750 | 125.52 | 238 | |
| 60+ | 36 | 22.5% | 22M–14F | 25% | 11 | 16 | 0 | >750 | 130.83 | 246.9 | |
| SNP Code | Nt. | Low | High | Low | High | |
|---|---|---|---|---|---|---|
| rs73084296 | A | 0.74 | 0.54 | χ2 | 0.10 | 6.37 |
| G | 0.26 | 0.46 | p-value | NS | ** | |
| rs7494440 | C | 0.64 | 0.28 | χ2 | 2.33 | 3.30 |
| G | 0.36 | 0.72 | p-value | NS | NS | |
| rs7494441 | C | 0.66 | 0.54 | χ2 | 3.54 | 25.77 |
| T | 0.34 | 0.46 | p-value | NS | *** | |
| rs61986170 | G | 0.80 | 0.77 | χ2 | 0.00 | 1.12 |
| A | 0.19 | 0.22 | p-value | NS | NS | |
| rs61986171 | C | 0.82 | 0.75 | χ2 | 1.14 | 0.24 |
| T | 0.71 | 0.25 | p-value | NS | NS | |
| rs12896746 | A | 0.38 | 0.32 | χ2 | 5.56 | 11.49 |
| G | 0.61 | 0.67 | p-value | ** | *** | |
| rs12896897 | C | 0.55 | 0.53 | χ2 | 5.37 | 24.33 |
| A | 0.44 | 0.46 | p-value | ** | *** | |
| rs7144089 | C | 0.46 | 0.51 | χ2 | 7.26 | 23.37 |
| G | 0.54 | 0.48 | p-value | ** | *** | |
| rs7143677 | A | 0.72 | 0.71 | χ2 | 3.31 | 2.68 |
| G | 0.27 | 0.28 | p-value | NS | NS |
| rs73084296 | rs7494441 | ||||||||||
| Low | High | Low | High | ||||||||
| Genotype | Obs. (%) | Exp. (%) | Obs. (%) | Exp. (%) | Δ(Low-High) | Genotype | Obs. (%) | Exp. (%) | Obs. (%) | Exp. (%) | Δ(Low-High) |
| A/A | 56 | 54.8 | 21.2 | 28.9 | 34.8 | C/C | 52 | 43.6 | 44.1 | 28.8 | 7.9 |
| A/G | 36 | 38.5 | 65.2 | 49.7 | −29.2 | C/T | 28 | 44.9 | 19.1 | 49.7 | 8.9 |
| G/G | 8 | 6.8 | 13.6 | 38.5 | −5.6 | T/T | 20 | 11.6 | 36.8 | 21.5 | −16.8 |
| rs12896746 | rs12896897 | ||||||||||
| Low | High | Low | High | ||||||||
| Genotype | Obs. (%) | Exp. (%) | Obs. (%) | Exp. (%) | Δ(Low-High) | Genotype | Obs. (%) | Exp. (%) | Obs. (%) | Exp. (%) | Δ(Low-High) |
| A/A | 3.8 | 14.8 | 1.5 | 10.5 | 2.3 | C/C | 43.1 | 28.6 | 42.3 | 31.1 | −0.8 |
| A/G | 69.2 | 47.3 | 61.8 | 43.8 | 7.4 | C/T | 20.8 | 49.8 | 26.9 | 49.3 | 6.1 |
| G/G | 26.9 | 37.9 | 36.8 | 45.8 | −9.9 | T/T | 36.1 | 21.6 | 30.8 | 19.6 | −5.3 |
| rs7144089 | |||||||||||
| Low | High | ||||||||||
| Genotype | Obs. (%) | Exp. (%) | Obs. (%) | Exp. (%) | Δ(Low-High) | ||||||
| C/C | 34.8 | 20.8 | 41.5 | 26.6 | −6.7 | ||||||
| C/G | 21.7 | 49.6 | 20 | 50 | 1.7 | ||||||
| G/G | 43.5 | 29.5 | 38.5 | 23.5 | 5 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colucci, M.; De Santis, E.; Totti, B.; Miroballo, M.; Tamiro, F.; Rossi, G.; Piepoli, A.; De Vincentis, G.; Greco, A.; Mangia, A.; et al. Associations between Allelic Variants of the Human IgH 3? Regulatory Region 1 and the Immune Response to BNT162b2 mRNA Vaccine. Vaccines 2021, 9, 1207. https://doi.org/10.3390/vaccines9101207
Colucci M, De Santis E, Totti B, Miroballo M, Tamiro F, Rossi G, Piepoli A, De Vincentis G, Greco A, Mangia A, et al. Associations between Allelic Variants of the Human IgH 3? Regulatory Region 1 and the Immune Response to BNT162b2 mRNA Vaccine. Vaccines. 2021; 9(10):1207. https://doi.org/10.3390/vaccines9101207
Chicago/Turabian StyleColucci, Mattia, Elisabetta De Santis, Beatrice Totti, Mattia Miroballo, Francesco Tamiro, Giovanni Rossi, Ada Piepoli, Gabriella De Vincentis, Antonio Greco, Alessandra Mangia, and et al. 2021. "Associations between Allelic Variants of the Human IgH 3? Regulatory Region 1 and the Immune Response to BNT162b2 mRNA Vaccine" Vaccines 9, no. 10: 1207. https://doi.org/10.3390/vaccines9101207
APA StyleColucci, M., De Santis, E., Totti, B., Miroballo, M., Tamiro, F., Rossi, G., Piepoli, A., De Vincentis, G., Greco, A., Mangia, A., Cianci, R., Di Mauro, L., Miscio, G., & Giambra, V. (2021). Associations between Allelic Variants of the Human IgH 3? Regulatory Region 1 and the Immune Response to BNT162b2 mRNA Vaccine. Vaccines, 9(10), 1207. https://doi.org/10.3390/vaccines9101207







