Evaluation of Immunogenicity and Efficacy of Fasciola hepatica Tetraspanin 2 (TSP2) Fused to E. coli Heat-Labile Enterotoxin B Subunit LTB Adjuvant Following Intranasal Vaccination of Cattle
Abstract
:1. Introduction
2. Materials and Methods
2.1. Integration of Constructs into Pichia pastoris
2.2. Expression and Purification of Recombinant Proteins
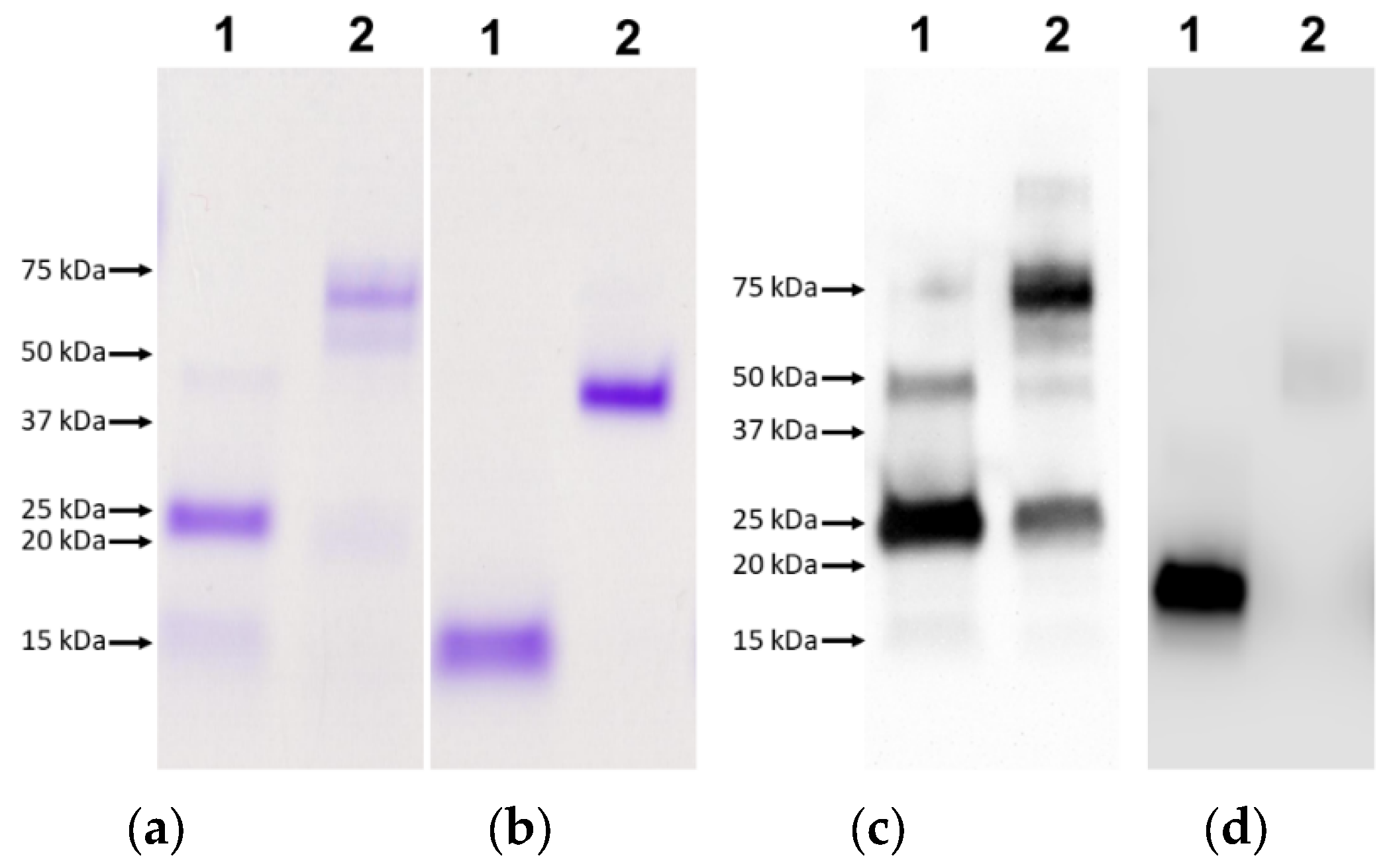
2.3. Protein Separation via SDS-PAGE and Western Blot Analysis
2.4. Characterisation of Antigenicity of Recombinant Proteins
2.5. Characterisation of GM1 Binding of Recombinant AB5 Toxin
2.6. Experimental Animals and Immunisation
2.7. Assessment of Protection
2.8. Assessment of Antibody Responses
2.9. Statistical Analysis
3. Results
3.1. Recombinant LTB-TSP2 Fusion Protein Expressed in Pichia pastoris
3.2. Characterisation of Oligomerisation and Antigenicity of rLTB-FhTSP2
3.3. Efficacy Assessment of rFhTSP2 and rLTB-FhTSP2 as Vaccines in Cattle
3.4. Humoral IgG and IgA Responses in Cattle and Correlations with Fluke Burdens
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schweizer, G.; Braun, U.; Deplazes, P.; Torgerson, P.R. Estimating the financial losses due to bovine fasciolosis in Switzerland. Vet. Rec. 2005, 157, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Molina-Hernández, V.; Mulcahy, G.; Pérez, J.; Martínez-Moreno, Á.; Donnelly, S.; O’Neill, S.M.; Dalton, J.P.; Cwiklinski, K. Fasciola hepatica vaccine: We may not be there yet but we’re on the right road. Vet. Parasitol. 2015, 208, 101–111. [Google Scholar] [CrossRef] [Green Version]
- Charlier, J.; Vercruysse, J.; Morgan, E.; Van Dijk, J.; Williams, D. Recent advances in the diagnosis, impact on production and prediction of Fasciola hepatica in cattle. Parasitology 2014, 141, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Zerna, G.; Spithill, T.W.; Beddoe, T. Current Status for Controlling the Overlooked Caprine Fasciolosis. Animals 2021, 11, 1819. [Google Scholar] [CrossRef] [PubMed]
- Cwiklinski, K.; O’Neill, S.M.; Donnelly, S.; Dalton, J.P. A prospective view of animal and human Fasciolosis. Parasite Immunol. 2016, 38, 558–568. [Google Scholar] [CrossRef] [Green Version]
- Brockwell, Y.M.; Elliott, T.P.; Anderson, G.R.; Stanton, R.; Spithill, T.W.; Sangster, N.C. Confirmation of Fasciola hepatica resistant to triclabendazole in naturally infected Australian beef and dairy cattle. Int. J. Parasitol-Drug 2014, 4, 48–54. [Google Scholar] [CrossRef] [Green Version]
- Kelley, J.M.; Elliott, T.P.; Beddoe, T.; Anderson, G.; Skuce, P.; Spithill, T.W. Current threat of triclabendazole resistance in Fasciola hepatica. Trends Parasitol. 2016, 32, 458–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fairweather, I.; Brennan, G.; Hanna, R.; Robinson, M.; Skuce, P. Drug resistance in liver flukes. Int. J. Parasitol. Drugs Drug Resist. 2020, 12, 39–59. [Google Scholar] [CrossRef]
- Toet, H.; Piedrafita, D.M.; Spithill, T.W. Liver fluke vaccines in ruminants: Strategies, progress and future opportunities. Int. J. Parasitol. 2014, 44, 915–927. [Google Scholar] [CrossRef]
- Golden, O.; Flynn, R.J.; Read, C.; Sekiya, M.; Donnelly, S.; Stack, C.; Dalton, J.P.; Mulcahy, G. Protection of cattle against a natural infection of Fasciola hepatica by vaccination with recombinant cathepsin L1 (rFhCL1). Vaccine 2010, 28, 5551–5557. [Google Scholar] [CrossRef]
- Morrison, C.A.; Colin, T.; Sexton, J.L.; Bowen, F.; Wicker, J.; Friedel, T.; Spithill, T.W. Protection of cattle against Fasciola hepatica infection by vaccination with glutathione S-transferase. Vaccine 1996, 14, 1603–1612. [Google Scholar] [CrossRef]
- Spithill, T.W.; Toet, H.; Rathinasamy, V.; Zerna, G.; Swan, J.; Cameron, T.; Smooker, P.M.; Piedrafita, D.M.; Dempster, R.; Beddoe, T. Vaccines for Fasciola (liver fluke): New thinking for an old problem. In Fasciolosis, 2nd ed.; CAB International: Wallingford, UK, 2021; submitted; accepted; in press. [Google Scholar]
- Sulaiman, A.A.; Zolnierczyk, K.; Japa, O.; Owen, J.P.; Maddison, B.C.; Emes, R.D.; Hodgkinson, J.E.; Gough, K.C.; Flynn, R.J. A trematode parasite derived growth factor binds and exerts influences on host immune functions via host cytokine receptor complexes. PLoS Path. 2016, 12, e1005991. [Google Scholar] [CrossRef] [Green Version]
- Wilson, R.A.; Wright, J.M.; de Castro-Borges, W.; Parker-Manuel, S.J.; Dowle, A.A.; Ashton, P.D.; Young, N.D.; Gasser, R.B.; Spithill, T.W. Exploring the Fasciola hepatica tegument proteome. Int. J. Parasitol. 2011, 41, 1347–1359. [Google Scholar] [CrossRef] [Green Version]
- McNulty, S.N.; Tort, J.F.; Rinaldi, G.; Fischer, K.; Rosa, B.A.; Smircich, P.; Fontenla, S.; Choi, Y.-J.; Tyagi, R.; Hallsworth-Pepin, K. Genomes of Fasciola hepatica from the Americas reveal colonization with Neorickettsia endobacteria related to the agents of Potomac horse and human Sennetsu fevers. PLoS Genet. 2017, 13, e1006537. [Google Scholar] [CrossRef]
- Cwiklinski, K.; Dalton, J.P.; Dufresne, P.J.; La Course, J.; Williams, D.J.; Hodgkinson, J.; Paterson, S. The Fasciola hepatica genome: Gene duplication and polymorphism reveals adaptation to the host environment and the capacity for rapid evolution. Genome Biol. 2015, 16, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemler, M.E. Specific tetraspanin functions. J. Cell. Biol. 2001, 155, 1103. [Google Scholar] [CrossRef]
- Van Deventer, S.J.; Dunlock, V.-M.E.; van Spriel, A.B. Molecular interactions shaping the tetraspanin web. Biochem. Soc. Trans. 2017, 45, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Braschi, S.; Curwen, R.S.; Ashton, P.D.; Verjovski-Almeida, S.; Wilson, A. The tegument surface membranes of the human blood parasite Schistosoma mansoni: A proteomic analysis after differential extraction. Proteomics 2006, 6, 1471–1482. [Google Scholar] [CrossRef] [PubMed]
- Braschi, S.; Wilson, R.A. Proteins exposed at the adult schistosome surface revealed by biotinylation. Mol. Cell. Proteomics 2006, 5, 347–356. [Google Scholar] [CrossRef] [Green Version]
- Mulvenna, J.; Sripa, B.; Brindley, P.J.; Gorman, J.; Jones, M.K.; Colgrave, M.L.; Jones, A.; Nawaratna, S.; Laha, T.; Suttiprapa, S. The secreted and surface proteomes of the adult stage of the carcinogenic human liver fluke Opisthorchis viverrini. Proteomics 2010, 10, 1063–1078. [Google Scholar] [CrossRef] [Green Version]
- Sotillo, J.; Pearson, M.; Becker, L.; Mulvenna, J.; Loukas, A. A quantitative proteomic analysis of the tegumental proteins from Schistosoma mansoni schistosomula reveals novel potential therapeutic targets. Int. J. Parasitol. 2015, 45, 505–516. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Yu, K.; Liang, A.; Huang, Y.; Ou, F.; Wei, H.; Wan, X.; Yang, Y.; Zhang, W.; Jiang, Z. Identification and analysis of the tegument protein and excretory-secretory products of the carcinogenic liver fluke Clonorchis Sinensis. Front. Microbiol. 2020, 11, 2298. [Google Scholar] [CrossRef]
- Mulvenna, J.; Moertel, L.; Jones, M.K.; Nawaratna, S.; Lovas, E.M.; Gobert, G.N.; Colgrave, M.; Jones, A.; Loukas, A.; McManus, D.P. Exposed proteins of the Schistosoma japonicum tegument. Int. J. Parasitol. 2010, 40, 543–554. [Google Scholar] [CrossRef]
- Tran, M.H.; Pearson, M.S.; Bethony, J.M.; Smyth, D.J.; Jones, M.K.; Duke, M.; Don, T.A.; McManus, D.P.; Correa-Oliveira, R.; Loukas, A. Tetraspanins on the surface of Schistosoma mansoni are protective antigens against schistosomiasis. Nat. Med. 2006, 12, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Pearson, M.S.; Pickering, D.A.; McSorley, H.J.; Bethony, J.M.; Tribolet, L.; Dougall, A.M.; Hotez, P.J.; Loukas, A. Enhanced protective efficacy of a chimeric form of the schistosomiasis vaccine antigen Sm-TSP-2. PLoS Negl. Trop. Dis. 2012, 6, e1564. [Google Scholar] [CrossRef] [Green Version]
- Pinheiro, C.S.; Ribeiro, A.P.; Cardoso, F.C.; Martins, V.P.; Figueiredo, B.C.; Assis, N.R.; Morais, S.B.; Caliari, M.V.; Loukas, A.; Oliveira, S.C. A multivalent chimeric vaccine composed of Schistosoma mansoni SmTSP-2 and Sm29 was able to induce protection against infection in mice. Parasite Immunol. 2014, 36, 303–312. [Google Scholar] [CrossRef] [Green Version]
- Keitel, W.; Potter, G.; Diemert, D.; Bethony, J.; El Sahly, H.; Kennedy, J.; Patel, S.; Plieskatt, J.; Jones, W.; Deye, G. A phase 1 study of the safety, reactogenicity, and immunogenicity of a Schistosoma mansoni vaccine with or without glucopyranosyl lipid A aqueous formulation (GLA-AF) in healthy adults from a non-endemic area. Vaccine 2019, 37, 6500–6509. [Google Scholar] [CrossRef]
- Eyayu, T.; Zeleke, A.J.; Worku, L. Current status and future prospects of protein vaccine candidates against Schistosoma mansoni infection. Parasite Epidemiol. Control. 2020, 11, e00176. [Google Scholar] [CrossRef] [PubMed]
- Chaiyadet, S.; Sotillo, J.; Krueajampa, W.; Thongsen, S.; Brindley, P.J.; Sripa, B.; Loukas, A.; Laha, T. Vaccination of hamsters with Opisthorchis viverrini extracellular vesicles and vesicle-derived recombinant tetraspanins induces antibodies that block vesicle uptake by cholangiocytes and reduce parasite burden after challenge infection. PLoS Negl. Trop. Dis. 2019, 13, e0007450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phung, L.T.; Chaiyadet, S.; Hongsrichan, N.; Sotillo, J.; Dieu, H.D.T.; Tran, C.Q.; Brindley, P.J.; Loukas, A.; Laha, T. Recombinant Opisthorchis viverrini tetraspanin expressed in Pichia pastoris as a potential vaccine candidate for opisthorchiasis. Parasitol. Res. 2019, 118, 3419–3427. [Google Scholar] [CrossRef]
- Cwiklinski, K.; de la Torre-Escudero, E.; Trelis, M.; Bernal, D.; Dufresne, P.J.; Brennan, G.P.; O’Neill, S.; Tort, J.; Paterson, S.; Marcilla, A.; et al. The Extracellular Vesicles of the Helminth Pathogen, Fasciola hepatica: Biogenesis Pathways and Cargo Molecules Involved in Parasite Pathogenesis. Mol. Cell. Proteom. 2015, 14, 3258–3273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalton, J.P.; Robinson, M.W.; Mulcahy, G.; O’Neill, S.M.; Donnelly, S. Immunomodulatory molecules of Fasciola hepatica: Candidates for both vaccine and immunotherapeutic development. Vet. Parasitol. 2013, 195, 272–285. [Google Scholar] [CrossRef]
- McNeilly, T.N.; Nisbet, A.J. Immune modulation by helminth parasites of ruminants: Implications for vaccine development and host immune competence. Parasite 2014, 21, 51. [Google Scholar] [CrossRef] [Green Version]
- Piedrafita, D.; Raadsma, H.W.; Prowse, R.; Spithill, T.W. Immunology of the host–parasite relationship in fasciolosis (Fasciola hepatica and Fasciola gigantica). Can. J. Zool. 2004, 82, 233–250. [Google Scholar] [CrossRef]
- Pleasance, J.; Raadsma, H.W.; Estuningsih, S.E.; Widjajanti, S.; Meeusen, E.; Piedrafita, D. Innate and adaptive resistance of Indonesian Thin Tail sheep to liver fluke: A comparative analysis of Fasciola gigantica and Fasciola hepatica infection. Vet. Parasitol. 2011, 178, 264–272. [Google Scholar] [CrossRef]
- Beddoe, T.; Paton, A.W.; Le Nours, J.; Rossjohn, J.; Paton, J.C. Structure, biological functions and applications of the AB5 toxins. Trends Biochem. Sci. 2010, 35, 411–418. [Google Scholar] [CrossRef] [Green Version]
- Williams, N.A.; Hirst, T.R.; Nashar, T.O. Immune modulation by the cholera-like enterotoxins: From adjuvant to therapeutic. Immunol. ToDay 1999, 20, 95–101. [Google Scholar] [CrossRef]
- Da Hora, V.P.; Conceicao, F.R.; Dellagostin, O.A.; Doolan, D.L. Non-toxic derivatives of LT as potent adjuvants. Vaccine 2011, 29, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- Conceição, F.R.; Moreira, Â.N.; Dellagostin, O.A. A recombinant chimera composed of R1 repeat region of Mycoplasma hyopneumoniae P97 adhesin with Escherichia coli heat-labile enterotoxin B subunit elicits immune response in mice. Vaccine 2006, 24, 5734–5743. [Google Scholar] [CrossRef]
- Mudrak, B.; Kuehn, M.J. Heat-labile enterotoxin: Beyond G M1 binding. Toxins 2010, 2, 1445–1470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchioro, S.B.; Fisch, A.; Gomes, C.K.; Jorge, S.; Galli, V.; Haesebrouck, F.; Maes, D.; Dellagostin, O.; Conceicao, F.R. Local and systemic immune responses induced by a recombinant chimeric protein containing Mycoplasma hyopneumoniae antigens fused to the B subunit of Escherichia coli heat-labile enterotoxin LTB. Vet. Microbiol. 2014, 173, 166–171. [Google Scholar] [CrossRef]
- Grassmann, A.A.; Felix, S.R.; dos Santos, C.X.; Amaral, M.G.; Seixas Neto, A.C.; Fagundes, M.Q.; Seixas, F.K.; da Silva, E.F.; Conceicao, F.R.; Dellagostin, O.A. Protection against lethal leptospirosis after vaccination with LipL32 coupled or coadministered with the B subunit of Escherichia coli heat-labile enterotoxin. Clin. Vaccine Immunol. 2012, 19, 740–745. [Google Scholar] [CrossRef] [Green Version]
- Cameron, T.C.; Cooke, I.; Faou, P.; Toet, H.; Piedrafita, D.; Young, N.; Rathinasamy, V.; Beddoe, T.; Anderson, G.; Dempster, R. A novel ex vivo immunoproteomic approach characterising Fasciola hepatica tegumental antigens identified using immune antibody from resistant sheep. Int. J. Parasitol. 2017, 47, 555–567. [Google Scholar] [CrossRef]
- Elliott, T.; Muller, A.; Brockwell, Y.; Murphy, N.; Grillo, V.; Toet, H.; Anderson, G.; Sangster, N.; Spithill, T. Evidence for high genetic diversity of NAD1 and COX1 mitochondrial haplotypes among triclabendazole resistant and susceptible populations and field isolates of Fasciola hepatica (liver fluke) in Australia. Vet. Parasitol. 2014, 200, 90–96. [Google Scholar] [CrossRef]
- Ng, N.M.; Littler, D.R.; Paton, A.W.; Le Nours, J.; Rossjohn, J.; Paton, J.C.; Beddoe, T. EcxAB is a founding member of a new family of metalloprotease AB5 toxins with a hybrid cholera-like B subunit. Structure 2013, 21, 2003–2013. [Google Scholar] [CrossRef] [Green Version]
- Brockwell, Y.; Spithill, T.; Anderson, G.; Grillo, V.; Sangster, N. Comparative kinetics of serological and coproantigen ELISA and faecal egg count in cattle experimentally infected with Fasciola hepatica and following treatment with triclabendazole. Vet. Parasitol. 2013, 196, 417–426. [Google Scholar] [CrossRef]
- McCusker, P.; Toet, H.; Rathinasamy, V.; Young, N.; Beddoe, T.; Anderson, G.; Dempster, R.; McVeigh, P.; McCammick, E.; Wells, D. Molecular characterisation and vaccine efficacy of two novel developmentally regulated surface tegument proteins of Fasciola hepatica. Vet. Parasitol. 2020, 286, 109244. [Google Scholar] [CrossRef]
- Sandkvist, M.; Bagdasarian, M. Suppression of temperature-sensitive assembly mutants of heat-labile enterotoxin B subunits. Mol. Microbiol. 1993, 10, 635–645. [Google Scholar] [CrossRef]
- Tran, M.H.; Freitas, T.C.; Cooper, L.; Gaze, S.; Gatton, M.L.; Jones, M.K.; Lovas, E.; Pearce, E.J.; Loukas, A. Suppression of mRNAs encoding tegument tetraspanins from Schistosoma mansoni results in impaired tegument turnover. PLoS Path. 2010, 6, e1000840. [Google Scholar] [CrossRef] [Green Version]
- Chaiyadet, S.; Krueajampa, W.; Hipkaeo, W.; PLosan, Y.; Piratae, S.; Sotillo, J.; Smout, M.; Sripa, B.; Brindley, P.J.; Loukas, A. Suppression of mRNAs encoding CD63 family tetraspanins from the carcinogenic liver fluke Opisthorchis viverrini results in distinct tegument phenotypes. Sci. Rep. 2017, 7, 14342. [Google Scholar] [CrossRef]
- Piedrafita, D.; Estuningsih, E.; Pleasance, J.; Prowse, R.; Raadsma, H.W.; Meeusen, E.N.; Spithill, T.W. Peritoneal lavage cells of Indonesian thin-tail sheep mediate antibody-dependent superoxide radical cytotoxicity in vitro against newly excysted juvenile Fasciola gigantica but not juvenile Fasciola hepatica. Infect. Immun. 2007, 75, 1954–1963. [Google Scholar] [CrossRef] [Green Version]
- Piedrafita, D.; Parsons, J.C.; Sandeman, R.M.; Wood, P.; Estuningsih, S.; Partoutomo, S.; Spithill, T.W. Antibody-dependent cell-mediated cytotoxicity to newly excysted juvenile Fasciola hepatica in vitro is mediated by reactive nitrogen intermediates. Parasite Immunol. 2001, 23, 473–482. [Google Scholar] [CrossRef] [Green Version]
- Lammas, D.; Duffus, W. The shedding of the outer glycocalyx of juvenile Fasciola hepatica. Vet. Parasitol. 1983, 12, 165–178. [Google Scholar] [CrossRef]
- González-Miguel, J.; Becerro-Recio, D.; Siles-Lucas, M. Insights into Fasciola hepatica Juveniles: Crossing the Fasciolosis Rubicon. Trends Parasitol. 2020, 37, 35–47. [Google Scholar] [CrossRef]
- Hanna, R. Fasciola hepatica: Glycocalyx replacement in the juvenile as a possible mechanism for protection against host immunity. Exp. Parasitol. 1980, 50, 103–114. [Google Scholar] [CrossRef]
- Zhang, W.; Li, J.; Duke, M.; Jones, M.K.; Kuang, L.; Zhang, J.; Blair, D.; Li, Y.; McManus, D.P. Inconsistent protective efficacy and marked polymorphism limits the value of Schistosoma japonicum tetraspanin-2 as a vaccine target. PLoS Negl. Trop. Dis. 2011, 5, e1166. [Google Scholar] [CrossRef]
- Yuan, C.; Fu, Y.-j.; Li, J.; Yue, Y.-f.; Cai, L.-l.; Xiao, W.-j.; Chen, J.-p.; Yang, L. Schistosoma japonicum: Efficient and rapid purification of the tetraspanin extracellular loop 2, a potential protective antigen against schistosomiasis in mammalian. Exp. Parasitol. 2010, 126, 456–461. [Google Scholar] [CrossRef]
- Cai, P.; Bu, L.; Wang, J.; Wang, Z.; Zhong, X.; Wang, H. Molecular characterization of Schistosoma japonicum tegument protein tetraspanin-2: Sequence variation and possible implications for immune evasion. Biochem. Biophys. Res. Commun. 2008, 372, 197–202. [Google Scholar] [CrossRef]
- Dominguez, M.F.; González-Miguel, J.; Carmona, C.; Dalton, J.P.; Cwiklinski, K.; Tort, J.; Siles-Lucas, M. Low allelic diversity in vaccine candidates genes from different locations sustain hope for Fasciola hepatica immunization. Vet. Parasitol. 2018, 258, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Jin, Z.; Gao, S.; Cui, X.; Sun, D.; Zhao, K. Adjuvants and delivery systems based on polymeric nanoparticles for mucosal vaccines. Int. J. Pharm. 2019, 572, 118731. [Google Scholar] [CrossRef]
- Chen, H. Recent advances in mucosal vaccine development. J. Controlled Release 2000, 67, 117–128. [Google Scholar] [CrossRef]
- Su, F.; Xu, L.; Xue, Y.; Li, J.; Fu, Y.; Yu, B.; Wang, S.; Yuan, X. Th1-biased immunoadjuvant effect of the recombinant B subunit of an Escherichia coli heat-labile enterotoxin on an inactivated PRRSV antigen via intranasal immunization in mice. J. Vet. Med. Sci. 2019, 81, 1475–1484. [Google Scholar] [CrossRef]
- Weltzin, R.; Guy, B.; Thomas, W.D., Jr.; Giannasca, P.J.; Monath, T.P. Parenteral adjuvant activities of Escherichia coli heat-labile toxin and its B subunit for immunization of mice against gastric Helicobacter pylori infection. Infect. Immun. 2000, 68, 2775–2782. [Google Scholar] [CrossRef] [Green Version]
- Boyaka, P.N.; Ohmura, M.; Fujihashi, K.; Koga, T.; Yamamoto, M.; Kweon, M.N.; Takeda, Y.; Jackson, R.J.; Kiyono, H.; Yuki, Y.; et al. Chimeras of labile toxin one and cholera toxin retain mucosal adjuvanticity and direct Th cell subsets via their B subunit. J. Immunol. 2003, 170, 454–462. [Google Scholar] [CrossRef] [Green Version]
- Duan, Q.; Xia, P.; Nandre, R.; Zhang, W.; Zhu, G. Review of newly identified functions associated with the heat-labile toxin of enterotoxigenic Escherichia coli. Front. Cell. Infect. Microbiol. 2019, 9, 292. [Google Scholar] [CrossRef] [Green Version]
- Chia, M.-Y.; Hsiao, S.-H.; Chan, H.-T.; Do, Y.-Y.; Huang, P.-L.; Chang, H.-W.; Tsai, Y.-C.; Lin, C.-M.; Pang, V.F.; Jeng, C.-R. Evaluation of the immunogenicity of a transgenic tobacco plant expressing the recombinant fusion protein of GP5 of porcine reproductive and respiratory syndrome virus and B subunit of Escherichia coli heat-labile enterotoxin in pigs. Vet. Immunol. Immunopathol. 2011, 140, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Yokomizo, Y.; Watanabe, F.; Imada, Y.; Inumaru, S.; Yanaka, T.; Tsuji, T. Mucosal immunoadjuvant activity of the low toxic recombinant Escherichia coli heat-labile enterotoxin produced by Bacillus brevis for the bacterial subunit or component vaccine in pigs and cattle. Vet. Immunol. Immunopathol. 2002, 87, 291–300. [Google Scholar] [CrossRef]
- Ayalew, S.; Step, D.; Montelongo, M.; Confer, A. Intranasal vaccination of calves with Mannheimia haemolytica chimeric protein containing the major surface epitope of outer membrane lipoprotein PlpE, the neutralizing epitope of leukotoxin, and cholera toxin subunit B. Vet. Immunol. Immunopathol. 2009, 132, 295–302. [Google Scholar] [CrossRef]
- Cunha, C.E.; Moreira, G.M.; Salvarani, F.M.; Neves, M.S.; Lobato, F.C.; Dellagostin, O.A.; Conceicao, F.R. Vaccination of cattle with a recombinant bivalent toxoid against botulism serotypes C and D. Vaccine 2014, 32, 214–216. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.; Estuningsih, E.; Wiedosari, E.; Spithill, T. Acquisition of resistance against Fasciola gigantica by Indonesian thin tail sheep. Vet. Parasitol. 1997, 73, 215–224. [Google Scholar] [CrossRef]
- Dalton, J.P.; McGonigle, S.; Rolph, T.P.; Andrews, S.J. Induction of protective immunity in cattle against infection with Fasciola hepatica by vaccination with cathepsin L proteinases and with hemoglobin. Infect. Immun. 1996, 64, 5066–5074. [Google Scholar] [CrossRef] [Green Version]
- Mendes, R.E.; Pérez-Écija, R.A.; Zafra, R.; Buffoni, L.; Martínez-Moreno, Á.; Dalton, J.P.; Mulcahy, G.; Pérez, J. Evaluation of hepatic changes and local and systemic immune responses in goats immunized with recombinant Peroxiredoxin (Prx) and challenged with Fasciola hepatica. Vaccine 2010, 28, 2832–2840. [Google Scholar] [CrossRef]
- Piacenza, L.; Acosta, D.; Basmadjian, I.; Dalton, J.P.; Carmona, C. Vaccination with cathepsin L proteinases and with leucine aminopeptidase induces high levels of protection against fascioliasis in sheep. Infect. Immun. 1999, 67, 1954–1961. [Google Scholar] [CrossRef]
- Wesolowska, A.; Kozak Ljunggren, M.; Jedlina, L.; Basalaj, K.; Legocki, A.; Wedrychowicz, H.; Kesik-Brodacka, M. A Preliminary Study of a Lettuce-Based Edible Vaccine Expressing the Cysteine Proteinase of Fasciola hepatica for Fasciolosis Control in Livestock. Front. Immunol. 2018, 9, 2592. [Google Scholar] [CrossRef] [PubMed]
- Wedrychowicz, H.; Kesik, M.; Kaliniak, M.; Kozak-Cieszczyk, M.; Jedlina-Panasiuk, L.; Jaros, S.; Plucienniczak, A. Vaccine potential of inclusion bodies containing cysteine proteinase of Fasciola hepatica in calves and lambs experimentally challenged with metacercariae of the fluke. Vet. Parasitol. 2007, 147, 77–88. [Google Scholar] [CrossRef]
- Norbury, L.J.; Basałaj, K.; Zawistowska-Deniziak, A.; Sielicka, A.; Wilkowski, P.; Wesołowska, A.; Smooker, P.M.; Wędrychowicz, H. Intranasal delivery of a formulation containing stage-specific recombinant proteins of Fasciola hepatica cathepsin L5 and cathepsin B2 triggers an anti-fecundity effect and an adjuvant-mediated reduction in fluke burden in sheep. Vet. Parasitol. 2018, 258, 14–23. [Google Scholar] [CrossRef] [PubMed]






| Group | Treatment (Delivery) | Fluke Burdens (mean ± SD) | Efficacay (% Protection) | Fluke Wet Weight (mean ± SD) | Fecal Egg Count (mean ± SD) | Egg Count Reduction | Liver Pathology Score (mean ± SD) |
|---|---|---|---|---|---|---|---|
| 1 | Control PBS (sc) | 48, 63, 66, 68, 77, 111 (72.2 ± 21.2) | - | 1.2, 1.8, 2, 2, 3.9, 4.4 (2.6 ± 1.3) | 1, 3, 3, 3, 4, 23 (6.2 ± 8.3) | - | 1, 2, 2, 2, 2, 3 (2 ± 0.6) |
| 2 | rLTB-FhTSP2 (in) | 23, 43, 46, 62, 91, 94 (59.8 ± 28.2) | 17% | 0.6, 1, 1.5, 2, 2, 2.1 (1.7 ± 0.4) | 1, 1, 1, 1, 2, 4 (1.7 ± 1.2) | 73% | 1, 2, 2, 2, 2, 2 (1.8 ± 0.4) |
| 3 | rFhTSP2 (sc) | 25, 66, 72, 88, 92, 97 (73.3 ± 26.5) | −1.6% | 0.6, 1.7, 2.4, 2.5, 3.1, 3.7 (2.3 ± 1.1) | 0, 1, 2, 6, 10, 16 (5.8 ± 6.2) | 5% | 1, 2, 2, 2, 2, 2 (1.8 ± 0.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zerna, G.; Rathinasamy, V.A.; Toet, H.; Anderson, G.; Dempster, R.; Spithill, T.W.; Beddoe, T. Evaluation of Immunogenicity and Efficacy of Fasciola hepatica Tetraspanin 2 (TSP2) Fused to E. coli Heat-Labile Enterotoxin B Subunit LTB Adjuvant Following Intranasal Vaccination of Cattle. Vaccines 2021, 9, 1213. https://doi.org/10.3390/vaccines9111213
Zerna G, Rathinasamy VA, Toet H, Anderson G, Dempster R, Spithill TW, Beddoe T. Evaluation of Immunogenicity and Efficacy of Fasciola hepatica Tetraspanin 2 (TSP2) Fused to E. coli Heat-Labile Enterotoxin B Subunit LTB Adjuvant Following Intranasal Vaccination of Cattle. Vaccines. 2021; 9(11):1213. https://doi.org/10.3390/vaccines9111213
Chicago/Turabian StyleZerna, Gemma, Vignesh A. Rathinasamy, Hayley Toet, Glenn Anderson, Robert Dempster, Terry W. Spithill, and Travis Beddoe. 2021. "Evaluation of Immunogenicity and Efficacy of Fasciola hepatica Tetraspanin 2 (TSP2) Fused to E. coli Heat-Labile Enterotoxin B Subunit LTB Adjuvant Following Intranasal Vaccination of Cattle" Vaccines 9, no. 11: 1213. https://doi.org/10.3390/vaccines9111213
APA StyleZerna, G., Rathinasamy, V. A., Toet, H., Anderson, G., Dempster, R., Spithill, T. W., & Beddoe, T. (2021). Evaluation of Immunogenicity and Efficacy of Fasciola hepatica Tetraspanin 2 (TSP2) Fused to E. coli Heat-Labile Enterotoxin B Subunit LTB Adjuvant Following Intranasal Vaccination of Cattle. Vaccines, 9(11), 1213. https://doi.org/10.3390/vaccines9111213







