Longitudinal Characterization of the Mumps-Specific HLA-A2 Restricted T-Cell Response after Mumps Virus Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. PBMC Isolation
2.3. Analysis of MuV-Specific T Cells by Flow Cytometry
2.4. Isolation of MuV-Specific T Cells for TCRβ Analysis
2.5. Preparing TCRβ cDNA Libraries for Sequencing
2.6. TCRβ Clonotype Analysis
2.7. Alignment of MuV Peptides
2.8. Statistical Analysis of Flow Cytometry Data
3. Results
3.1. Characteristics of Study Population
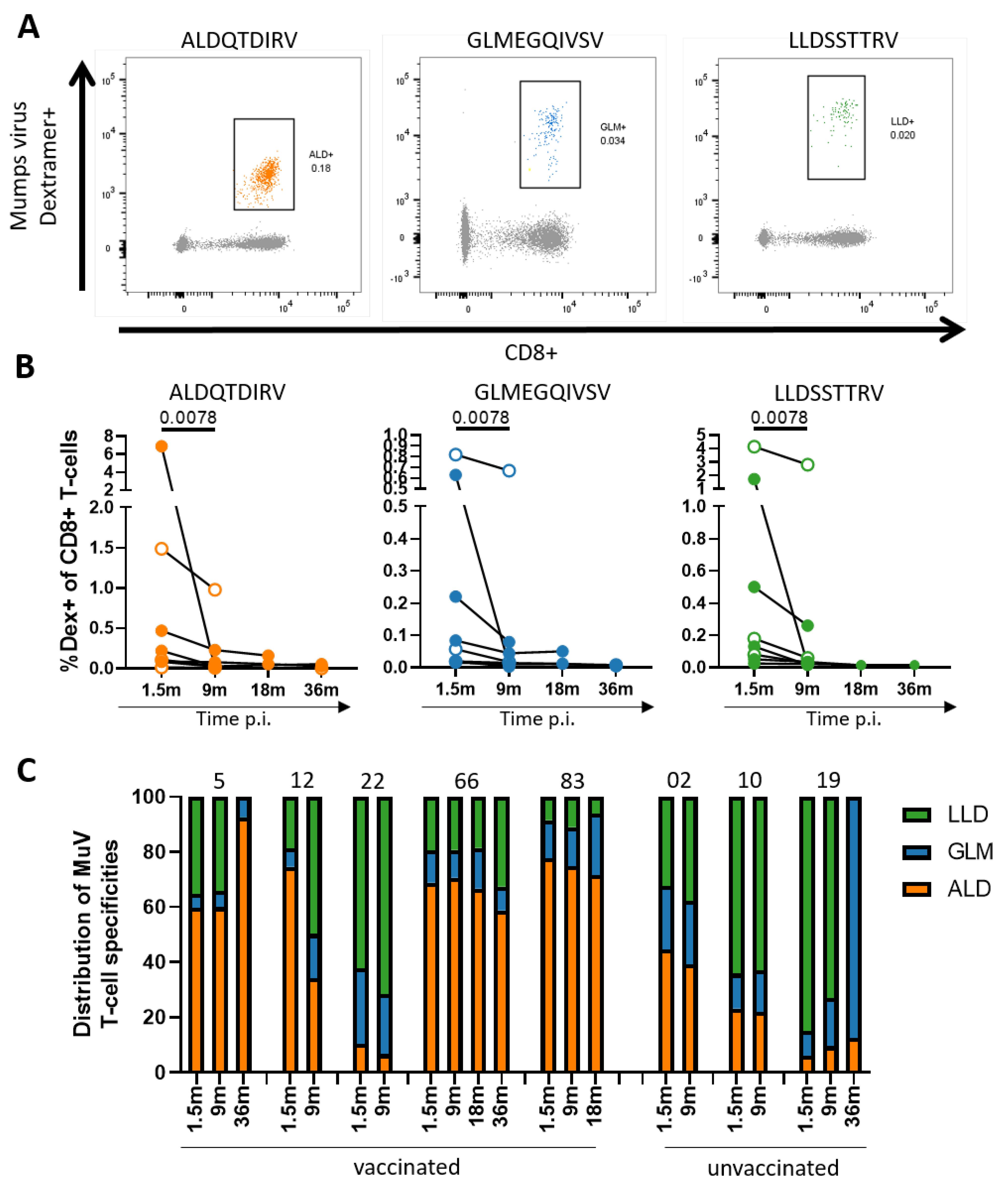
3.2. Decrease in MuV-Specific CD8+ T-Cell Frequencies over Time after Infection
3.3. Phenotype of MuV-Specific CD8+ T Cells Shifts from Short-Lived Effector to Memory Cells
3.4. The Decline in MuV-Specific CD8+ T-Cell Frequencies Is Not Explained by the Expression of Inhibitory Markers
3.5. The Decline in MuV-Specific CD8+ T-Cell Frequencies Is Not Explained by Increased Expression of Bone Marrow Homing Markers
3.6. MuV-Specific TCRβ Repertoire Is Maintained in the Memory Phase
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- MacDonald, N.; Hatchette, T.; Elkout, L.; Sarwal, S. Mumps is Back: Why is Mumps Eradication Not Working? Adv. Exp. Med. Biol. 2011, 7, 197–220. [Google Scholar]
- Plotkin, S.A.; Orenstein, W.A.; Offit, P.A. Vaccines; Elsevier: Philadelphia, PA, USA, 2018. [Google Scholar]
- Kaaijk, P.; Van Der Zeijst, B.; Boog, M.; Hoitink, C. Increased mumps incidence in the Netherlands: Review on the possible role of vaccine strain and genotype. Eurosurveillance 2008, 13, 18914. [Google Scholar] [CrossRef]
- Dayan, G.H.; Quinlisk, M.P.; Parker, A.A.; Barskey, A.E.; Harris, M.L.; Schwartz, J.M.H.; Hunt, K.; Finley, C.G.; Leschinsky, D.P.; O’Keefe, A.L.; et al. Recent Resurgence of Mumps in the United States. N. Engl. J. Med. 2008, 358, 1580–1589. [Google Scholar] [CrossRef] [Green Version]
- Karagiannis, I.; Van Lier, A.; Van Binnendijk, R.; Ruijs, H.; Fanoy, E.; Spaendonck, M.A.E.C.-V.; De Melker, H.; Hahné, S. Mumps in a community with low vaccination coverage in the Netherlands. Eurosurveillance 2008, 13, 18901. [Google Scholar] [CrossRef]
- Sane, J.; Gouma, S.; Koopmans, M.; De Melker, H.; Swaan, C.; Van Binnendijk, R.; Hahné, S. Epidemic of Mumps among Vaccinated Persons, the Netherlands, 2009–2012. Emerg. Infect. Dis. 2014, 20, 643–648. [Google Scholar] [CrossRef] [Green Version]
- Gans, H.; Yasukawa, L.; Rinki, M.; DeHovitz, R.; Forghani, B.; Beeler, J.; Audet, S.; Maldonado, Y.; Arvin, A.M. Immune Responses to Measles and Mumps Vaccination of Infants at 6, 9, and 12 Months. J. Infect. Dis. 2001, 184, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Kaaijk, P.; Gouma, S.; Hulscher, H.I.T.; Han, W.G.; Kleijne, D.E.; Van Binnendijk, R.S.; Van Els, C.A. Dynamics of the serologic response in vaccinated and unvaccinated mumps cases during an epidemic. Hum. Vaccines Immunother. 2015, 11, 1754–1761. [Google Scholar] [CrossRef] [Green Version]
- Dayan, G.H.; Rubin, S. Mumps outbreaks in vaccinated populations: Are available mumps vaccines effective enough to prevent outbreaks? Clin. Infect. Dis. 2008, 47, 1458–1467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Šantak, M.; Lang-Balija, M.; Ivancic-Jelecki, J.; Košutić-Gulija, T.; Ljubin-Sternak, S.; Forcic, D. Antigenic differences between vaccine and circulating wild-type mumps viruses decreases neutralization capacity of vaccine-induced antibodies. Epidemiol. Infect. 2013, 141, 1298–1309. [Google Scholar] [CrossRef] [PubMed]
- Gouma, S.; Sane, J.; Gijselaar, D.; Cremer, J.; Hahné, S.; Koopmans, M.; Van Binnendijk, R. Two major mumps genotype G variants dominated recent mumps outbreaks in the Netherlands (2009–2012). J. Gen. Virol. 2014, 95, 1074–1082. [Google Scholar] [CrossRef] [PubMed]
- de Wit, J.; Emmelot, M.E.; Poelen, M.C.; Lanfermeijer, J.; Han, W.G.; van Els, C.A.; Kaaijk, P. The Human CD4+ T Cell Response against Mumps Virus Targets a Broadly Recognized Nucleoprotein Epitope. J. Virol. 2019, 93, e01883-18. [Google Scholar] [CrossRef] [Green Version]
- Rasheed, M.A.U.; Hickman, C.J.; McGrew, M.; Sowers, S.B.; Mercader, S.; Hopkins, A.; Grimes, V.; Yu, T.; Wrammert, J.; Mulligan, M.J.; et al. Decreased humoral immunity to mumps in young adults immunized with MMR vaccine in childhood. Proc. Natl. Acad. Sci. USA 2019, 116, 19071–19076. [Google Scholar] [CrossRef] [Green Version]
- Mühlemann, K. The molecular epidemiology of mumps virus. Infect. Genet. Evol. 2004, 4, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Kaaijk, P.; Emmelot, M.; Kerkhof, J.; van Els, C.; Meiring, H.; de Wit, J.; Bodewes, R. Genetic Analysis Reveals Differences in CD8+ T Cell Epitope Regions That May Impact Cross-Reactivity of Vaccine-Induced T Cells against Wild-Type Mumps Viruses. Vaccines 2021, 9, 699. [Google Scholar] [CrossRef] [PubMed]
- Slifka, M.K.; Amanna, I. How advances in immunology provide insight into improving vaccine efficacy. Vaccine 2014, 32, 2948–2957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crooke, S.N.; Ovsyannikova, I.G.; Poland, G.A.; Kennedy, R.B. Immunosenescence and human vaccine immune responses. Immun. Ageing 2019, 16, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaaijk, P.; Emmelot, M.E.; Meiring, H.D.; van Els, C.A.C.M.; de Wit, J. Novel mumps virus epitopes reveal robust cytotoxic T cell responses after natural infection but not after vaccination. Sci. Rep. 2021, 11, 1–7. [Google Scholar]
- Jokinen, S.; Österlund, P.; Julkunen, I.; Davidkin, I. Cellular Immunity to Mumps Virus in Young Adults 21 Years after Measles-Mumps-Rubella Vaccination. J. Infect. Dis. 2007, 196, 861–867. [Google Scholar] [CrossRef]
- Vandermeulen, C.; Clement, F.; Roelants, M.; Van Damme, P.; Hoppenbrouwers, K.; Leroux-Roels, G. Evaluation of Cellular Immunity to Mumps in Vaccinated Individuals with or without Circulating Antibodies up to 16 Years after Their Last Vaccination. J. Infect. Dis. 2009, 199, 1457–1460. [Google Scholar] [CrossRef] [Green Version]
- De Wit, J.; Emmelot, M.E.; Poelen, M.C.; van Binnendijk, R.S.; van der Lee, S.; van Baarle, D.; Han, W.G.; van Els, C.A.; Kaaijk, P. Mumps infection but not childhood vaccination induces persistent polyfunctional CD8+ T-cell memory. J. Allergy Clin. Immunol. 2018, 141, 1908–1911. [Google Scholar] [CrossRef] [Green Version]
- de Wit, J.; Emmelot, M.E.; Meiring, H.; van Gaans-van den Brink, J.A.; van Els, C.A.; Kaaijk, P. Identification of Naturally Processed Mumps Virus Epitopes by Mass Spectrometry: Confirmation of Multiple CD8+ T-Cell Responses in Mumps Patients. J. Infect. Dis. 2020, 221, 474–482. [Google Scholar] [CrossRef] [Green Version]
- Mamedov, I.Z.; Britanova, O.V.; Zvyagin, I.V.; Turchaninova, M.A.; Bolotin, D.A.; Putintseva, E.V.; Lebedev, Y.B.; Chudakov, D.M. Preparing Unbiased T-Cell Receptor and Antibody cDNA Libraries for the Deep Next Generation Sequencing Profiling. Front. Immunol. 2013, 4, 456. [Google Scholar] [CrossRef] [Green Version]
- Gerritsen, B.; Pandit, A.; Andeweg, A.C.; de Boer, R.J. RTCR: A pipeline for complete and accurate recovery of T cell repertoires from high throughput sequencing data. Bioinformatics 2016, 32, 3098–3106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanfermeijer, J.; de Greef, P.C.; Hendriks, M.; Vos, M.; van Beek, J.; Borghans, J.A.M.; van Baarle, D. Age and CMV-Infection Jointly Affect the EBV-Specific CD8+ T-Cell Repertoire. Front. Aging 2021, 2, 11. [Google Scholar] [CrossRef]
- Al-Azzam, S.; Ding, Y.; Liu, J.; Pandya, P.; Ting, J.P.; Afshar, S. Peptides to combat viral infectious diseases. Peptides 2020, 134, 170402. [Google Scholar] [CrossRef]
- Wherry, E.J.; Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 2015, 15, 486–499. [Google Scholar] [CrossRef] [PubMed]
- McLane, L.M.; Hakeem, M.A.; Wherry, E.J. CD8 T Cell Exhaustion during Chronic Viral Infection and Cancer. Annu. Rev. Immunol. 2019, 37, 457–495. [Google Scholar] [CrossRef] [Green Version]
- David, P.; Megger, D.A.; Kaiser, T.; Werner, T.; Liu, J.; Chen, L.; Sitek, B.; Dittmer, U.; Zelinskyy, G. The PD-1/PD-L1 Pathway Affects the Expansion and Function of Cytotoxic CD8+ T Cells during an Acute Retroviral Infection. Front. Immunol. 2019, 10, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jubel, J.M.; Barbati, Z.R.; Burger, C.; Wirtz, D.C.; Schildberg, F.A. The Role of PD-1 in Acute and Chronic Infection. Front. Immunol. 2020, 11, 487. [Google Scholar] [CrossRef] [Green Version]
- Pauken, K.E.; Godec, J.; Odorizzi, P.M.; Brown, K.E.; Yates, K.B.; Ngiow, S.F.; Burke, K.P.; Maleri, S.; Grande, S.M.; Francisco, L.M.; et al. The PD-1 Pathway Regulates Development and Function of Memory CD8+ T Cells following Respiratory Viral Infection. Cell Rep. 2020, 31, 107827. [Google Scholar] [CrossRef]
- Schonrich, G.; Raftery, M.J. The PD-1/PD-L1 Axis and Virus Infections: A Delicate Balance. Front. Cell. Infect. Microbiol. 2019, 9, 207. [Google Scholar] [CrossRef] [Green Version]
- Ahn, E.; Araki, K.; Hashimoto, M.; Li, W.; Riley, J.; Cheung, J.; Sharpe, A.H.; Freeman, G.J.; Irving, B.A.; Ahmed, R. Role of PD-1 during effector CD8 T cell differentiation. Proc. Natl. Acad. Sci. USA 2018, 115, 4749–4754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okhrimenko, A.; Grün, J.R.; Westendorf, K.; Fang, Z.; Reinke, S.; von Roth, P.; Wassilew, G.; Kühl, A.A.; Kudernatsch, R.; Demski, S.; et al. Human memory T cells from the bone marrow are resting and maintain long-lasting systemic memory. Proc. Natl. Acad. Sci. USA 2014, 111, 9229–9234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sallusto, F.; Geginat, J.; Lanzavecchia, A. Central Memory and Effector Memory T Cell Subsets: Function, Generation, and Maintenance. Annu. Rev. Immunol. 2004, 22, 745–763. [Google Scholar] [CrossRef]
- Kurachi, M.; Kurachi, J.; Suenaga, F.; Tsukui, T.; Abe, J.; Ueha, S.; Tomura, M.; Sugihara, K.; Takamura, S.; Kakimi, K.; et al. Chemokine receptor CXCR3 facilitates CD8+ T cell differentiation into short-lived effector cells leading to memory degeneration. J. Exp. Med. 2011, 208, 1605–1620. [Google Scholar] [CrossRef] [Green Version]
- Goedhart, M.; Gessel, S.; van der Voort, R.; Slot, E.; Lucas, B.; Gielen, E.; Hoogenboezem, M.; Rademakers, T.; Geerman, S.; van Buul, J.D.; et al. CXCR4, but not CXCR3, drives CD8+ T-cell entry into and migration through the murine bone marrow. Eur. J. Immunol. 2019, 49, 576–589. [Google Scholar] [CrossRef]
- Badovinac, V.; Porter, B.B.; Harty, J. Programmed contraction of CD8+ T cells after infection. Nat. Immunol. 2002, 3, 619–626. [Google Scholar] [CrossRef]
- Kaech, S.M.; Hemby, S.; Kersh, E.; Ahmed, R. Molecular and Functional Profiling of Memory CD8 T Cell Differentiation. Cell 2002, 111, 837–851. [Google Scholar] [CrossRef] [Green Version]
- Martin, M.D.; Condotta, S.A.; Harty, J.; Badovinac, V.P. Population Dynamics of Naive and Memory CD8 T Cell Responses after Antigen Stimulations In Vivo. J. Immunol. 2011, 188, 1255–1265. [Google Scholar] [CrossRef] [Green Version]
- Badovinac, V.; Messingham, K.; Hamilton, S.E.; Harty, J. Regulation of CD8+ T Cells Undergoing Primary and Secondary Responses to Infection in the Same Host. J. Immunol. 2003, 170, 4933–4942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Förster, R.; Davalos-Misslitz, A.C.; Rot, A. CCR7 and its ligands: Balancing immunity and tolerance. Nat. Rev. Immunol. 2008, 8, 362–371. [Google Scholar] [CrossRef]
- Rot, A.; von Andrian, U.H. Chemokines in innate and adaptive host defense: Basic chemokinese grammar for immune cells. Annu. Rev. Immunol. 2004, 22, 891–928. [Google Scholar] [CrossRef] [PubMed]
- Becker, T.C.; Coley, S.M.; Wherry, E.J.; Ahmed, R. Bone Marrow Is a Preferred Site for Homeostatic Proliferation of Memory CD8 T Cells. J. Immunol. 2005, 174, 1269–1273. [Google Scholar] [CrossRef]
- Palendira, U.; Chinn, R.; Raza, W.; Piper, K.; Pratt, G.; Machado, L.; Bell, A.; Khan, N.; Hislop, A.D.; Steyn, R.; et al. Selective accumulation of virus-specific CD8+ T cells with unique homing phenotype within the human bone marrow. Blood 2008, 112, 3293–3302. [Google Scholar] [CrossRef] [Green Version]
- Sercan Alp, Ö.; Durlanik, S.; Schulz, D.; McGrath, M.; Grün, J.R.; Bardua, M.; Ikuta, K.; Sgouroudis, E.; Riedel, R.; Zehentmeier, S.; et al. Memory CD8+ T cells colocalize with IL-7+ stromal cells in bone marrow and rest in terms of pro-liferation and transcription. Eur. J. Immunol. 2015, 45, 975–987. [Google Scholar] [CrossRef] [PubMed]
- Groom, J.; Luster, A.D. CXCR3 in T cell function. Exp. Cell Res. 2011, 317, 620–631. [Google Scholar] [CrossRef]
- Remmerswaal, E.B.; Hombrink, P.; Nota, B.; Pircher, H.; Ten Berge, I.J.; van Lier, R.A.; van Aalderen, M.C. Expression of IL-7Ralpha and KLRG1 defines functionally distinct CD8+ T-cell populations in humans. Eur. J. Immunol. 2019, 49, 694–708. [Google Scholar] [CrossRef] [Green Version]
- Renkema, K.R.; Huggins, M.A.; Da Silva, H.B.; Knutson, T.P.; Henzler, C.; Hamilton, S.E. KLRG1+ Memory CD8 T Cells Combine Properties of Short-Lived Effectors and Long-Lived Memory. J. Immunol. 2020, 205, 1059–1069. [Google Scholar] [CrossRef]
- Co, M.D.T.; Kilpatrick, E.D.; Rothman, A.L. Dynamics of the CD8 T-cell response following yellow fever virus 17D immunization. Immunology 2009, 128, e718–e727. [Google Scholar] [CrossRef] [PubMed]
- DeWitt, W.; Emerson, R.O.; Lindau, P.; Vignali, M.; Snyder, T.; Desmarais, C.; Sanders, C.; Utsugi, H.; Warren, E.H.; McElrath, J.; et al. Dynamics of the Cytotoxic T Cell Response to a Model of Acute Viral Infection. J. Virol. 2015, 89, 4517–4526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minervina, A.A.; Pogorelyy, M.V.; Komech, E.A.; Karnaukhov, V.K.; Bacher, P.; Rosati, E.; Franke, A.; Chudakov, D.M.; Mamedov, I.Z.; Lebedev, Y.B.; et al. Primary and secondary anti-viral response captured by the dynamics and phenotype of individual T cell clones. eLife 2020, 9, e53704. [Google Scholar] [CrossRef] [PubMed]
- Annels, N.E.; Callan, M.F.C.; Tan, L.; Rickinson, A.B. Changing Patterns of Dominant TCR Usage with Maturation of an EBV-Specific Cytotoxic T Cell Response. J. Immunol. 2000, 165, 4831–4841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iancu, E.M.; Corthesy, P.; Baumgaertner, P.; Devevre, E.; Voelter, V.; Romero, P.; Speiser, D.E.; Rufer, N. Clonotype Selection and Composition of Human CD8 T Cells Specific for Persistent Herpes Viruses Varies with Differentiation but Is Stable over Time. J. Immunol. 2009, 183, 319–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klarenbeek, P.L.; Remmerswaal, E.B.M.; Berge, I.J.M.T.; Doorenspleet, M.E.; Van Schaik, B.D.C.; Esveldt, R.E.E.; Koch, S.D.; Brinke, A.T.; Van Kampen, A.H.C.; Bemelman, F.J.; et al. Deep Sequencing of Antiviral T-Cell Responses to HCMV and EBV in Humans Reveals a Stable Repertoire That Is Maintained for Many Years. PLoS Pathog. 2012, 8, e1002889. [Google Scholar] [CrossRef] [PubMed]



| Donor | Sex | Age (yrs) | Time Points | Vaccination Status | IgG Concentration, 1.5 Months after Infection (RU/mL) | IgG Concentration, 7–10 Months after Infection (RU/mL) | Clinical Symptoms |
|---|---|---|---|---|---|---|---|
| 263-05 | M | 21 | 1.5 m, 9 m, and 36 m | Vaccinated | 4436 | 3010 | Parotitis, swollen neck glands, fever, cold, cough |
| 263-12 | M | 25 | 1.5 m and 9 m | Vaccinated | 7265 | 5928 | Parotitis, swollen neck glands, fever |
| 263-22 | M | 26 | 1.5 m and 9 m | Vaccinated | 21,683 | 7108 | Orchitis |
| 274-66 | F | 30 | 1.5 m, 9 m, 18 m, and 36 m | Vaccinated | 34,843 | 10,579 | Parotitis, swollen neck glands |
| 274-83 | F | 20 | 1.5 m, 9 m, and 18 m | Vaccinated | 12,785 | 23,669 | Parotitis, fever, permanent unilateral deafness |
| 263-02 | F | 26 | 1.5 m and 9 m | Unvaccinated | 358 | 1856 | Parotitis, swollen neck glands, abdominal pain, cold, otitis |
| 263-10 | M | 40 | 1.5 m and 9 m | Unvaccinated | 2396 | 663 | Orchitis, parotitis, swollen neck glands, fever, sore throat |
| 263-19 | F | 53 | 1.5 m and 9 m | Unvaccinated | 449 | 3704 | Swollen neck glands, fever, cough, vertigo, temporary deafness |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lanfermeijer, J.; Nühn, M.M.; Emmelot, M.E.; Poelen, M.C.M.; van Els, C.A.C.M.; Borghans, J.A.M.; van Baarle, D.; Kaaijk, P.; de Wit, J. Longitudinal Characterization of the Mumps-Specific HLA-A2 Restricted T-Cell Response after Mumps Virus Infection. Vaccines 2021, 9, 1431. https://doi.org/10.3390/vaccines9121431
Lanfermeijer J, Nühn MM, Emmelot ME, Poelen MCM, van Els CACM, Borghans JAM, van Baarle D, Kaaijk P, de Wit J. Longitudinal Characterization of the Mumps-Specific HLA-A2 Restricted T-Cell Response after Mumps Virus Infection. Vaccines. 2021; 9(12):1431. https://doi.org/10.3390/vaccines9121431
Chicago/Turabian StyleLanfermeijer, Josien, Marieke M. Nühn, Maarten E. Emmelot, Martien C. M. Poelen, Cécile A. C. M. van Els, José A. M. Borghans, Debbie van Baarle, Patricia Kaaijk, and Jelle de Wit. 2021. "Longitudinal Characterization of the Mumps-Specific HLA-A2 Restricted T-Cell Response after Mumps Virus Infection" Vaccines 9, no. 12: 1431. https://doi.org/10.3390/vaccines9121431
APA StyleLanfermeijer, J., Nühn, M. M., Emmelot, M. E., Poelen, M. C. M., van Els, C. A. C. M., Borghans, J. A. M., van Baarle, D., Kaaijk, P., & de Wit, J. (2021). Longitudinal Characterization of the Mumps-Specific HLA-A2 Restricted T-Cell Response after Mumps Virus Infection. Vaccines, 9(12), 1431. https://doi.org/10.3390/vaccines9121431






