Construction of Recombinant Lactococcus lactis Strain Expressing VP1 Fusion Protein of Duck Hepatitis A Virus Type 1 and Evaluation of Its Immune Effect
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Vector
2.2. Main Reagents and Antibodies
2.3. Expression Plasmid Construction in E. coli
2.4. Construction of Recombinant L. lactis MG1363-VP1
2.5. Fluorescence Microscopy
2.6. Western Blot Analysis
2.7. Intestinal Colonization of Recombinant L. lactis
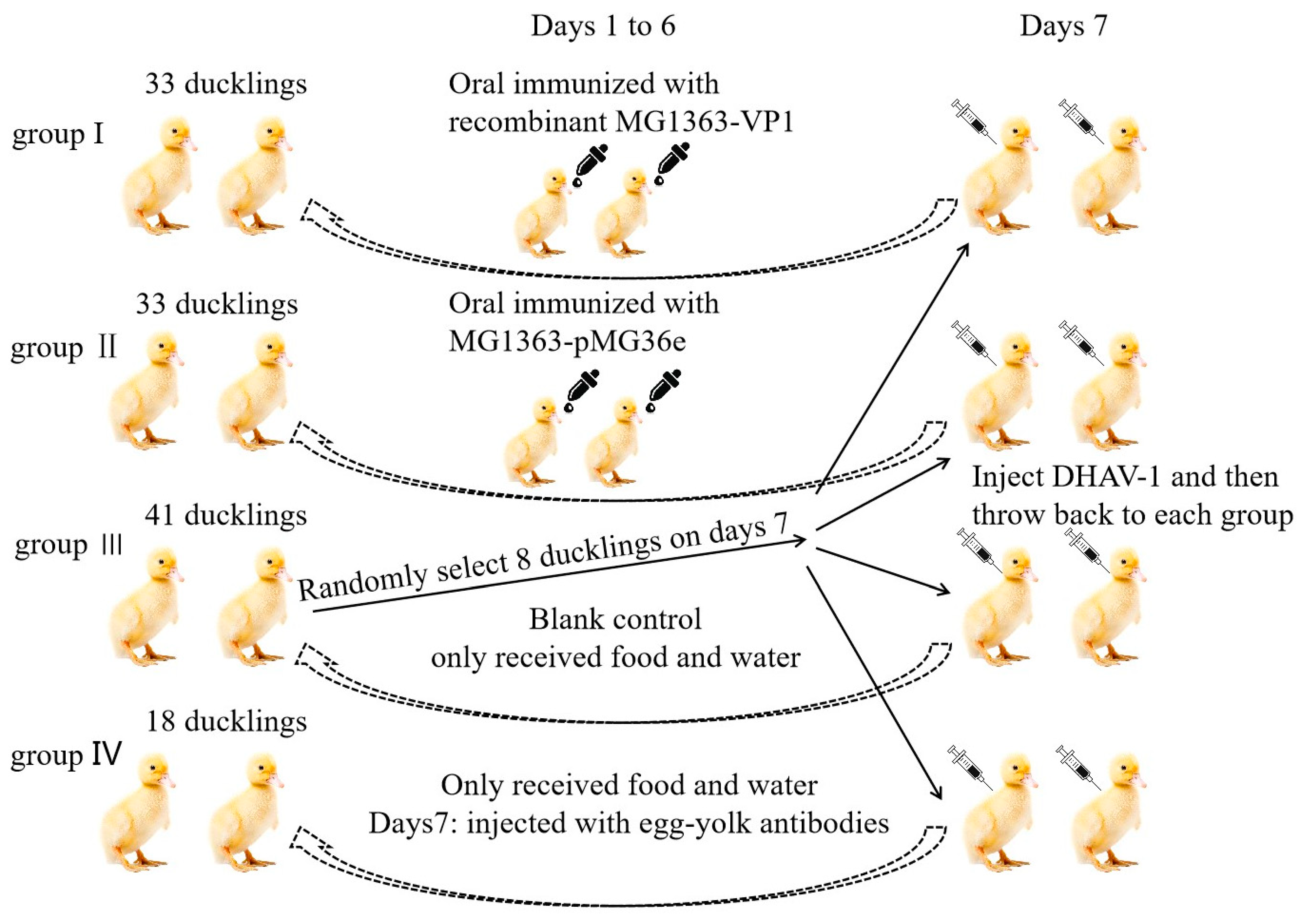
2.8. Oral Vaccination of Ducklings
2.9. Detection of Cytokines and Specific Antibody in Ducklings
3. Results
3.1. Construction of Recombinant Plasmid Usp45-VP1-eGFP-pMG36e
3.2. Construction of Recombinant L. lactis MG1363-VP1
3.3. Immune Response and Protection Effect in Ducklings after Oral Immunization
3.4. The Intestinal Colonization of L. lactis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Bosch, A.; Guix, S.; Krishna, N.K.; Méndez, E.; Monroe, S.S.; Pantin-Jackwood, M.; Schultz-Cherry, S. Classification and Nomenclature of Viruses: Ninth Report of the International Committee on the Taxonomy of Viruses; Ninth Report of the ICTV; Elsevier Academic Press: London, UK, 2011; pp. 953–959. [Google Scholar]
- Kim, M.C.; Kwon, Y.K.; Joh, S.J.; Lindberg, A.M.; Kwon, J.H.; Kim, J.H.; Kim, S.J. Molecular analysis of duck hepatitis virus type 1 reveals a novel lineage close to the genus Parechovirus in the family Picornaviridae. J. Gen. Virol. 2006, 87, 3307–3316. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.C.; Kwon, Y.K.; Joh, S.J.; Kim, S.J.; Tolf, C.; Kim, J.H.; Sung, H.W.; Lindberg, A.M.; Kwon, J.H. Recent Korean isolates of duck hepatitis virus reveal the presence of a new geno- and serotype when compared to duck hepatitis virus type 1 type strains. Arch. Virol. 2007, 152, 2059–2072. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Pan, M.; Wang, X.; Xu, Y.; Yang, H.; Zhang, D. Molecular detection and typing of duck hepatitis A virus directly from clinical specimens. Vet. Microbiol. 2008, 131, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Zhang, D. Molecular analysis of duck hepatitis virus type 1. Virology 2007, 361, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Zhang, W.; Zhang, W.; Gu, C.; Cheng, G.; Hu, X. Identification and molecular analysis of the highly pathogenic duck hepatitis virus type 1 in Hubei province of China. Res. Vet. Sci. 2008, 85, 595–598. [Google Scholar] [CrossRef]
- Tseng, C.H.; Knowles, N.J.; Tsai, H.J. Molecular analysis of duck hepatitis virus type 1 indicates that it should be assigned to a new genus. Virus Res. 2007, 123, 190–203. [Google Scholar] [CrossRef]
- Gao, J.; Chen, J.; Si, X.; Xie, Z.; Zhu, Y.; Zhang, X.; Wang, S.; Jiang, S. Genetic variation of the VP1 gene of the virulent duck hepatitis A virus type 1 (DHAV-1) isolates in Shandong province of China. Virol. Sin. 2012, 27, 248–253. [Google Scholar] [CrossRef]
- Alonso, C.; Vicario, M.; Pigrau, M.; Lobo, B.; Santos, J. Intestinal barrier function and the brain-gut axis. Adv. Exp. Med. Biol. 2014, 817, 73–113. [Google Scholar] [PubMed]
- Maldonado Galdeano, C.; Cazorla, S.I.; Lemme Dumit, J.M.; Vélez, E.; Perdigón, G. Beneficial effects of probiotic consumption on the immune system. Ann. Nutr. Metab. 2019, 74, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Wells, J. Mucosal vaccination and therapy with genetically modified lactic acid bacteria. Annu. Rev. Food Sci. Technol. 2011, 2, 423–445. [Google Scholar] [CrossRef]
- Pontes, D.S.; de Azevedo, M.S.; Chatel, J.M.; Langella, P.; Azevedo, V.; Miyoshi, A. Lactococcus lactis as a live vector: Heterologous protein production and DNA delivery systems. Protein Expr. Purif. 2011, 79, 165–175. [Google Scholar] [CrossRef]
- Zhang, R.; Zhou, G.; Xin, Y.; Chen, J.; Lin, S.; Tian, Y.; Xie, Z.; Jiang, S. Identification of a conserved neutralizing linear B-cell epitope in the VP1 proteins of duck hepatitis A virus type 1 and 3. Vet. Microbiol. 2015, 180, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.M.; Wilson, P.W.; Le Page, R.W. Improved cloning vectors and transformation procedure for Lactococcus lactis. J. Appl. Microbiol. 1993, 74, 629–636. [Google Scholar]
- Morello, E.; Bermudez-Humaran, L.G.; Llull, D.; Sole, V.; Miraglio, N.; Langella, P.; Poquet, I. Lactococcus lactis, an efficient cell factory for recombinant protein production and secretion. Mol. Microbiol. Biotechnol. 2008, 14, 48–58. [Google Scholar] [CrossRef]
- Chen, G.; Yang, Z.; Cao, C.; Xiao, X.; Huang, Y.; Tian, L.; Bai, W. Subacute safety assessment of recombinant Lactococcus lactis on the gut microbiota of male Sprague-Dawley rats. J. Sci Food Agric. 2021, 101, 5807–5812. [Google Scholar] [CrossRef]
- Rezaei, M.; Rabbani Khorasgani, M.; Zarkesh Esfahani, S.H.; Emamzadeh, R.; Abtahi, H. Production of Brucella melitensis Omp16 protein fused to the human interleukin 2 in Lactococcus lactis MG1363 toward developing a Lactococcus-based vaccine against brucellosis. Can. J. Microbiol. 2020, 66, 39–45. [Google Scholar] [CrossRef]
- Steidler, L.; Hans, W.; Schotte, L.; Neirynck, S.; Obermeier, F.; Falk, W.; Fiers, W.; Remaut, E. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science 2000, 289, 1352–1355. [Google Scholar] [CrossRef]
- Villatoro-Hernández, J.; Kuipers, O.P.; Saucedo-Cárdenas, O.; Montes-de-Oca-Luna, R. Heterologous protein expression by Lactococcus lactis. Methods Mol. Biol. 2012, 824, 155–165. [Google Scholar]
- Budin-Verneuil, A.; Pichereau, V.; Auffray, Y.; Ehrlich, D.S.; Maguin, E. Proteomic characterization of the acid tolerance response in Lactococcus lactis MG1363. Proteomics 2005, 5, 4794–4807. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Li, P.; Zhang, R.; Chen, J.; Lan, J.; Lin, S.; Guo, G.; Xie, Z.; Jiang, S. Oral vaccine of recombinant Lactococcus lactis expressing the VP1 protein of duck hepatitis A virus type 3 induces mucosal and systemic immune responses. Vaccine 2019, 37, 4364–4369. [Google Scholar] [CrossRef]
- Ng, D.T.; Sarkar, C.A. Engineering signal peptides for enhanced protein secretion from Lactococcus lactis. Appl. Environ. Microbiol. 2013, 79, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Van de Guchte, M.; Kodde, J.; Van der Vossen, J.M.; Kok, J.; Venema, G. Heterologous gene expression in Lactococcus lactis subsp. lactis: Synthesis, secretion, and processing of the Bacillus subtilis neutral protease. Appl. Environ. Microbiol. 1990, 56, 2606–2611. [Google Scholar] [CrossRef] [PubMed]
- Avall-Jääskeläinen, S.; Palva, A. Secretion of biologically active porcine interleukin-2 by Lactococcus lactis. Vet. Microbiol. 2006, 115, 278–283. [Google Scholar] [CrossRef]
- Freitas, D.A.; Leclerc, S.; Miyoshi, A.; Oliveira, S.C.; Sommer, P.S.; Rodrigues, L.; Correa Junior, A.; Gautier, M.; Langella, P.; Azevedo, V.A.; et al. Secretion of streptomyces tendae antifungal protein 1 by Lactococcus lactis. Braz. J. Med. Biol. Res. 2005, 38, 1585–1592. [Google Scholar] [CrossRef]
- Simonen, M.; Palva, I. Protein secretion in Bacillus species. Microbiol. Rev. 1993, 57, 109–137. [Google Scholar] [CrossRef] [PubMed]
- Olins, P.O.; Rangwala, S.H. A novel sequence element derived from bacteriophage T7 mRNA acts as an enhancer of translation of the lacZ gene in Escherichia coli. J. Biol. Chem. 1989, 264, 16973–16976. [Google Scholar] [CrossRef]
- Olins, P.O.; Devine, C.S.; Rangwala, S.H.; Kavka, K.S. The T7 phage gene 10 leader RNA, a ribosome-binding site that dramatically enhances the expression of foreign genes in Escherichia coli. Gene 1988, 73, 227–235. [Google Scholar] [CrossRef]
- Chai, Y.B.; Liu, H.P.; Zhao, Z.L.; Chen, S.M.; Chen, N.C.; Gao, H.; Liu, X.P. Construction of the expression vector pSC34 containing the prokaryotic translation enhancer T7g10L and its preliminary application. Lett. Biotechnol. 1997, 8, 138–139. [Google Scholar]
- Maqsood, I.; Shi, W.; Wang, L.; Wang, X.; Han, B.; Zhao, H.; Nadeem, A.M.; Moshin, B.S.; Saima, K.; Jamal, S.S.; et al. Immunogenicity and protective efficacy of orally administered recombinant Lactobacillus plantarum expressing VP2 protein against IBDV in chicken. J. Appl. Microbiol. 2018, 125, 1670–1681. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, C.W.; Du, J.; Lu, X.Y. Study on the genetic stability of expression plasmid vector pMG36e in host bacteria. Health Res. 2005, 34, 214–216. [Google Scholar]
- Lei, H.; Xu, Y.; Chen, J.; Wei, X.; Lam, D.M. Immunoprotection against influenza H5N1 virus by oral administration of enteric-coated recombinant Lactococcus lactis mini-capsules. Virology 2010, 407, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Quigley, B.R.; Hatkoff, M.; Thanassi, D.G.; Ouattara, M.; Eichenbaum, Z.; Scott, J.R. A foreign protein incorporated on the tip of T3 pili in Lactococcus lactis elicits systemic and mucosal immunity. Infect. Immun. 2009, 78, 1294–1303. [Google Scholar] [CrossRef] [PubMed]
- Underhill, D.M.; Ozinsky, A. Toll-like receptors: Key mediators of microbe detection. Curr. Opin. Immunol. 2002, 14, 103–110. [Google Scholar] [CrossRef]
- Bahey-El-Din, M.; Casey, P.G.; Griffin, B.T.; Gahan, C.G. Expression of two Listeria monocytogenes antigens (P60 and LLO) in Lactococcus lactis and examination for use as live vaccine vectors. J. Med. Microbiol. 2010, 59, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, S.A.; Yasawardena, S.G.; Ramasamy, R. Age-dependent systemic antibody responses and immunisation-associated changes in mice orally and nasally immunised with Lactococcus lactis expressing a malaria parasite protein. Vaccine 2009, 27, 4947–4952. [Google Scholar] [CrossRef]
- Parlane, N.A.; Grage, K.; Lee, J.W.; Buddle, B.M.; Denis, M.; Rehm, B.H. Production of a particulate hepatitis C vaccine candidate by an engineered Lactococcus lactis strain. Appl. Environ. Microb. 2011, 77, 8516–8522. [Google Scholar] [CrossRef]
- Mosmann, T.R.; Cherwinski, H.; Bond, M.W.; Giedlin, M.A.; Coffman, R.L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 1986, 136, 2348–2357. [Google Scholar] [PubMed]
- Saraiva, M.; O’Garra, A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010, 10, 170–181. [Google Scholar] [CrossRef]






| Primers | Sequences (5′ to 3′) |
|---|---|
| Usp45-VP1-F1 | cagtgatactttctgctgcagccccgttgtcaggtgtttacgctGGTGATTCTAACCAGT |
| Usp45-VP1-F2 | atgaaaaaaaagattatctcagctattttaatgtctacagtgatactttctg |
| VP1-R | aacagctcctcgcccttgctcaCTTCAATTTCCAAATTGAGTTC |
| T7g10L-F | TCTAGAaataattttgtttaactttaagATGAAAAAAAAGATTATCTCA |
| eGFP-F | AATTTGGAAATTGAAgtgagcaagggcgaggagctgttcac |
| eGFP-R | AAGCTTtcagttatctagatccggtggatcccgggcccgcg |
| F’ | ctcgcccggggatcgatccTCTAGAaataattttgtttaactttaag |
| R’ | accttcgttttcagactttgcAAGCTTtcagttatctagatccggtggatc |
| MG1363-F | GATGATACATAGCCGACCTGA |
| MG1363-R | TTAGCCGTCCCTTTCTGG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zhang, R.; Wang, J.; Sui, N.; Xu, G.; Yan, H.; Zhu, Y.; Xie, Z.; Jiang, S. Construction of Recombinant Lactococcus lactis Strain Expressing VP1 Fusion Protein of Duck Hepatitis A Virus Type 1 and Evaluation of Its Immune Effect. Vaccines 2021, 9, 1479. https://doi.org/10.3390/vaccines9121479
Zhang X, Zhang R, Wang J, Sui N, Xu G, Yan H, Zhu Y, Xie Z, Jiang S. Construction of Recombinant Lactococcus lactis Strain Expressing VP1 Fusion Protein of Duck Hepatitis A Virus Type 1 and Evaluation of Its Immune Effect. Vaccines. 2021; 9(12):1479. https://doi.org/10.3390/vaccines9121479
Chicago/Turabian StyleZhang, Xiaoting, Ruihua Zhang, Jingyu Wang, Nana Sui, Guige Xu, Hui Yan, Yanli Zhu, Zhijing Xie, and Shijin Jiang. 2021. "Construction of Recombinant Lactococcus lactis Strain Expressing VP1 Fusion Protein of Duck Hepatitis A Virus Type 1 and Evaluation of Its Immune Effect" Vaccines 9, no. 12: 1479. https://doi.org/10.3390/vaccines9121479
APA StyleZhang, X., Zhang, R., Wang, J., Sui, N., Xu, G., Yan, H., Zhu, Y., Xie, Z., & Jiang, S. (2021). Construction of Recombinant Lactococcus lactis Strain Expressing VP1 Fusion Protein of Duck Hepatitis A Virus Type 1 and Evaluation of Its Immune Effect. Vaccines, 9(12), 1479. https://doi.org/10.3390/vaccines9121479






