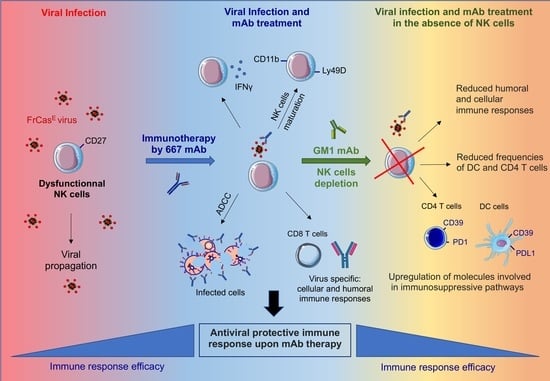
Immunomodulatory Role of NK Cells during Antiviral Antibody Therapy
Abstract
:1. Introduction
2. Materials and Methods
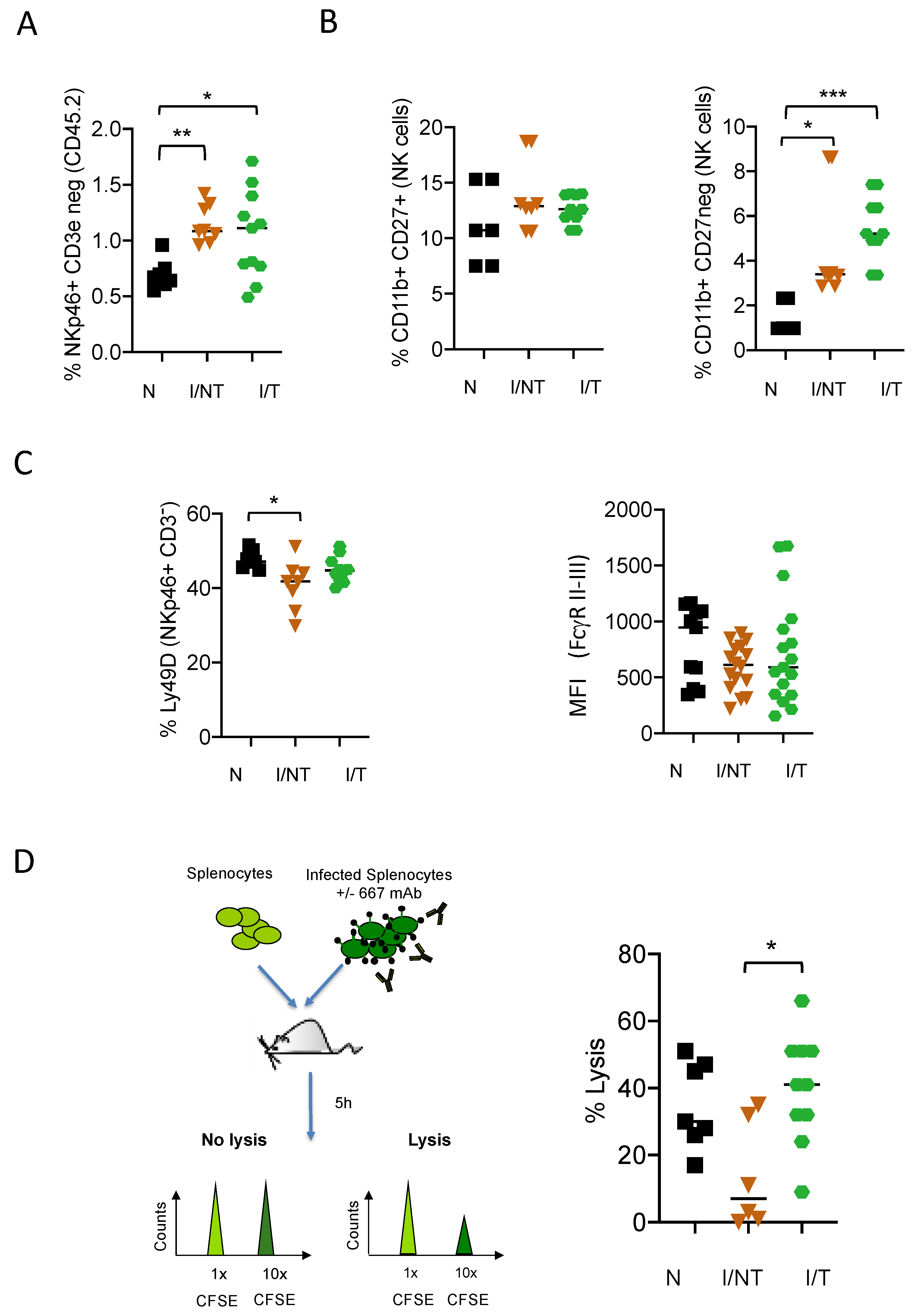
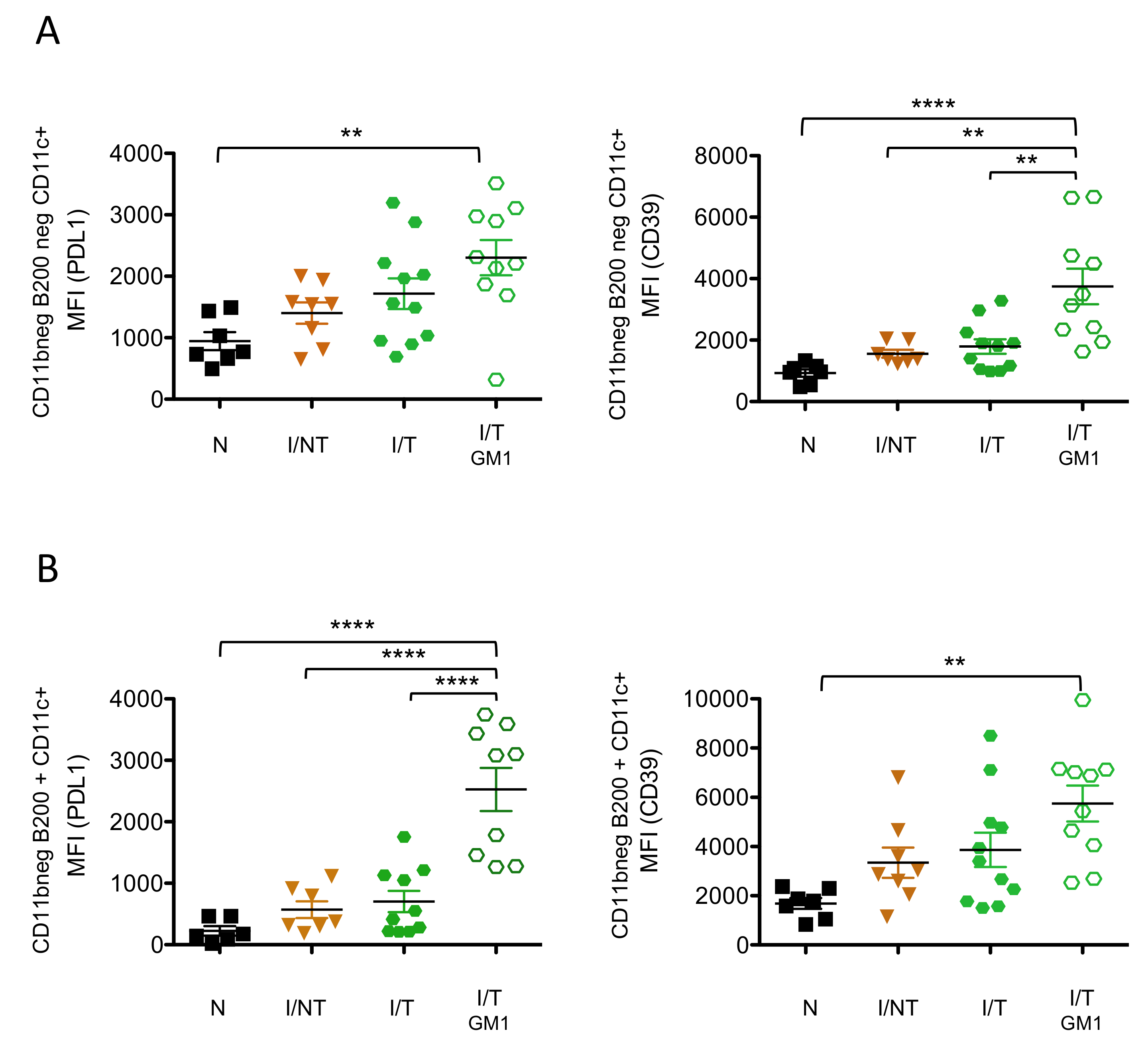
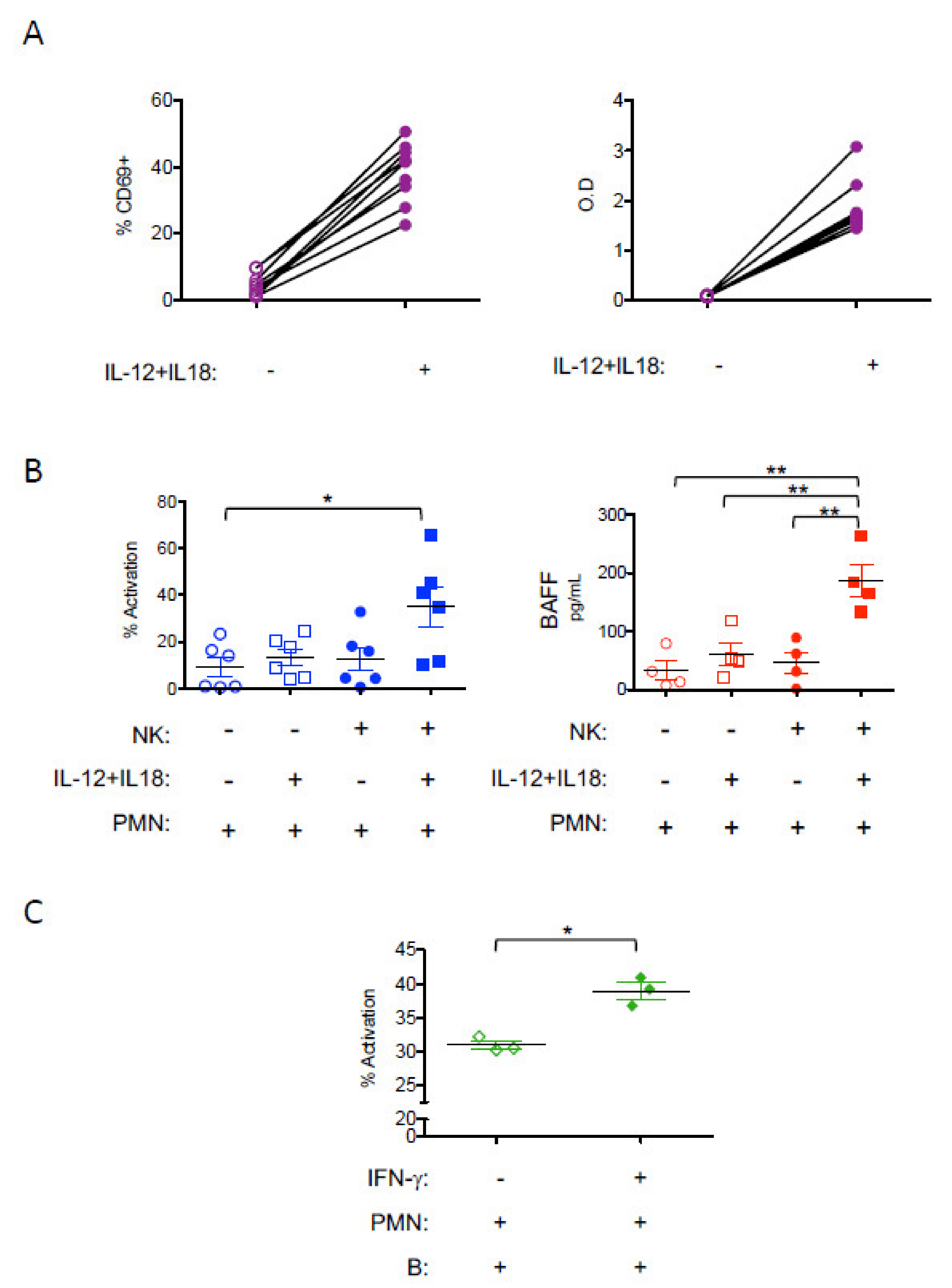
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Dibo, M.; Battocchio, E.C.; Dos Santos Souza, L.M.; da Silva, M.D.V.; Banin-Hirata, B.K.; Sapla, M.M.M.; Marinello, P.; Rocha, S.P.D.; Faccin-Galhardi, L.C. Antibody Therapy for the Control of Viral Diseases: An Update. Curr. Pharm. Biotechnol. 2019, 20, 1108–1121. [Google Scholar] [CrossRef]
- Salazar, G.; Zhang, N.; Fu, T.-M.; An, Z. Antibody therapies for the prevention and treatment of viral infections. NPJ Vaccines 2017, 2, 19. [Google Scholar] [CrossRef] [Green Version]
- Van Huijsduijnen, R.F.; Kojima, S.; Carter, D.; Okabe, H.; Sato, A.; Akahata, W.; Wells, T.N.C.; Katsuno, K. Reassessing therapeutic antibodies for neglected and tropical diseases. PLoS Negl. Trop. Dis. 2020, 14, e0007860. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.L.; Suscovich, T.J.; Fortune, S.M.; Alter, G. Beyond binding: Antibody effector functions in infectious diseases. Nat. Rev. Immunol. 2018, 18, 46–61. [Google Scholar] [CrossRef]
- Marasco, W.A.; Sui, J. The growth and potential of human antiviral monoclonal antibody therapeutics. Nat. Biotechnol. 2007, 25, 1421–1434. [Google Scholar] [CrossRef]
- Pelegrin, M.; Naranjo-Gomez, M.; Piechaczyk, M. Antiviral Monoclonal Antibodies: Can They Be More Than Simple Neutralizing Agents? Trends Microbiol. 2015, 23, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Nasser, R.; Pelegrin, M.; Michaud, H.A.; Plays, M.; Piechaczyk, M.; Gros, L. Long-lasting protective antiviral immunity induced by passive immunotherapies requires both neutralizing and effector functions of the administered monoclonal antibody. J. Virol. 2010, 84, 10169–10181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michaud, H.-A.; Gomard, T.; Gros, L.; Thiolon, K.; Nasser, R.; Jacquet, C.; Hernandez, J.; Piechaczyk, M.; Pelegrin, M. A crucial role for infected-cell/antibody immune complexes in the enhancement of endogenous antiviral immunity by short passive immunotherapy. PLoS Pathog. 2010, 6, e1000948. [Google Scholar] [CrossRef]
- Nasser, R.; Pelegrin, M.; Plays, M.; Gros, L.; Piechaczyk, M. Control of regulatory T cells is necessary for vaccine-like effects of antiviral immunotherapy by monoclonal antibodies. Blood 2013, 121, 1102–1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bournazos, S.; Corti, D.; Virgin, H.W.; Ravetch, J.V. Fc-optimized antibodies elicit CD8 immunity to viral respiratory infection. Nature 2020, 588, 485–490. [Google Scholar] [CrossRef]
- Ng, C.T.; Jaworski, J.P.; Jayaraman, P.; Sutton, W.F.; Delio, P.; Kuller, L.; Anderson, D.; Landucci, G.; Richardson, B.A.; Burton, D.R.; et al. Passive neutralizing antibody controls SHIV viremia and enhances B cell responses in infant macaques. Nat. Med. 2010, 16, 1117–1119. [Google Scholar] [CrossRef] [PubMed]
- Watkins, J.D.; Siddappa, N.B.; Lakhashe, S.K.; Humbert, M.; Sholukh, A.; Hemashettar, G.; Wong, Y.L.; Yoon, J.K.; Wang, W.; Novembre, F.J.; et al. An anti-HIV-1 V3 loop antibody fully protects cross-clade and elicits T-cell immunity in macaques mucosally challenged with an R5 clade C SHIV. PLoS ONE 2011, 6, e18207. [Google Scholar] [CrossRef] [PubMed]
- Bossart, K.N.; Geisbert, T.W.; Feldmann, H.; Zhu, Z.; Feldmann, F.; Geisbert, J.B.; Yan, L.; Feng, Y.R.; Brining, D.; Scott, D.; et al. A neutralizing human monoclonal antibody protects african green monkeys from hendra virus challenge. Sci. Transl. Med. 2011, 3, 105ra103. [Google Scholar] [CrossRef] [Green Version]
- Barouch, D.H.; Whitney, J.B.; Moldt, B.; Klein, F.; Oliveira, T.Y.; Liu, J.; Stephenson, K.E.; Chang, H.W.; Shekhar, K.; Gupta, S.; et al. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature 2013, 503, 224–228. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, Y.; Gautam, R.; Chun, T.-W.; Sadjadpour, R.; Foulds, K.E.; Shingai, M.; Klein, F.; Gazumyan, A.; Golijanin, J.; Donaldson, M.; et al. Early antibody therapy can induce long-lasting immunity to SHIV. Nature 2017, 543, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Schoofs, T.; Klein, F.; Braunschweig, M.; Kreider, E.F.; Feldmann, A.; Nogueira, L.; Oliveira, T.; Lorenzi, J.C.; Parrish, E.H.; Learn, G.H.; et al. HIV-1 therapy with monoclonal antibody 3BNC117 elicits hoost immune responses against HIV-1. Science 2016. [Google Scholar] [CrossRef] [Green Version]
- Niessl, J.; Baxter, A.E.; Mendoza, P.; Jankovic, M.; Cohen, Y.Z.; Butler, A.L.; Lu, C.-L.; Dubé, M.; Shimeliovich, I.; Gruell, H.; et al. Combination anti-HIV-1 antibody therapy is associated with increased virus-specific T cell immunity. Nat. Med. 2020, 26, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Naranjo-Gomez, M.; Pelegrin, M. Vaccinal effect of HIV-1 antibody therapy. Curr. Opin. HIV AIDS 2019, 14, 325–333. [Google Scholar] [CrossRef]
- Naranjo-Gomez, M.; Lambour, J.; Piechaczyk, M.; Pelegrin, M. Neutrophils are essential for induction of vaccine-like effects by antiviral monoclonal antibody immunotherapies. JCI Insight 2018, 3, e97339. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Sheng, Z.; Sreenivasan, C.C.; Wang, D.; Li, F. Influenza A Virus Antibodies with Antibody-Dependent Cellular Cytotoxicity Function. Viruses 2020, 12, 276. [Google Scholar] [CrossRef] [Green Version]
- Van Erp, E.A.; Luytjes, W.; Ferwerda, G.; van Kasteren, P.B. Fc-Mediated Antibody Effector Functions During Respiratory Syncytial Virus Infection and Disease. Front. Immunol. 2019, 10, 548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.S.; Kent, S.J. Anti-HIV-1 antibody-dependent cellular cytotoxicity: Is there more to antibodies than neutralization? Curr. Opin. HIV AIDS 2018, 13, 160–166. [Google Scholar] [CrossRef]
- Dorner, B.G.; Smith, H.R.C.; French, A.R.; Kim, S.; Poursine-Laurent, J.; Beckman, D.L.; Pingel, J.T.; Kroczek, R.A.; Yokoyama, W.M. Coordinate Expression of Cytokines and Chemokines by NK Cells during Murine Cytomegalovirus Infection. J. Immunol. 2004, 172, 3119–3131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fauriat, C.; Long, E.O.; Ljunggren, H.-G.; Bryceson, Y.T. Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood 2010, 115, 2167–2176. [Google Scholar] [CrossRef] [Green Version]
- Roda, J.M.; Parihar, R.; Magro, C.; Nuovo, G.J.; Tridandapani, S.; Carson, W.E. Natural Killer Cells Produce T Cell–Recruiting Chemokines in Response to Antibody-Coated Tumor Cells. Cancer Res. 2006, 66, 517–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiossone, L.; Dumas, P.-Y.; Vienne, M.; Vivier, E. Natural killer cells and other innate lymphoid cells in cancer. Nat. Rev. Immunol. 2018, 18, 671–688. [Google Scholar] [CrossRef]
- Abel, A.M.; Yang, C.; Thakar, M.S.; Malarkannan, S. Natural Killer Cells: Development, Maturation, and Clinical Utilization. Front. Immunol. 2018, 9, 1869. [Google Scholar] [CrossRef] [Green Version]
- Ferlazzo, G.; Morandi, B. Cross-Talks between Natural Killer Cells and Distinct Subsets of Dendritic Cells. Front. Immunol. 2014, 5, 159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agaugué, S.; Marcenaro, E.; Ferranti, B.; Moretta, L.; Moretta, A. Human natural killer cells exposed to IL-2, IL-12, IL-18, or IL-4 differently modulate priming of naive T cells by monocyte-derived dendritic cells. Blood 2008, 112, 1776–1783. [Google Scholar] [CrossRef] [Green Version]
- Thorén, F.B.; Riise, R.E.; Ousbäck, J.; Della Chiesa, M.; Alsterholm, M.; Marcenaro, E.; Pesce, S.; Prato, C.; Cantoni, C.; Bylund, J.; et al. Human NK Cells Induce Neutrophil Apoptosis via an NKp46- and Fas-Dependent Mechanism. J. Immunol. 2012, 188, 1668–1674. [Google Scholar] [CrossRef] [PubMed]
- Mailliard, R.B.; Son, Y.-I.; Redlinger, R.; Coates, P.T.; Giermasz, A.; Morel, P.A.; Storkus, W.J.; Kalinski, P. Dendritic Cells Mediate NK Cell Help for Th1 and CTL Responses: Two-Signal Requirement for the Induction of NK Cell Helper Function. J. Immunol. 2003, 171, 2366–2373. [Google Scholar] [CrossRef]
- Costantini, C.; Cassatella, M.A. The defensive alliance between neutrophils and NK cells as a novel arm of innate immunity. J. Leukoc Biol. 2011, 89, 221–233. [Google Scholar] [CrossRef]
- Bald, T.; Krummel, M.F.; Smyth, M.J.; Barry, K.C. The NK cell-cancer cycle: Advances and new challenges in NK cell-based immunotherapies. Nat. Immunol. 2020, 21, 835–847. [Google Scholar] [CrossRef]
- Shimasaki, N.; Jain, A.; Campana, D. NK cells for cancer immunotherapy. Nat. Rev. Drug Discov. 2020, 19, 200–218. [Google Scholar] [CrossRef] [PubMed]
- Lucar, O.; Reeves, R.K.; Jost, S. A Natural Impact: NK Cells at the Intersection of Cancer and HIV Disease. Front. Immunol. 2019, 10, 1850. [Google Scholar] [CrossRef] [Green Version]
- Flórez-Álvarez, L.; Hernandez, J.C.; Zapata, W. NK Cells in HIV-1 Infection: From Basic Science to Vaccine Strategies. Front. Immunol. 2018, 9, 2290. [Google Scholar] [CrossRef]
- Schuster, I.S.; Coudert, J.D.; Andoniou, C.E.; Degli-Esposti, M.A. “Natural Regulators”: NK Cells as Modulators of T Cell Immunity. Front. Immunol. 2016, 7, 235. [Google Scholar] [CrossRef] [Green Version]
- Cichicki, F.; Schlums, H.; Theorell, J.; Tesi, B.; Miller, J.S.; Ljunggren, H.-G.; Bryceson, Y.T. Diversification and Functional Specialization of Human NK Cell Subsets. Curr. Top. Microbiol. Immunol. 2016, 395, 63–94. [Google Scholar] [PubMed]
- McAtee, F.J.; Portis, J.L. Monoclonal antibodies specific for wild mouse neurotropic retrovirus: Detection of comparable levels of virus replication in mouse strains susceptible and resistant to paralytic disease. J. Virol. 1985, 56, 1018–1022. [Google Scholar] [CrossRef] [Green Version]
- Portis, J.L.; Czub, S.; Garon, C.F.; McAtee, F.J. Neurodegenerative disease induced by the wild mouse ecotropic retrovirus is markedly accelerated by long terminal repeat and gag-pol sequences from nondefective Friend murine leukemia virus. J. Virol. 1990, 64, 1648–1656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelegrin, M.; Marin, M.; Oates, A.; Noel, D.; Saller, R.; Salmons, B.; Piechaczyk, M. Immunotherapy of a viral disease by in vivo production of therapeutic monoclonal antibodies. Hum. Gene Ther. 2000, 11, 1407–1415. [Google Scholar] [CrossRef]
- Gros, L.; Dreja, H.; Fiser, A.L.; Plays, M.; Pelegrin, M.; Piechaczyk, M. Induction of long-term protective antiviral endogenous immune response by short neutralizing monoclonal antibody treatment. J. Virol. 2005, 79, 6272–6280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chesebro, B. Characterization of mouse monoclonal antibodies specific for Friend murine leukemia virus-induced erythroleukemia cells: Friend-specific and FMR-specific antigens. Virology 1981, 112, 131–144. [Google Scholar] [CrossRef]
- Carlyle, J.R.; Mesci, A.; Ljutic, B.; Belanger, S.; Tai, L.-H.; Rousselle, E.; Troke, A.D.; Proteau, M.-F.; Makrigiannis, A.P. Molecular and genetic basis for strain-dependent NK1.1 alloreactivity of mouse NK cells. J. Immunol. 2006, 176, 7511–7524. [Google Scholar] [CrossRef] [Green Version]
- Guyre, C.A.; Gomes, D.; Smith, K.A.; Kaplan, J.M.; Perricone, M.A. Development of an in vivo antibody-mediated killing (IVAK) model, a flow cytometric method to rapidly evaluate therapeutic antibodies. J. Immunol. Methods 2008, 333, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Qin, H.; Chesebro, B.; Cheever, M.A. Identification of a gag-encoded cytotoxic T-lymphocyte epitope from FBL-3 leukemia shared by Friend, Moloney, and Rauscher murine leukemia virus-induced tumors. J. Virol. 1996, 70, 7773–7782. [Google Scholar] [CrossRef] [Green Version]
- Cortez, V.S.; Robinette, M.L.; Colonna, M. Innate lymphoid cells: New insights into function and development. Curr. Opin. Immunol. 2015, 32, 71–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruhns, P.; Jonsson, F. Mouse and human FcR effector functions. Immunol. Rev. 2015, 268, 25–51. [Google Scholar] [CrossRef] [Green Version]
- Bodhankar, S.; Woolard, M.D.; Sun, X.; Simecka, J.W. NK cells interfere with the generation of resistance against mycoplasma respiratory infection following nasal-pulmonary immunization. J. Immunol. 2009, 183, 2622–2631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ong, S.; Ligons, D.L.; Barin, J.G.; Wu, L.; Talor, M.V.; Diny, N.; Fontes, J.A.; Gebremariam, E.; Kass, D.A.; Rose, N.R.; et al. Natural killer cells limit cardiac inflammation and fibrosis by halting eosinophil infiltration. Am. J. Pathol. 2015, 185, 847–861. [Google Scholar] [CrossRef] [Green Version]
- Parsa, R.; Lund, H.; Georgoudaki, A.M.; Zhang, X.M.; Ortlieb Guerreiro-Cacais, A.; Grommisch, D.; Warnecke, A.; Croxford, A.L.; Jagodic, M.; Becher, B.; et al. BAFF-secreting neutrophils drive plasma cell responses during emergency granulopoiesis. J. Exp. Med. 2016, 213, 1537–1553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikulak, J.; Oriolo, F.; Zaghi, E.; Di Vito, C.; Mavilio, D. Natural killer cells in HIV-1 infection and therapy. AIDS 2017, 31, 2317–2330. [Google Scholar] [CrossRef]
- Market, M.; Angka, L.; Martel, A.B.; Bastin, D.; Olanubi, O.; Tennakoon, G.; Boucher, D.M.; Ng, J.; Ardolino, M.; Auer, R.C. Flattening the COVID-19 Curve With Natural Killer Cell Based Immunotherapies. Front. Immunol. 2020, 11, 1512. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Sun, H.; Xiao, W.; Zhang, C.; Tian, Z. Natural killer cell dysfunction in hepatocellular carcinoma and NK cell-based immunotherapy. Acta Pharmacol. Sin. 2015, 36, 1191–1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Han, Q.; Hou, Z.; Zhang, C.; Tian, Z.; Zhang, J. Exosomes mediate hepatitis B virus (HBV) transmission and NK-cell dysfunction. Cell Mol. Immunol. 2017, 14, 465–475. [Google Scholar] [CrossRef]
- Drabczyk-Pluta, M.; Werner, T.; Hoffmann, D.; Leng, Q.; Chen, L.; Dittmer, U.; Zelinskyy, G. Granulocytic myeloid-derived suppressor cells suppress virus-specific CD8+ T cell responses during acute Friend retrovirus infection. Retrovirology 2017, 14, 42. [Google Scholar] [CrossRef] [Green Version]
- Mayoux, M.; Roller, A.; Pulko, V.; Sammicheli, S.; Chen, S.; Sum, E.; Jost, C.; Fransen, M.F.; Buser, R.B.; Kowanetz, M.; et al. Dendritic cells dictate responses to PD-L1 blockade cancer immunotherapy. Sci. Transl. Med. 2020, 12, eaav7431. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Qiu, X.; Zhang, Z.; Zhang, S.; Zhang, Y.; Liang, Y.; Guo, J.; Peng, H.; Chen, M.; Fu, Y.-X.; et al. PD-L1 on dendritic cells attenuates T cell activation and regulates response to immune checkpoint blockade. Nat. Commun. 2020, 11, 4835. [Google Scholar] [CrossRef]
- Gehrie, E.; Van der Touw, W.; Bromberg, J.S.; Ochando, J.C. Plasmacytoid Dendritic Cells in Tolerance. In Suppression and Regulation of Immune Responses; Cuturi, M.C., Anegon, I., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2010; Volume 677, pp. 127–147. ISBN 978-1-60761-868-3. [Google Scholar]
- Tian, Y.; Meng, L.; Wang, Y.; Li, B.; Yu, H.; Zhou, Y.; Bui, T.; Li, A.; Abraham, C.; Zhang, Y.; et al. Graft-versus-host disease depletes plasmacytoid dendritic cell progenitors to impair tolerance induction. J. Clin. Investig. 2020, 131. [Google Scholar] [CrossRef]
- Riemann, D.; Schütte, W.; Turzer, S.; Seliger, B.; Möller, M. High PD-L1/CD274 Expression of Monocytes and Blood Dendritic Cells Is a Risk Factor in Lung Cancer Patients Undergoing Treatment with PD1 Inhibitor Therapy. Cancers 2020, 12, 2966. [Google Scholar] [CrossRef]
- Benlahrech, A.; Yasmin, A.; Westrop, S.J.; Coleman, A.; Herasimtschuk, A.; Page, E.; Kelleher, P.; Gotch, F.; Imami, N.; Patterson, S. Dysregulated immunophenotypic attributes of plasmacytoid but not myeloid dendritic cells in HIV-1 infected individuals in the absence of highly active anti-retroviral therapy. Clin. Exp. Immunol. 2012, 170, 212–221. [Google Scholar] [CrossRef]
- Barber, D.L.; Wherry, E.J.; Masopust, D.; Zhu, B.; Allison, J.P.; Sharpe, A.H.; Freeman, G.J.; Ahmed, R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 2006, 439, 682–687. [Google Scholar] [CrossRef]
- Ahn, E.; Araki, K.; Hashimoto, M.; Li, W.; Riley, J.L.; Cheung, J.; Sharpe, A.H.; Freeman, G.J.; Irving, B.A.; Ahmed, R. Role of PD-1 during effector CD8 T cell differentiation. Proc. Natl. Acad. Sci. USA 2018, 115, 4749–4754. [Google Scholar] [CrossRef] [Green Version]
- Day, C.L.; Kaufmann, D.E.; Kiepiela, P.; Brown, J.A.; Moodley, E.S.; Reddy, S.; Mackey, E.W.; Miller, J.D.; Leslie, A.J.; DePierres, C.; et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 2006, 443, 350–354. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, M.; Fontenot, A.P.; Mack, D.G.; Lozupone, C.; Dillon, S.; Meditz, A.; Wilson, C.C.; Connick, E.; Palmer, B.E. Programmed death 1 expression on HIV-specific CD4+ T cells is driven by viral replication and associated with T cell dysfunction. J. Immunol. 2007, 179, 1979–1987. [Google Scholar] [CrossRef] [Green Version]
- Porichis, F.; Kwon, D.S.; Zupkosky, J.; Tighe, D.P.; McMullen, A.; Brockman, M.A.; Pavlik, D.F.; Rodriguez-Garcia, M.; Pereyra, F.; Freeman, G.J.; et al. Responsiveness of HIV-specific CD4 T cells to PD-1 blockade. Blood 2011, 118, 965–974. [Google Scholar] [CrossRef] [Green Version]
- Porichis, F.; Hart, M.G.; Massa, A.; Everett, H.L.; Morou, A.; Richard, J.; Brassard, N.; Veillette, M.; Hassan, M.; Ly, N.L.; et al. Immune Checkpoint Blockade Restores HIV-Specific CD4 T Cell Help for NK Cells. J. Immunol. 2018, 201, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Teigler, J.E.; Zelinskyy, G.; Eller, M.A.; Bolton, D.L.; Marovich, M.; Gordon, A.D.; Alrubayyi, A.; Alter, G.; Robb, M.L.; Martin, J.N.; et al. Differential Inhibitory Receptor Expression on T Cells Delineates Functional Capacities in Chronic Viral Infection. J. Virol. 2017, 91, e01263-17. [Google Scholar] [CrossRef] [Green Version]
- Brooks, D.G.; Teyton, L.; Oldstone, M.B.A.; McGavern, D.B. Intrinsic Functional Dysregulation of CD4 T Cells Occurs Rapidly following Persistent Viral Infection. J. Virol. 2005, 79, 10514–10527. [Google Scholar] [CrossRef] [Green Version]
- Balança, C.-C.; Salvioni, A.; Scarlata, C.-M.; Michelas, M.; Martinez-Gomez, C.; Gomez-Roca, C.; Sarradin, V.; Tosolini, M.; Valle, C.; Pont, F.; et al. PD-1 blockade restores helper activity of tumor-infiltrating exhausted PD-1hiCD39+ CD4 T cells. JCI Insight 2020. [Google Scholar]
- Dwyer, K.M.; Deaglio, S.; Gao, W.; Friedman, D.; Strom, T.B.; Robson, S.C. CD39 and control of cellular immune responses. Purinergic Signal. 2007, 3, 171–180. [Google Scholar] [CrossRef] [Green Version]
- Nikolova, M.; Carriere, M.; Jenabian, M.-A.; Limou, S.; Younas, M.; Kök, A.; Huë, S.; Seddiki, N.; Hulin, A.; Delaneau, O.; et al. CD39/adenosine pathway is involved in AIDS progression. PLoS Pathog. 2011, 7, e1002110. [Google Scholar] [CrossRef] [Green Version]
- Song, J.-W.; Huang, H.-H.; Zhang, C.; Yang, H.-G.; Zhang, J.-Y.; Xu, R.-N.; Jin, L.; Shi, M.; Wang, F.-S.; Jiao, Y.-M. Expression of CD39 Is Correlated With HIV DNA Levels in Naïve Tregs in Chronically Infected ART Naïve Patients. Front. Immunol. 2019, 10, 2465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, P.K.; Godec, J.; Wolski, D.; Adland, E.; Yates, K.; Pauken, K.E.; Cosgrove, C.; Ledderose, C.; Junger, W.G.; Robson, S.C.; et al. CD39 Expression Identifies Terminally Exhausted CD8+ T Cells. PLoS Pathog. 2015, 11, e1005177. [Google Scholar] [CrossRef] [Green Version]
- Trad, M.; Gautheron, A.; Fraszczak, J.; Alizadeh, D.; Larmonier, C.; LaCasse, C.J.; Centuori, S.; Audia, S.; Samson, M.; Ciudad, M.; et al. T Lymphocyte Inhibition by Tumor-Infiltrating Dendritic Cells Involves Ectonucleotidase CD39 but Not Arginase-1. BioMed Res. Int. 2015, 2015, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, O.; Kimura, S.; Jackson, E.K.; Robson, S.C.; Geller, D.A.; Murase, N.; Thomson, A.W. CD39 expression by hepatic myeloid dendritic cells attenuates inflammation in liver transplant ischemia-reperfusion injury in mice. Hepatology 2013, 58, 2163–2175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mascanfroni, I.D.; Yeste, A.; Vieira, S.M.; Burns, E.J.; Patel, B.; Sloma, I.; Wu, Y.; Mayo, L.; Ben-Hamo, R.; Efroni, S.; et al. IL-27 acts on DCs to suppress the T cell response and autoimmunity by inducing expression of the immunoregulatory molecule CD39. Nat. Immunol. 2013, 14, 1054–1063. [Google Scholar] [CrossRef]
- Vitale, M.; Chiesa, M.D.; Carlomagno, S.; Pende, D.; Aricò, M.; Moretta, L.; Moretta, A. NK-dependent DC maturation is mediated by TNFα and IFNγ released upon engagement of the NKp30 triggering receptor. Blood 2005, 106, 566–571. [Google Scholar] [CrossRef]
- Bhatnagar, N.; Hong, H.S.; Krishnaswamy, J.K.; Haghikia, A.; Behrens, G.M.; Schmidt, R.E.; Jacobs, R. Cytokine-activated NK cells inhibit PMN apoptosis and preserve their functional capacity. Blood 2010, 116, 1308–1316. [Google Scholar] [CrossRef]
- Puga, I.; Cols, M.; Barra, C.M.; He, B.; Cassis, L.; Gentile, M.; Comerma, L.; Chorny, A.; Shan, M.; Xu, W.; et al. B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat. Immunol. 2011, 13, 170–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giordano, D.; Kuley, R.; Draves, K.E.; Roe, K.; Holder, U.; Giltiay, N.V.; Clark, E.A. BAFF Produced by Neutrophils and Dendritic Cells Is Regulated Differently and Has Distinct Roles in Antibody Responses and Protective Immunity against West Nile Virus. J. Immunol. 2020, 204, 1508–1520. [Google Scholar] [CrossRef]
- Nishikado, H.; Mukai, K.; Kawano, Y.; Minegishi, Y.; Karasuyama, H. NK Cell-Depleting Anti-Asialo GM1 Antibody Exhibits a Lethal Off-Target Effect on Basophils In Vivo. J. Immunol. 2011, 186, 5766–5771. [Google Scholar] [CrossRef] [Green Version]
- Voehringer, D. Regulation of type 2 immunity by basophils. Adv. Exp. Med. Biol 2013, 785, 37–41. [Google Scholar]
- Reitz, M.; Brunn, M.-L.; Voehringer, D.; Breloer, M. Basophils are dispensable for the establishment of protective adaptive immunity against primary and challenge infection with the intestinal helminth parasite Strongyloides ratti. PLoS Negl. Trop. Dis. 2018, 12, e0006992. [Google Scholar] [CrossRef]
- Deligne, C.; Metidji, A.; Fridman, W.H.; Teillaud, J.L. Anti-CD20 therapy induces a memory Th1 response through the IFN-gamma/IL-12 axis and prevents protumor regulatory T-cell expansion in mice. Leukemia 2015, 29, 947–957. [Google Scholar] [CrossRef]
- Srivastava, R.M.; Lee, S.C.; Andrade Filho, P.A.; Lord, C.A.; Jie, H.B.; Davidson, H.C.; Lopez-Albaitero, A.; Gibson, S.P.; Gooding, W.E.; Ferrone, S.; et al. Cetuximab-activated natural killer and dendritic cells collaborate to trigger tumor antigen-specific T-cell immunity in head and neck cancer patients. Clin. Cancer Res. 2013, 19, 1858–1872. [Google Scholar] [CrossRef] [Green Version]
- Perrot, I.; Michaud, H.-A.; Giraudon-Paoli, M.; Augier, S.; Docquier, A.; Gros, L.; Courtois, R.; Déjou, C.; Jecko, D.; Becquart, O.; et al. Blocking Antibodies Targeting the CD39/CD73 Immunosuppressive Pathway Unleash Immune Responses in Combination Cancer Therapies. Cell Rep. 2019, 27, 2411–2425.e9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velu, V.; Titanji, K.; Zhu, B.; Husain, S.; Pladevega, A.; Lai, L.; Vanderford, T.H.; Chennareddi, L.; Silvestri, G.; Freeman, G.J.; et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature 2009, 458, 206–210. [Google Scholar] [CrossRef]
- Klausz, K.; Cieker, M.; Kellner, C.; Rösner, T.; Otte, A.; Krohn, S.; Lux, A.; Nimmerjahn, F.; Valerius, T.; Gramatzki, M.; et al. Fc-engineering significantly improves the recruitment of immune effector cells by anti-ICAM-1 antibody MSH-TP15 for myeloma therapy. Haematologica 2020. [Google Scholar] [CrossRef] [PubMed]
- Schmied, B.J.; Riegg, F.; Zekri, L.; Grosse-Hovest, L.; Bühring, H.-J.; Jung, G.; Salih, H.R. An Fc-Optimized CD133 Antibody for Induction of Natural Killer Cell Reactivity Against Colorectal Cancer. Cancers 2019, 11, 789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turini, M.; Chames, P.; Bruhns, P.; Baty, D.; Kerfelec, B. A FcγRIII-engaging bispecific antibody expands the range of HER2-expressing breast tumors eligible to antibody therapy. Oncotarget 2014, 5, 5304–5319. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naranjo-Gomez, M.; Cahen, M.; Lambour, J.; Boyer-Clavel, M.; Pelegrin, M. Immunomodulatory Role of NK Cells during Antiviral Antibody Therapy. Vaccines 2021, 9, 137. https://doi.org/10.3390/vaccines9020137
Naranjo-Gomez M, Cahen M, Lambour J, Boyer-Clavel M, Pelegrin M. Immunomodulatory Role of NK Cells during Antiviral Antibody Therapy. Vaccines. 2021; 9(2):137. https://doi.org/10.3390/vaccines9020137
Chicago/Turabian StyleNaranjo-Gomez, Mar, Marine Cahen, Jennifer Lambour, Myriam Boyer-Clavel, and Mireia Pelegrin. 2021. "Immunomodulatory Role of NK Cells during Antiviral Antibody Therapy" Vaccines 9, no. 2: 137. https://doi.org/10.3390/vaccines9020137