A Fermented Milk Matrix Containing Postbiotics Supports Th1- and Th17-Type Immunity In Vitro and Modulates the Influenza-Specific Vaccination Response In Vivo in Association with Altered Serum Galectin Ratios
Abstract
:1. Introduction
2. Materials and Methods
2.1. In Vitro IEC/PBMC Co-Culture Model
2.1.1. Intestinal Epithelial Cell Culture
2.1.2. Peripheral Blood Mononuclear Cells Isolation
2.1.3. IEC/PBMC Co-Culture Model
2.1.4. Enzyme-Linked Immunosorbent Assay (ELISA)
2.2. In Vivo Influenza Vaccination Model
2.2.1. Animals
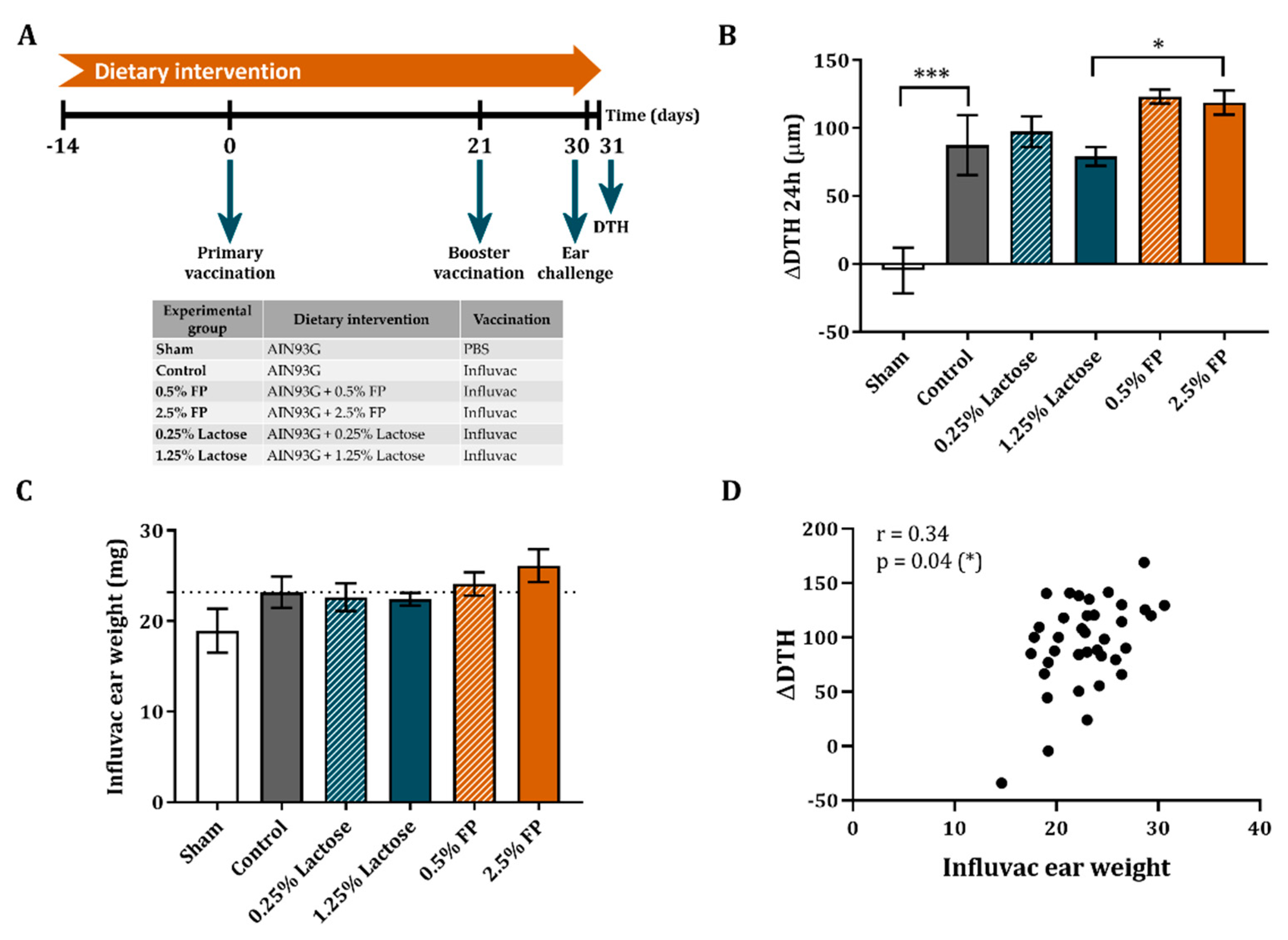
2.2.2. Vaccination Protocol and Dietary Intervention
2.2.3. Vaccine-Specific Immunoglobulins and Galectins in Serum
2.2.4. Cell Isolation from Tissues
2.2.5. Flow Cytometry of Immune Cells
2.2.6. Generation of Bone Marrow-Derived Dendritic Cells (BMDC)
2.2.7. Ex Vivo Re-Stimulation Assay
2.2.8. qPCR Analysis of Gene Expression
2.3. Statistical Analysis
3. Results
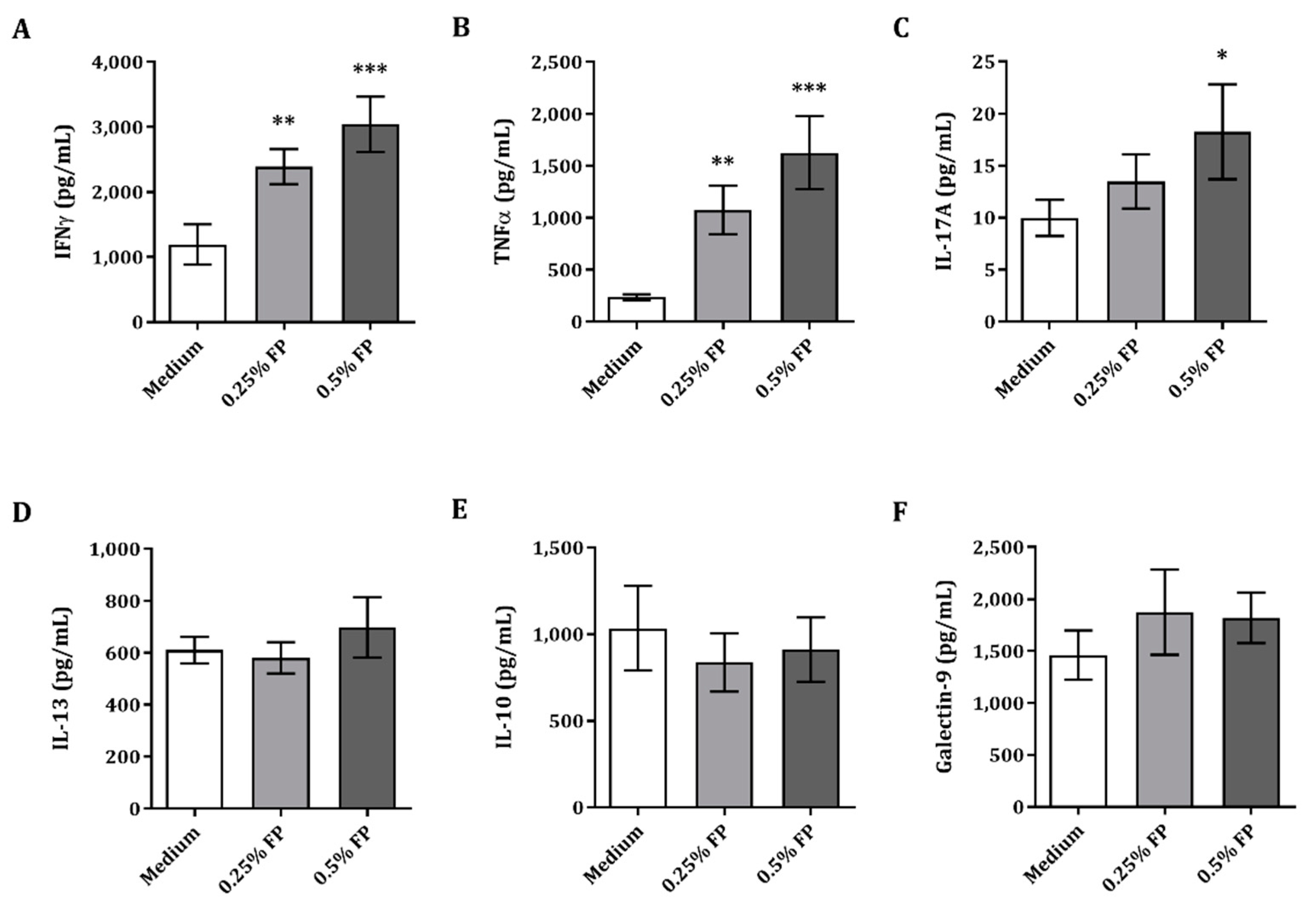
3.1. Exposure of IEC to FP Enhances Th1- and Th17-Type Cytokines Iin the IEC/PBMC Co-Culture
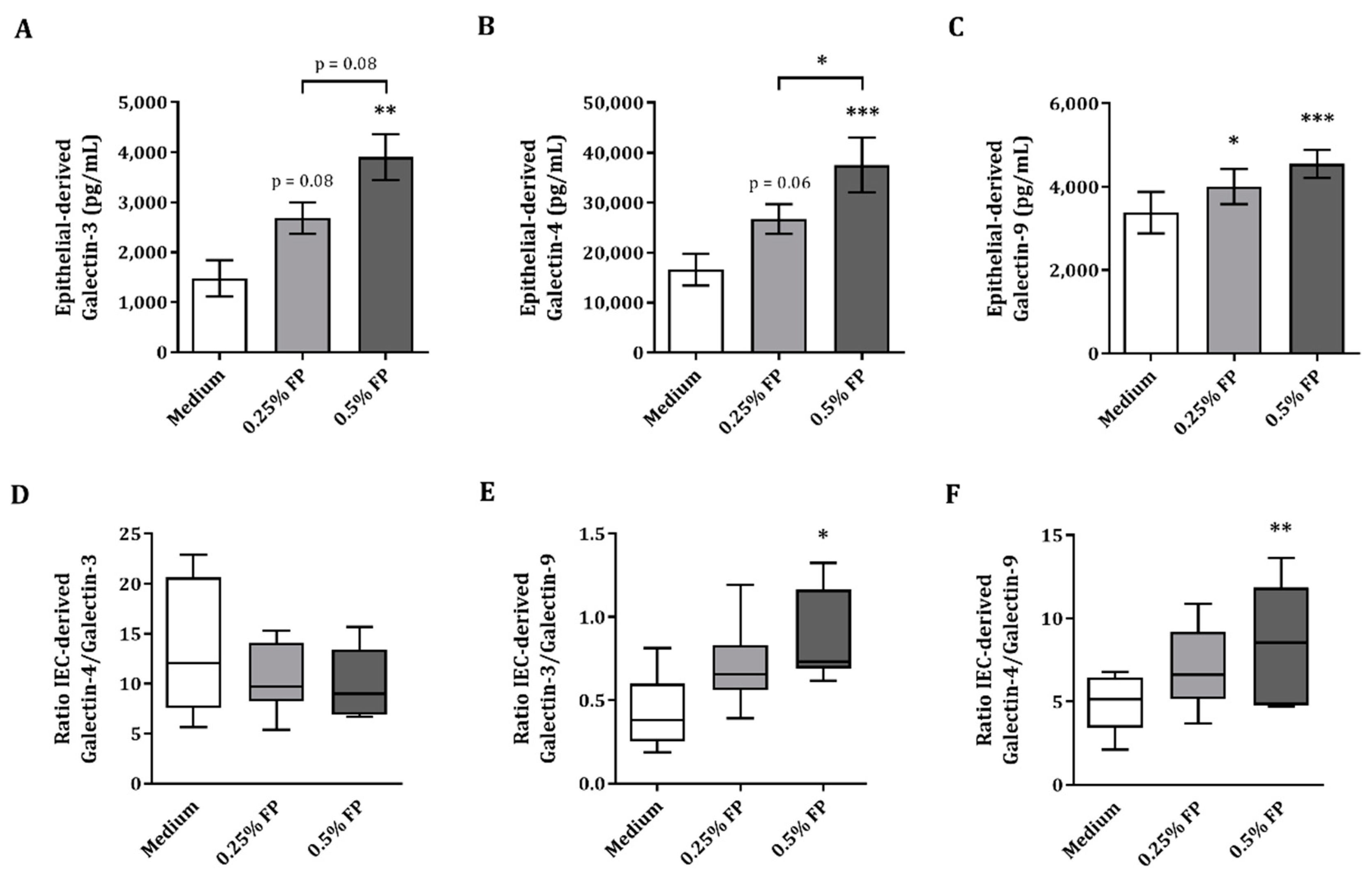
3.2. IEC-Derived Galectin-3, -4 and-9 after IEC/PBMC Co-Culture
3.3. Dietary Intervention with FP Improves the Vaccine-Specific DTH Response
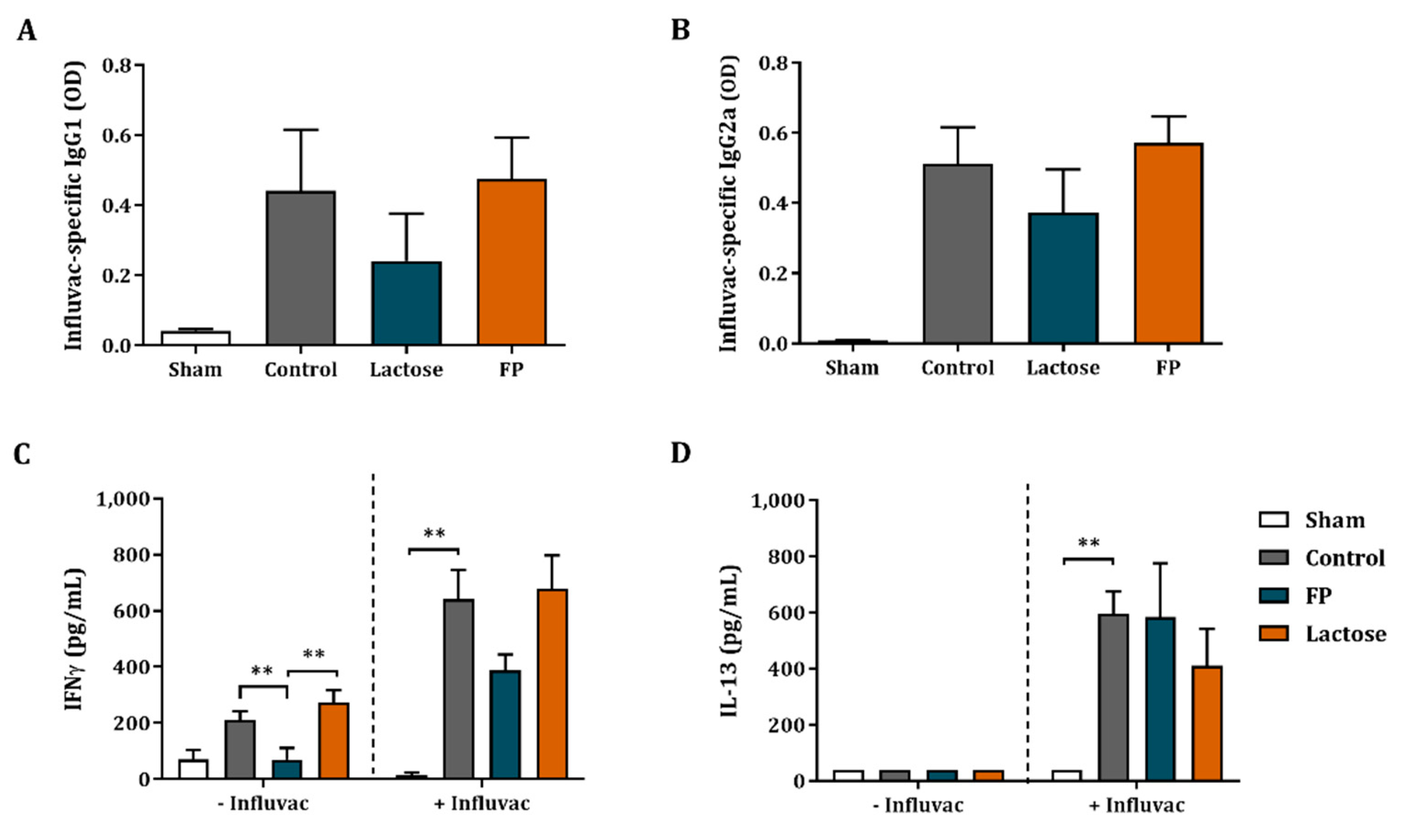
3.4. Influvac-Specific Igg1 and Igg2a in Serum and Ex Vivo Cytokine Secretion
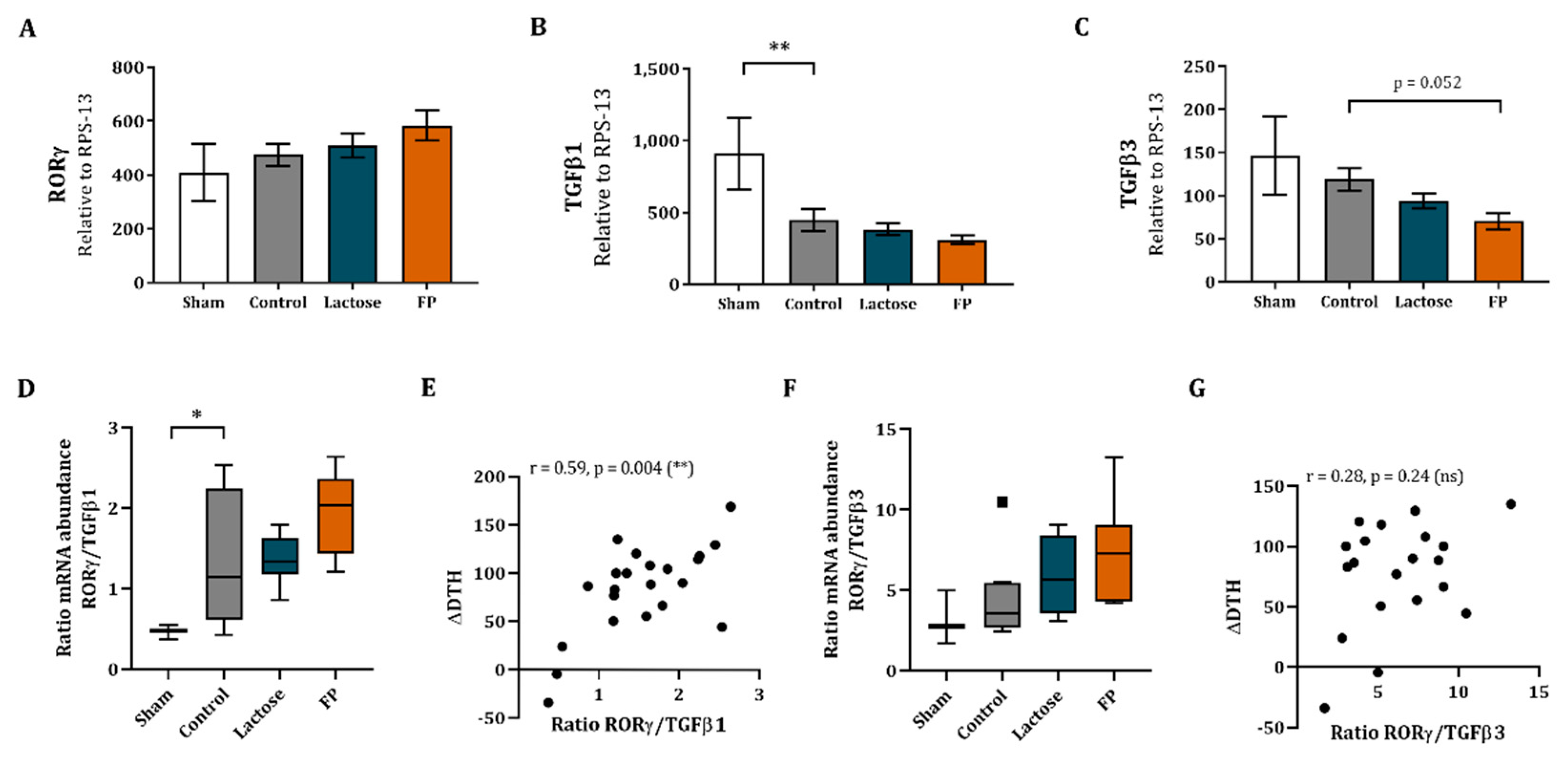
3.5. Shift in Th17/T-Regulatory Mrna Expression in Ileum
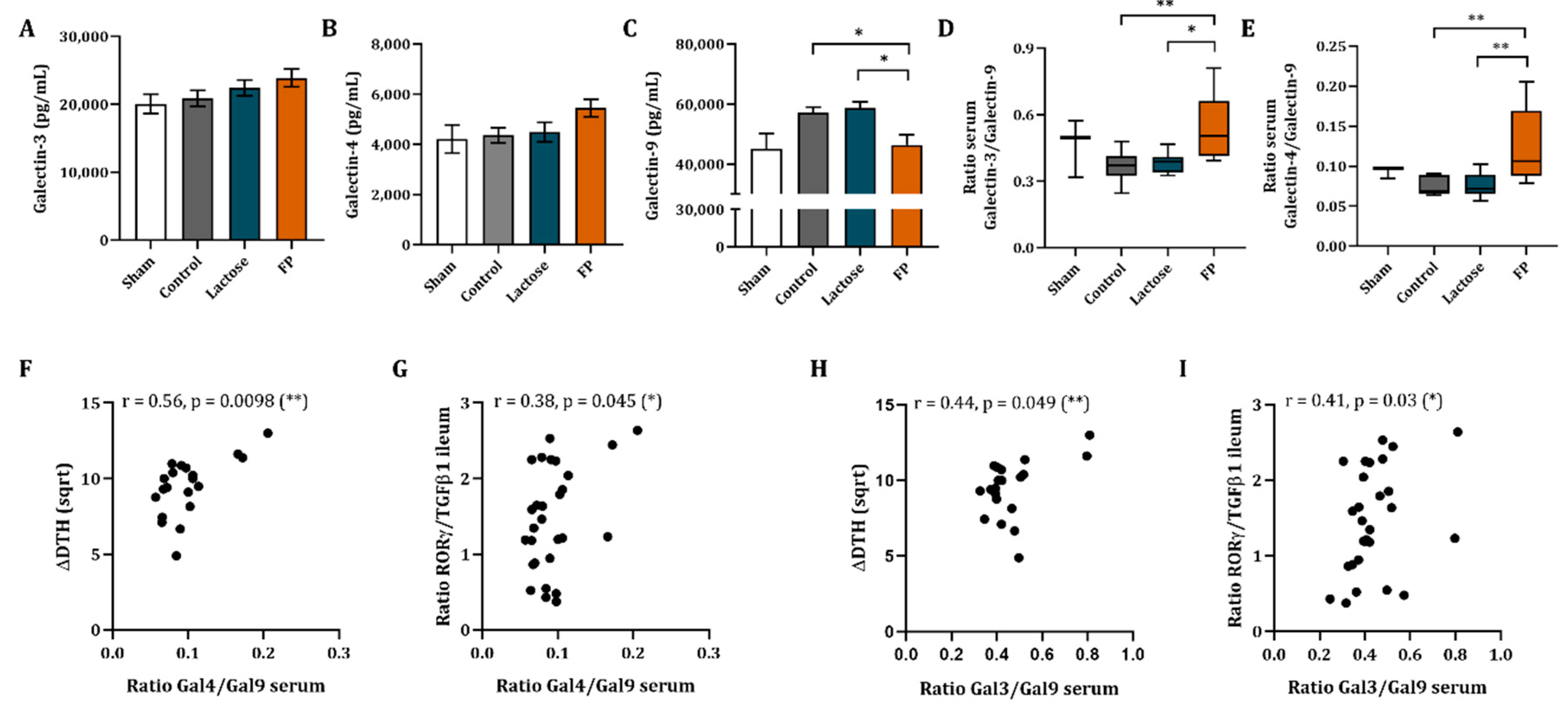
3.6. Galectin-3, -4 and -9 Mrna Expression in Ileum and Concentrations in Serum
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martin, R.; Nauta, A.J.; Ben Amor, K.; Knippels, L.M.J.; Knol, J.; Garssen, J. Early life: Gut microbiota and immune development in infancy. Benef. Microbes 2010, 1, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Torow, N.; Marsland, B.J.; Hornef, M.W.; Gollwitzer, E.S. Neonatal mucosal immunology. Mucosal Immunol. 2017, 10, 5–17. [Google Scholar] [CrossRef]
- Victora, C.G.; Bahl, R.; Barros, A.J.D.; França, G.V.A.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C.; et al. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef] [Green Version]
- Salminen, S.; Stahl, B.; Vinderola, G.; Szajewska, H. Infant Formula Supplemented with Biotics: Current Knowledge and Future Perspectives. Nutrients 2020, 12, 1952. [Google Scholar] [CrossRef] [PubMed]
- Granier, A.; Goulet, O.; Hoarau, C. Fermentation products: Immunological effects on human and animal models. Pediatr. Res. 2013, 74, 238–244. [Google Scholar] [CrossRef] [Green Version]
- Collado, M.C.; Vinderola, G.; Salminen, S. Postbiotics: Facts and open questions. A position paper on the need for a consensus definition. Benef. Microbes 2019, 10, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Toalá, J.E.; Garcia-Varela, R.; Garcia, H.S.; Mata-Haro, V.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Technol. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- Wegh, C.A.M.; Geerlings, S.Y.; Knol, J.; Roeselers, G.; Belzer, C. Postbiotics and their potential applications in early life nutrition and beyond. Int. J. Mol. Sci. 2019, 20, 4673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szajewska, H.; Skórka, A.; Pieścik-Lech, M. Fermented infant formulas without live bacteria: A systematic review. Eur. J. Pediatr. 2015, 174, 1413–1420. [Google Scholar] [CrossRef]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar] [CrossRef]
- Rodriguez-Herrera, A.; Mulder, K.; Bouritius, H.; Rubio, R.; Muñoz, A.; Agosti, M.; Lista, G.; Corvaglia, L.; Ludwig, T.; Abrahamse-Berkeveld, M.; et al. Gastrointestinal tolerance, growth and safety of a partly fermented formula with specific prebiotics in healthy infants: A double-blind, randomized, controlled trial. Nutrients 2019, 11, 1530. [Google Scholar] [CrossRef] [Green Version]
- Thibault, H.; Aubert-Jacquin, C.; Goulet, O. Effects of Long-term Consumption of a Fermented Infant Formula (with Bifidobacterium breve c50 and Streptococcus thermophilus 065) on Acute Diarrhea in Healthy Infants. J. Pediatr. Gastroenterol. Nutr. 2004, 39, 147–152. [Google Scholar] [CrossRef]
- Indrio, F.; Ladisa, G.; Mautone, A.; Montagna, O. Effect of a fermented formula on thymus size and stool pH in healthy term infants. Pediatr. Res. 2007, 62, 98–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campeotto, F.; Suau, A.; Kapel, N.; Magne, F.; Viallon, V.; Ferraris, L.; Waligora-Dupriet, A.J.; Soulaines, P.; Leroux, B.; Kalach, N.; et al. A fermented formula in pre-term infants: Clinical tolerance, gut microbiota, down-regulation of faecal calprotectin and up-regulation of faecal secretory IgA. Br. J. Nutr. 2011, 105, 1843–1851. [Google Scholar] [CrossRef] [PubMed]
- Mullié, C.; Yazourh, A.; Thibault, H.; Odou, M.F.; Singer, E.; Kalach, N.; Kremp, O.; Romond, M.B. Increased poliovirus-specific intestinal antibody response coincides with promotion of Bifidobacterium longum-infantis and Bifidobacterium breve in infants: A randomized, double-blind, placebo-controlled trial. Pediatr. Res. 2004, 56, 791–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morisset, M.; Aubert-Jacquin, C.; Soulaines, P.; Moneret-Vautrin, D.A.; Dupont, C. A non-hydrolyzed, fermented milk formula reduces digestive and respiratory events in infants at high risk of allergy. Eur. J. Clin. Nutr. 2011, 65, 175–183. [Google Scholar] [CrossRef]
- Hoarau, C.; Lagaraine, C.; Martin, L.; Velge-Roussel, F.; Lebranchu, Y. Supernatant of Bifidobacterium breve induces dendritic cell maturation, activation, and survival through a Toll-like receptor 2 pathway. J. Allergy Clin. Immunol. 2006, 117, 696–702. [Google Scholar] [CrossRef]
- Ménard, S.; Laharie, D.; Asensio, C.; Vidal-Martinez, T.; Candalh, C.; Rullier, A.; Zerbib, F.; Mégraud, F.; Matysiak-Budnik, T.; Heyman, M. Bifidobacterium breve and Streptococcus thermophilus secretion products enhance T helper 1 immune response and intestinal barrier in mice. Exp. Biol. Med. 2005, 230, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Vos, A.P.; Haarman, M.; Buco, A.; Govers, M.; Knol, J.; Garssen, J.; Stahl, B.; Boehm, G.; M’Rabet, L. A specific prebiotic oligosaccharide mixture stimulates delayed-type hypersensitivity in a murine influenza vaccination model. Int. Immunopharmacol. 2006, 6, 1277–1286. [Google Scholar] [CrossRef]
- Vos, A.P.; Haarman, M.; Van Ginkel, J.-W.H.; Knol, J.; Garssen, J.; Stahl, B.; Boehm, G.; Rabet, L.M. Dietary supplementation of neutral and acidic oligosaccharides enhances Th1-dependent vaccination responses in mice. Pediatr. Allergy Immunol. 2007, 18, 304–312. [Google Scholar] [CrossRef]
- Vos, A.P.; Knol, J.; Stahl, B.; M’Rabet, L.; Garssen, J. Specific prebiotic oligosaccharides modulate the early phase of a murine vaccination response. Int. Immunopharmacol. 2010, 10, 619–625. [Google Scholar] [CrossRef]
- Schijf, M.A.; Kerperien, J.A.; Bastiaans, J.; Szklany, K.; Meerding, J.; Hofman, G.; Boon, L.; van Wijk, F.; Garssen, J.; van’t Land, B. Alterations in Regulatory T Cells Induced by Specific Oligosaccharides Improve Vaccine Responsiveness in Mice. PLoS ONE 2013, 8, e75148. [Google Scholar] [CrossRef]
- Xiao, L.; Leusink-Muis, T.; Kettelarij, N.; van Ark, I.; Blijenberg, B.; Hesen, N.A.; Stahl, B.; Overbeek, S.A.; Garssen, J.; Folkerts, G.; et al. Human milk oligosaccharide 2′-Fucosyllactose improves innate and adaptive immunity in an influenza-specific murine vaccination model. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Kivit, S.; Kraneveld, A.D.; Knippels, L.M.J.; Van Kooyk, Y.; Garssen, J.; Willemsen, L.E.M. Intestinal epithelium-derived galectin-9 is involved in the immunomodulating effects of nondigestible oligosaccharides. J. Innate Immun. 2013, 5, 625–638. [Google Scholar] [CrossRef]
- Ayechu-Muruzabal, V.; Overbeek, S.A.; Kostadinova, A.I.; Stahl, B.; Garssen, J.; Van’t Land, B.; Willemsen, L.E.M. Exposure of intestinal epithelial cells to 2′-fucosyllactose and CpG enhances galectin release and instructs dendritic cells to drive Th1 and regulatory-type immune development. Biomolecules 2020, 10, 784. [Google Scholar] [CrossRef]
- Schouten, B.; Van Esch, B.C.A.M.; Hofman, G.A.; Van Doorn, S.A.C.M.; Knol, J.; Nauta, A.J.; Garssen, J.; Willemsen, L.E.M.; Knippels, L.M.J. Cow milk allergy symptoms are reduced in mice fed dietary synbiotics during oral sensitization with whey. J. Nutr. 2009, 139, 1398–1403. [Google Scholar] [CrossRef]
- De Kivit, S.; Saeland, E.; Kraneveld, A.D.; Van De Kant, H.J.G.; Schouten, B.; Van Esch, B.C.A.M.; Knol, J.; Sprikkelman, A.B.; Van Der Aa, L.B.; Knippels, L.M.J.; et al. Galectin-9 induced by dietary synbiotics is involved in suppression of allergic symptoms in mice and humans. Allergy Eur. J. Allergy Clin. Immunol. 2012, 67, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Hayen, S.M.; Otten, H.G.; Overbeek, S.A.; Knulst, A.C.; Garssen, J.; Willemsen, L.E.M. Exposure of intestinal epithelial cells to short- and long-chain fructo-oligosaccharides and CpG oligodeoxynucleotides enhances peanut-specific T Helper 1 polarization. Front. Immunol. 2018, 9, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van’t Land, B.; Schijf, M.; van Esch, B.C.A.M.; van Bergenhenegouwen, J.; Bastiaans, J.; Schouten, B.; Boon, L.; Garssen, J. Regulatory T-cells have a prominent role in the immune modulated vaccine response by specific oligosaccharides. Vaccine 2010, 28, 5711–5717. [Google Scholar] [CrossRef]
- Lutz, M.B.; Kukutsch, N.; Ogilvie, A.L.J.; Rößner, S.; Koch, F.; Romani, N.; Schuler, G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 1999, 223, 77–92. [Google Scholar] [CrossRef]
- Smit, J.; Zeeuw-Brouwer, M. lène de; van Roest, M.; de Jong, G.; van Bilsen, J. Evaluation of the sensitizing potential of food proteins using two mouse models. Toxicol. Lett. 2016, 262, 62–69. [Google Scholar] [CrossRef] [PubMed]
- García-Vallejo, J.J.; Van Het Hof, B.; Robben, J.; Van Wijk, J.A.E.; Van Die, I.; Joziasse, D.H.; Van Dijk, W. Approach for defining endogenous reference genes in gene expression experiments. Anal. Biochem. 2004, 329, 293–299. [Google Scholar] [CrossRef]
- Jijon, H.; Backer, J.; Diaz, H.; Yeung, H.; Thiel, D.; McKaigney, C.; De Simone, C.; Madsen, K. DNA from Probiotic Bacteria Modulates Murine and Human Epithelial and Immune Function. Gastroenterology 2004, 126, 1358–1373. [Google Scholar] [CrossRef] [PubMed]
- Dwinell, M.B.; Lügering, N.; Eckmann, L.; Kagnoff, M.F. Regulated production of interferon-inducible T-cell chemoattractants by human intestinal epithelial cells. Gastroenterology 2001, 120, 49–59. [Google Scholar] [CrossRef] [PubMed]
- de Kivit, S.; van Hoffen, E.; Korthagen, N.; Garssen, J.; Willemsen, L.E.M. Apical TLR ligation of intestinal epithelial cells drives a Th1-polarized regulatory or inflammatory type effector response in vitro. Immunobiology 2011, 216, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Thiemann, S.; Baum, L.G. Galectins and Immune Responses-Just How Do They Do Those Things They Do? Annu. Rev. Immunol. 2016, 34, 243–264. [Google Scholar] [CrossRef]
- Cerliani, J.P.; Stowell, S.R.; Mascanfroni, I.D.; Arthur, C.M.; Cummings, R.D.; Rabinovich, G.A. Expanding the universe of cytokines and pattern recognition receptors: Galectins and glycans in innate immunity. J. Clin. Immunol. 2011, 31, 10–21. [Google Scholar] [CrossRef]
- Cao, Z.Q.; Guo, X.L. The role of galectin-4 in physiology and diseases. Protein Cell 2016, 7, 314–324. [Google Scholar] [CrossRef] [Green Version]
- Hokama, A.; Mizoguchi, E.; Sugimoto, K.; Shimomura, Y.; Tanaka, Y.; Yoshida, M.; Rietdijk, S.T.; De Jong, Y.P.; Snapper, S.B.; Terhorst, C.; et al. Induced reactivity of intestinal CD4+ T cells with an epithelial cell lectin, galectin-4, contributes to exacerbation of intestinal inflammation. Immunity 2004, 20, 681–693. [Google Scholar] [CrossRef] [Green Version]
- Paclik, D.; Danese, S.; Berndt, U.; Wiedenmann, B.; Dignass, A.; Sturm, A. Galectin-4 controls intestinal inflammation by selective regulation of peripheral and mucosal T cell apoptosis and cell cycle. PLoS ONE 2008, 3, e2629. [Google Scholar] [CrossRef] [Green Version]
- Tsai, H.F.; Wu, C.S.; Chen, Y.L.; Liao, H.J.; Chyuan, I.T.; Hsu, P.N. Galectin-3 suppresses mucosal inflammation and reduces disease severity in experimental colitis. J. Mol. Med. 2016, 94, 545–556. [Google Scholar] [CrossRef]
- Lv, K.; Zhang, Y.; Zhang, M.; Zhong, M.; Suo, Q. Galectin-9 promotes TGF-β1-dependent induction of regulatory T cells via the TGF-β/Smad signaling pathway. Mol. Med. Rep. 2013, 7, 205–210. [Google Scholar] [CrossRef]
- Seki, M.; Oomizu, S.; Sakata, K.M.; Sakata, A.; Arikawa, T.; Watanabe, K.; Ito, K.; Takeshita, K.; Niki, T.; Saita, N.; et al. Galectin-9 suppresses the generation of Th17, promotes the induction of regulatory T cells, and regulates experimental autoimmune arthritis. Clin. Immunol. 2008, 127, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Slight, S.R.; Khader, S.A. Th17 cytokines and vaccine-induced immunity. Semin. Immunopathol. 2010, 32, 79–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khader, S.A.; Gaffen, S.L.; Kolls, J.K. Th17 cells at the crossroads of innate and adaptive immunity against infectious diseases at the mucosa. Mucosal Immunol. 2009, 2, 403–411. [Google Scholar] [CrossRef] [Green Version]
- Ivanov, I.I.; McKenzie, B.S.; Zhou, L.; Tadokoro, C.E.; Lepelley, A.; Lafaille, J.J.; Cua, D.J.; Littman, D.R. The Orphan Nuclear Receptor RORγt Directs the Differentiation Program of Proinflammatory IL-17+ T Helper Cells. Cell 2006, 126, 1121–1133. [Google Scholar] [CrossRef] [Green Version]
- Veldhoen, M.; Hocking, R.J.; Atkins, C.J.; Locksley, R.M.; Stockinger, B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 2006, 24, 179–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bettelli, E.; Carrier, Y.; Gao, W.; Korn, T.; Strom, T.B.; Oukka, M.; Weiner, H.L.; Kuchroo, V.K. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006, 441, 235–238. [Google Scholar] [CrossRef]
- Gruson, D.; Ko, G. Galectins testing: New promises for the diagnosis and risk stratification of chronic diseases? Clin. Biochem. 2012, 45, 719–726. [Google Scholar] [CrossRef]
- Katoh, S.; Ikeda, M.; Shimizu, H.; Mouri, K.; Obase, Y.; Kobashi, Y.; Fukushima, K.; Hirashima, M.; Oka, M. Increased levels of plasma galectin-9 in patients with influenza virus infection. Tohoku J. Exp. Med. 2014, 232, 263–267. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.H.; Lin, C.Y.; Chang, M.R.; Urbina, A.N.; Assavalapsakul, W.; Thitithanyanont, A.; Chen, Y.H.; Liu, F.T.; Wang, S.F. The role of galectins in virus infection-A systemic literature review. J. Microbiol. Immunol. Infect. 2019, 53, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Machala, E.A.; McSharry, B.P.; Rouse, B.T.; Abendroth, A.; Slobedman, B. Gal power: The diverse roles of galectins in regulating viral infections. J. Gen. Virol. 2019, 100, 333–349. [Google Scholar] [CrossRef] [PubMed]
| Gene ID | Accession Number | Forward Primer Sequence (5′−3′) | Reverse Primer Sequence (5′−3′) |
|---|---|---|---|
| TNFα | NM_013693.3 | AACGGCATGGATCTCAAAGA– | TTTCTCCTGGTATGAGATAGCAAATC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayechu-Muruzabal, V.; Xiao, L.; Wehkamp, T.; van Ark, I.; Hoogendoorn, E.J.; Leusink-Muis, T.; Folkerts, G.; Garssen, J.; Willemsen, L.E.M.; van’t Land, B. A Fermented Milk Matrix Containing Postbiotics Supports Th1- and Th17-Type Immunity In Vitro and Modulates the Influenza-Specific Vaccination Response In Vivo in Association with Altered Serum Galectin Ratios. Vaccines 2021, 9, 254. https://doi.org/10.3390/vaccines9030254
Ayechu-Muruzabal V, Xiao L, Wehkamp T, van Ark I, Hoogendoorn EJ, Leusink-Muis T, Folkerts G, Garssen J, Willemsen LEM, van’t Land B. A Fermented Milk Matrix Containing Postbiotics Supports Th1- and Th17-Type Immunity In Vitro and Modulates the Influenza-Specific Vaccination Response In Vivo in Association with Altered Serum Galectin Ratios. Vaccines. 2021; 9(3):254. https://doi.org/10.3390/vaccines9030254
Chicago/Turabian StyleAyechu-Muruzabal, Veronica, Ling Xiao, Tjalling Wehkamp, Ingrid van Ark, Elisabeth J. Hoogendoorn, Thea Leusink-Muis, Gert Folkerts, Johan Garssen, Linette E. M. Willemsen, and Belinda van’t Land. 2021. "A Fermented Milk Matrix Containing Postbiotics Supports Th1- and Th17-Type Immunity In Vitro and Modulates the Influenza-Specific Vaccination Response In Vivo in Association with Altered Serum Galectin Ratios" Vaccines 9, no. 3: 254. https://doi.org/10.3390/vaccines9030254






