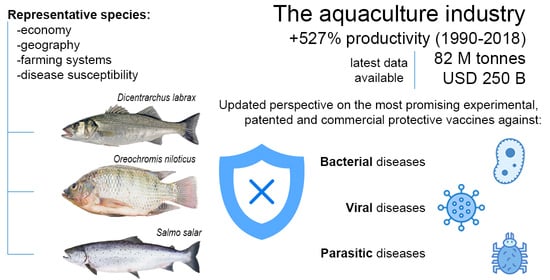
State-of-the-Art Vaccine Research for Aquaculture Use: The Case of Three Economically Relevant Fish Species
Abstract
:1. Introduction
2. Vaccine Research against Diseases in European Sea Bass Dicentrarchus labrax (Linnaeus 1758)
2.1. Bacterial Diseases
2.2. Viral Diseases
2.3. Parasitic Diseases
3. Vaccine Research against Diseases in Nile Tilapia Oreochromis niloticus (Linnaeus 1758)
3.1. Bacterial Diseases
3.2. Viral Diseases
3.3. Parasitic Diseases
4. Vaccine Research against Diseases in Atlantic Salmon Salmo salar (Linnaeus 1758)
4.1. Bacterial Diseases
4.2. Viral Diseases
4.3. Parasitic Diseases
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2020; FAO: Roma, Italy, 2020; ISBN 978-92-5-132692-3. [Google Scholar]
- Jackson, J.B.C.; Kirby, M.X.; Berger, W.H.; Bjorndal, K.A.; Botsford, L.W.; Bourque, B.J.; Bradbury, R.H.; Cooke, R.; Erlandson, J.; Estes, J.A.; et al. Historical overfishing and the recent collapse of coastal ecosystems. Science 2001, 293, 629–637. [Google Scholar] [CrossRef] [Green Version]
- Daskalov, G.M.; Grishin, A.N.; Rodionov, S.; Mihneva, V. Trophic cascades triggered by overfishing reveal possible mechanisms of ecosystem regime shifts. Proc. Natl. Acad. Sci. USA 2007, 104, 10518–10523. [Google Scholar] [CrossRef] [Green Version]
- United Nations. World Population Prospects; United Nations: New York, NY, USA, 2019. [Google Scholar]
- Kobayashi, M.; Msangi, S.; Batka, M.; Vannuccini, S.; Dey, M.M.; Anderson, J.L. Fish to 2030: The Role and Opportunity for Aquaculture. Aquac. Econ. Manag. 2015, 19, 282–300. [Google Scholar] [CrossRef] [Green Version]
- Leung, T.L.F.; Bates, A.E. More rapid and severe disease outbreaks for aquaculture at the tropics: Implications for food security. J. Appl. Ecol. 2013, 50, 215–222. [Google Scholar] [CrossRef]
- Raja, R.A.; Jithendran, K.P. Aquaculture disease diagnosis and health management. Adv. Mar. Brac. Aquac. 2015, 247–254. [Google Scholar] [CrossRef]
- Sharma, M.; Shrivastav, A.B.; Sahni, Y.P.; Pandey, G. Overviews of the Treatment and Control of Common Fish Diseases. Int. Res. J. Pharm. 2012, 3, 123–127. [Google Scholar]
- Lulijwa, R.; Rupia, E.J.; Alfaro, A.C. Antibiotic use in aquaculture, policies and regulation, health and environmental risks: A review of the top 15 major producers. Rev. Aquac. 2019, 12, 640–663. [Google Scholar] [CrossRef]
- Jia, B.; St-Hilaire, S.; Singh, K.; Gardner, I.A. Biosecurity knowledge, attitudes and practices of farmers culturing yellow catfish (Pelteobagrus fulvidraco) in Guangdong and Zhejiang provinces, China. Aquaculture 2017, 471, 146–156. [Google Scholar] [CrossRef]
- Sharrer, M.J.; Summerfelt, S.T. Ozonation followed by ultraviolet irradiation provides effective bacteria inactivation in a freshwater recirculating system. Aquac. Eng. 2007, 37, 180–191. [Google Scholar] [CrossRef] [Green Version]
- Irhayyim, T.; Beliczky, G.; Havasi, M.; Bercsényi, M. Impacts of magnetic water treatment on water quality, feeding efficiency and growth performance of common carp in integrated recirculating aquaculture systems. J. Cent. Eur. Agric. 2020, 21, 246–255. [Google Scholar] [CrossRef]
- Amenyogbe, E.; Chen, G.; Wang, Z.; Huang, J.S.; Huang, B.; Li, H. The exploitation of probiotics, prebiotics and synbiotics in aquaculture: Present study, limitations and future directions: A review. Aquac. Int. 2020, 28, 1017–1041. [Google Scholar] [CrossRef]
- Gudding, R.; Van Muiswinkel, W.B. A history of fish vaccination: Science-based disease prevention in aquaculture. Fish Shellfish Immunol. 2013, 35, 1683–1688. [Google Scholar] [CrossRef]
- Sommerset, I.; Krossøy, B.; Biering, E.; Frost, P. Vaccines for fish in aquaculture. Expert Rev. Vaccines 2005, 4, 89–101. [Google Scholar] [CrossRef]
- Miccoli, A.; Saraceni, P.R.; Scapigliati, G. Vaccines and immune protection of principal Mediterranean marine fish species. Fish Shellfish Immunol. 2019, 94, 800–809. [Google Scholar] [CrossRef]
- Ma, J.; Bruce, T.J.; Jones, E.M.; Cain, K.D. A Review of Fish Vaccine Development Strategies: Conventional Methods and Modern Biotechnological Approaches. Microorganisms 2019, 7, 569. [Google Scholar] [CrossRef] [Green Version]
- Brudeseth, B.E.; Wiulsrød, R.; Fredriksen, B.N.; Lindmo, K.; Løkling, K.E.; Bordevik, M.; Steine, N.; Klevan, A.; Gravningen, K. Status and future perspectives of vaccines for industrialised fin-fish farming. Fish Shellfish Immunol. 2013, 35, 1759–1768. [Google Scholar] [CrossRef] [PubMed]
- Bøgwald, J.; Dalmo, R.A. Review on immersion vaccines for fish: An update 2019. Microorganisms 2019, 7, 627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravid-Peretz, S.; Colorni, A.; Sharon, G.; Ucko, M. Vaccination of European sea bass Dicentrarchus labrax with avirulent Mycobacterium marinum (iipA::kan mutant). Fish Shellfish Immunol. 2019, 90, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Ziklo, N.; Colorni, A.; Gao, L.Y.; Du, S.J.; Ucko, M. Humoral and Cellular Immune Response of European Seabass Dicentrarchus labrax Vaccinated with Heat-Killed Mycobacterium marinum (iipA::kan Mutant). J. Aquat. Anim. Health 2018, 30, 312–324. [Google Scholar] [CrossRef]
- Khalil, R.H.; Diab, A.M.; Shakweer, M.S.; Ghetas, H.A.; Khallaf, M.M.; Omar, A.A.E.D. New perspective to control of tenacibaculosis in sea bass Dicentrarchus labrax L. Aquac. Res. 2018, 49, 2357–2365. [Google Scholar] [CrossRef]
- Salati, F.; Cubadda, C.; Viale, I.; Kusuda, R. Immune response of sea bass Dicentrarchus labrax to Tenacibaculum maritimum antigens. Fish. Sci. 2005, 71, 563–567. [Google Scholar] [CrossRef]
- Galindo-Villegas, J.; Mulero, I.; García-Alcazar, A.; Muñoz, I.; Peñalver-Mellado, M.; Streitenberger, S.; Scapigliati, G.; Meseguer, J.; Mulero, V. Recombinant TNFα as oral vaccine adjuvant protects European sea bass against vibriosis: Insights into the role of the CCL25/CCR9 axis. Fish Shellfish Immunol. 2013, 35, 1260–1271. [Google Scholar] [CrossRef]
- Spinos, E.; Kokkoris, G.D.; Bakopoulos, V. Prevention of sea bass (Dicentrarchus labrax, L. 1758) photobacteriosis and vibriosis. Long term efficacy study of intraperitoneally administered bivalent commercial vaccines. Aquaculture 2017, 471, 172–184. [Google Scholar] [CrossRef]
- Nuñez-Ortiz, N.; Pascoli, F.; Picchietti, S.; Buonocore, F.; Bernini, C.; Toson, M.; Scapigliati, G.; Toffan, A. A formalin-inactivated immunogen against viral encephalopathy and retinopathy (VER) disease in European sea bass (Dicentrarchus labrax): Immunological and protection effects. Vet. Res. 2016, 47, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Valero, Y.; Mokrani, D.; Chaves-Pozo, E.; Arizcun, M.; Oumouna, M.; Meseguer, J.; Esteban, M.Á.; Cuesta, A. Vaccination with UV-inactivated nodavirus partly protects European sea bass against infection, while inducing few changes in immunity. Dev. Comp. Immunol. 2018, 86, 171–179. [Google Scholar] [CrossRef]
- Pascoli, F.; Guazzo, A.; Buratin, A.; Toson, M.; Buonocore, F.; Scapigliati, G.; Toffan, A. Lack of in vivo cross-protection of two different betanodavirus species RGNNV and SJNNV in European sea bass Dicentrachus labrax. Fish Shellfish Immunol. 2019, 85, 85–89. [Google Scholar] [CrossRef]
- Gonzalez-Silvera, D.; Guardiola, F.A.; Espinosa, C.; Chaves-Pozo, E.; Esteban, M.Á.; Cuesta, A. Recombinant nodavirus vaccine produced in bacteria and administered without purification elicits humoral immunity and protects European sea bass against infection. Fish Shellfish Immunol. 2019, 88, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Marsian, J.; Hurdiss, D.L.; Ranson, N.A.; Ritala, A.; Paley, R.; Cano, I.; Lomonossoff, G.P. Plant-made nervous necrosis virus-like particles protect fish against disease. Front. Plant Sci. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Valero, Y.; Awad, E.; Buonocore, F.; Arizcun, M.; Esteban, M.Á.; Meseguer, J.; Chaves-Pozo, E.; Cuesta, A. An oral chitosan DNA vaccine against nodavirus improves transcription of cell-mediated cytotoxicity and interferon genes in the European sea bass juveniles gut and survival upon infection. Dev. Comp. Immunol. 2016, 65, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Pridgeon, J.W.; Klesius, P.H. Development and efficacy of a novobiocin-resistant Streptococcus iniae as a novel vaccine in Nile tilapia (Oreochromis niloticus). Vaccine 2011, 29, 5986–5993. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zou, L.L.; Li, A.X. Construction of a Streptococcus iniae sortase A mutant and evaluation of its potential as an attenuated modified live vaccine in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2014, 40, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, C.A.; LaFrentz, B.R.; Klesius, P.H.; Evans, J.J. Protection against heterologous Streptococcus iniae isolates using a modified bacterin vaccine in Nile tilapia, Oreochromis niloticus (L.). J. Fish Dis. 2010, 33, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, C.A.; Vandenberg, G.W.; Désormeaux, A.; Klesius, P.H.; Evans, J.J. Efficacy of a Streptococcus iniae modified bacterin delivered using OraljectTM technology in Nile tilapia (Oreochromis niloticus). Aquaculture 2006, 255, 151–156. [Google Scholar] [CrossRef]
- Kayansamruaj, P.; Dong, H.T.; Pirarat, N.; Nilubol, D.; Rodkhum, C. Efficacy of α-enolase-based DNA vaccine against pathogenic Streptococcus iniae in Nile tilapia (Oreochromis niloticus). Aquaculture 2017, 468, 102–106. [Google Scholar] [CrossRef]
- Shelby, R.A.; Klesius, P.H.; Shoemaker, C.A.; Evans, J.J. Passive immunization of tilapia, Oreochromis niloticus (L.), with anti-Streptococcus iniae whole sera. J. Fish Dis. 2002, 25, 1–6. [Google Scholar] [CrossRef]
- Huang, L.Y.; Wang, K.Y.; Xiao, D.; Chen, D.F.; Geng, Y.; Wang, J.; He, Y.; Wang, E.L.; Huang, J.L.; Xiao, G.Y. Safety and immunogenicity of an oral DNA vaccine encoding Sip of Streptococcus agalactiae from Nile tilapia Oreochromis niloticus delivered by live attenuated Salmonella typhimurium. Fish Shellfish Immunol. 2014, 38, 34–41. [Google Scholar] [CrossRef]
- Liu, L.; Lu, D.Q.; Xu, J.; Luo, H.L.; Li, A.X. Development of attenuated erythromycin-resistant Streptococcus agalactiae vaccine for tilapia (Oreochromis niloticus) culture. J. Fish Dis. 2019, 42, 693–701. [Google Scholar] [CrossRef]
- Li, L.P.; Wang, R.; Liang, W.W.; Huang, T.; Huang, Y.; Luo, F.G.; Lei, A.Y.; Chen, M.; Gan, X. Development of live attenuated Streptococcus agalactiae vaccine for tilapia via continuous passage in vitro. Fish Shellfish Immunol. 2015, 45, 955–963. [Google Scholar] [CrossRef]
- Abu-Elala, N.M.; Samir, A.; Wasfy, M.; Elsayed, M. Efficacy of Injectable and Immersion Polyvalent Vaccine against Streptococcal Infections in Broodstock and Offspring of Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2019, 88, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Paredes, J.G.; Mendoza-Roldan, M.A.; Lopez-Jimena, B.; Shahin, K.; Metselaar, M.; Thompson, K.D.; Penman, D.J.; Richards, R.H.; Adams, A. Whole cell inactivated autogenous vaccine effectively protects red Nile tilapia (Oreochromis niloticus) against francisellosis via intraperitoneal injection. J. Fish Dis. 2019, 42, 1191–1200. [Google Scholar] [CrossRef]
- Shahin, K.; Shinn, A.P.; Metselaar, M.; Ramirez-Paredes, J.G.; Monaghan, S.J.; Thompson, K.D.; Hoare, R.; Adams, A. Efficacy of an inactivated whole-cell injection vaccine for nile tilapia, Oreochromis niloticus (L), against multiple isolates of Francisella noatunensis subsp. orientalis from diverse geographical regions. Fish Shellfish Immunol. 2019, 89, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Pulpipat, T.; Maekawa, S.; Wang, P.C.; Chen, S.C. Immune responses and protective efficacy of a formalin-killed Francisella noatunensis subsp. orientalis vaccine evaluated through intraperitoneal and immersion challenge methods in Oreochromis niloticus. Vaccines 2020, 8, 163. [Google Scholar] [CrossRef] [Green Version]
- Bactol, I.D.C.; Padilla, L.V.; Hilario, A.L. Immune response of tilapia (Oreochromis niloticus) after vaccination with autoclavekilled, heat-killed, and formalin-killed whole cell Aeromonas hydrophila vaccines as possible serotype-independent vaccines. Int. J. Agric. Biol. 2018, 20, 846–850. [Google Scholar] [CrossRef]
- Pridgeon, J.W.; Klesius, P.H. Development and efficacy of novobiocin and rifampicin-resistant Aeromonas hydrophila as novel vaccines in channel catfish and Nile tilapia. Vaccine 2011, 29, 7896–7904. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.; Olivares-Fuster, O.; LaFrentz, S.; Arias, C.R. New attenuated vaccine against columnaris disease in fish: Choosing the right parental strain is critical for vaccine efficacy. Vaccine 2013, 31, 5276–5280. [Google Scholar] [CrossRef]
- Kwon, H.C.; Kang, Y.J. Effects of a subunit vaccine (FlaA) and immunostimulant (CpG-ODN 1668) against Vibrio anguillarum in tilapia (Oreochromis niloticus). Aquaculture 2016, 454, 125–129. [Google Scholar] [CrossRef]
- Cao, T.T.; Tsai, M.A.; Da Yang, C.; Wang, P.C.; Kuo, T.Y.; Chen, H.C.G.; Chen, S.C. Vaccine efficacy of glyceraldehyde-3-phosphate dehydrogenase (Gapdh) from Edwardsiella ictaluri against E. tarda in tilapia. J. Gen. Appl. Microbiol. 2015, 60, 241–250. [Google Scholar] [CrossRef] [Green Version]
- Igarashi, A.; Iida, T. A vaccination trial using live cells of Edwardsiella tarda in tilapia. Fish Pathol. 2002, 37, 145–148. [Google Scholar] [CrossRef] [Green Version]
- Yield Improvement of the Sea Lice MY32/Cr Novel Antigen Production and IgM Immune Response Characterization in Oreochromis Niloticus as a Model. Available online: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S1027-28522014000100004 (accessed on 4 February 2021).
- Småge, S.B.; Frisch, K.; Vold, V.; Duesund, H.; Brevik, Ø.J.; Olsen, R.H.; Sjaatil, S.T.; Klevan, A.; Brudeseth, B.; Watanabe, K.; et al. Induction of tenacibaculosis in Atlantic salmon smolts using Tenacibaculum finnmarkense and the evaluation of a whole cell inactivated vaccine. Aquaculture 2018, 495, 858–864. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Crosbie, P.B.B.; Nowak, B.F.; Bridle, A.R. The effects of inactivation methods of Yersinia ruckeri on the efficacy of single dip vaccination in Atlantic salmon (Salmo salar). J. Fish Dis. 2018, 41, 1173–1176. [Google Scholar] [CrossRef]
- Hoare, R.; Jung, S.J.; Ngo, T.P.H.; Bartie, K.; Bailey, J.; Thompson, K.D.; Adams, A. Efficacy and safety of a non-mineral oil adjuvanted injectable vaccine for the protection of Atlantic salmon (Salmo salar L.) against Flavobacterium psychrophilum. Fish Shellfish Immunol. 2019, 85, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Lund, H.; Bakke, A.F.; Sommerset, I.; Afanasyev, S.; Schriwer, G.; Thorisdottir, A.; Boysen, P.; Krasnov, A. A time-course study of gene expression and antibody repertoire at early time post vaccination of Atlantic salmon. Mol. Immunol. 2019, 106, 99–107. [Google Scholar] [CrossRef]
- Caruffo, M.; Maturana, C.; Kambalapally, S.; Larenas, J.; Tobar, J.A. Protective oral vaccination against infectious salmon anaemia virus in Salmo salar. Fish Shellfish Immunol. 2016, 54, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Tobar, I.; Arancibia, S.; Torres, C.; Vera, V.; Soto, P.; Carrasco, C.; Alvarado, M.; Neira, E.; Arcos, S.; Tobar, J.A. Successive oral immunizations against Piscirickettsia salmonis and infectious salmon anemia virus are required to maintain a long-term protection in farmed salmonids. Front. Immunol. 2015, 6, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Robertsen, B.; Chang, C.J.; Bratland, L. IFN-adjuvanted DNA vaccine against infectious salmon anemia virus: Antibody kinetics and longevity of IFN expression. Fish Shellfish Immunol. 2016, 54, 328–332. [Google Scholar] [CrossRef]
- Reyes, M.; Ramírez, C.; Ñancucheo, I.; Villegas, R.; Schaffeld, G.; Kriman, L.; Gonzalez, J.; Oyarzun, P. A novel “in-feed” delivery platform applied for oral DNA vaccination against IPNV enables high protection in Atlantic salmon (Salmo salar). Vaccine 2017, 35, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Vendramin, N.; Alencar, A.L.F.; Iburg, T.M.; Dahle, M.K.; Wessel, Ø.; Olsen, A.B.; Rimstad, E.; Olesen, N.J. Piscine orthoreovirus infection in atlantic salmon (Salmo salar) protects against subsequent challenge with infectious hematopoietic necrosis virus (ihnv). Vet. Res. 2018, 49, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Lund, M.; Røsæg, M.V.; Krasnov, A.; Timmerhaus, G.; Nyman, I.B.; Aspehaug, V.; Rimstad, E.; Dahle, M.K. Experimental Piscine orthoreovirus infection mediates protection against pancreas disease in Atlantic salmon (Salmo salar). Vet. Res. 2016. [Google Scholar] [CrossRef] [Green Version]
- Wessel, Ø.; Haugland, Ø.; Rode, M.; Fredriksen, B.N.; Dahle, M.K.; Rimstad, E. Inactivated Piscine orthoreovirus vaccine protects against heart and skeletal muscle inflammation in Atlantic salmon. J. Fish Dis. 2018, 41, 1411–1419. [Google Scholar] [CrossRef]
- Haatveit, H.M.; Hodneland, K.; Braaen, S.; Hansen, E.F.; Nyman, I.B.; Dahle, M.K.; Frost, P.; Rimstad, E. DNA vaccine expressing the non-structural proteins of Piscine orthoreovirus delay the kinetics of PRV infection and induces moderate protection against heart -and skeletal muscle inflammation in Atlantic salmon (Salmo salar). Vaccine 2018, 36, 7599–7608. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.J.; Gu, J.; Robertsen, B. Protective effect and antibody response of DNA vaccine against salmonid alphavirus 3 (SAV3) in Atlantic salmon. J. Fish Dis. 2017, 40, 1775–1781. [Google Scholar] [CrossRef]
- Chin, A.; Woo, P.T.K. Innate cell-mediated immune response and peripheral leukocyte populations in Atlantic salmon, Salmo salar L., to a live Cryptobia salmositica vaccine. Parasitol. Res. 2005, 95, 299–304. [Google Scholar] [CrossRef]
- Carpio, Y.; Basabe, L.; Acosta, J.; Rodríguez, A.; Mendoza, A.; Lisperger, A.; Zamorano, E.; González, M.; Rivas, M.; Contreras, S.; et al. Novel gene isolated from Caligus rogercresseyi: A promising target for vaccine development against sea lice. Vaccine 2011, 29, 2810–2820. [Google Scholar] [CrossRef] [PubMed]
- Valdenegro-Vega, V.A.; Cook, M.; Crosbie, P.; Bridle, A.R.; Nowak, B.F. Vaccination with recombinant protein (r22C03), a putative attachment factor of Neoparamoeba perurans, against AGD in Atlantic salmon (Salmo salar) and implications of a co-infection with Yersinia ruckeri. Fish Shellfish Immunol. 2015, 44, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Swain, J.K.; Carpio, Y.; Johansen, L.-H.; Velazquez, J.; Hernandez, L.; Leal, Y.; Kumar, A.; Estrada, M.P. Impact of a candidate vaccine on the dynamics of salmon lice (Lepeophtheirus salmonis) infestation and immune response in Atlantic salmon (Salmo salar L.). PLoS ONE 2020, 15, e0239827. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, M.; Gagnaire, P.A.; Allal, F. The European sea bass: A key marine fish model in the wild and in aquaculture. Anim. Genet. 2019, 50, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Muniesa, A.; Basurco, B.; Aguilera, C.; Furones, D.; Reverté, C.; Sanjuan-Vilaplana, A.; Jansen, M.D.; Brun, E.; Tavornpanich, S. Mapping the knowledge of the main diseases affecting sea bass and sea bream in Mediterranean. Transbound. Emerg. Dis. 2020, 67, 1089–1100. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, D.T.; Rhodes, M.W. Mycobacteriosis in fishes: A review. Vet. J. 2009, 180, 33–47. [Google Scholar] [CrossRef]
- Avendaño-Herrera, R.; Toranzo, A.E.; Magariños, B. Tenacibaculosis infection in marine fish caused by Tenacibaculum maritimum: A review. Dis. Aquat. Organ. 2006, 71, 255–266. [Google Scholar] [CrossRef]
- Ina-Salwany, M.Y.; Al-saari, N.; Mohamad, A.; Mursidi, F.A.; Mohd-Aris, A.; Amal, M.N.A.; Kasai, H.; Mino, S.; Sawabe, T.; Zamri-Saad, M. Vibriosis in Fish: A Review on Disease Development and Prevention. J. Aquat. Anim. Health 2019, 31, 3–22. [Google Scholar] [CrossRef]
- Barnes, A.; Dos Santos, N.; Ellis, A. Update on bacterial vaccines: Photobacterium damselae subsp. piscicida. Dev. Biol. 2005, 121, 75–84. [Google Scholar]
- Gao, L.Y.; Pak, M.; Kish, R.; Kajihara, K.; Brown, E.J. A mycobacterial operon essential for virulence in vivo and invasion and intracellular persistence in macrophages. Infect. Immun. 2006, 74, 1757–1767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiery, R.; Cozien, J.; Cabon, J.; Lamour, F.; Baud, M.; Schneemann, A. Induction of a Protective Immune Response against Viral Nervous Necrosis in the European Sea Bass Dicentrarchus labrax by Using Betanodavirus Virus-Like Particles. J. Virol. 2006, 80, 10201–10207. [Google Scholar] [CrossRef] [Green Version]
- Lai, Y.X.; Jin, B.L.; Xu, Y.; Huang, L.J.; Huang, R.Q.; Zhang, Y.; Kwang, J.; He, J.G.; Xie, J.F. Immune responses of orange-spotted grouper, Epinephelus coioides, against virus-like particles of betanodavirus produced in Escherichia coli. Vet. Immunol. Immunopathol. 2014, 157, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.S.; Lu, M.W.; Tang, L.; Liu, W.; Chao, C.B.; Lin, C.J.; Krishna, N.K.; Johnson, J.E.; Schneemann, A. Characterization of virus-like particles assembled in a recombinant baculovirus system expressing the capsid protein of a fish nodavirus. Virology 2001, 290, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Nozzi, V.; Strofaldi, S.; Piquer, I.F.; Di Crescenzo, D.; Olivotto, I.; Carnevali, O. Amyloodinum ocellatum in Dicentrarchus labrax: Study of infection in salt water and freshwater aquaponics. Fish Shellfish Immunol. 2016, 57, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Byadgi, O.; Beraldo, P.; Volpatti, D.; Massimo, M.; Bulfon, C.; Galeotti, M. Expression of infection-related immune response in European sea bass (Dicentrarchus labrax) during a natural outbreak from a unique dinoflagellate Amyloodinium ocellatum. Fish Shellfish Immunol. 2019, 84, 62–72. [Google Scholar] [CrossRef]
- Gupta, M.V.; Acosta, B.O. A review of global tilapia farming practices. Aquac. Asia 2004, IX, 1–14. [Google Scholar]
- Prabu, E.; Rajagopalsamy, C.B.T.; Ahilan, B.; Jeevagan, I.J.M.A.; Renuhadevi, M. Tilapia—An Excellent Candidate Species for World Aquaculture: A Review. Annu. Res. Rev. Biol. 2019, 31, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Bierne, H.; Mazmanian, S.K.; Trost, M.; Pucciarelli, M.G.; Liu, G.; Dehoux, P.; Jänsch, L.; Garcia-del Portillo, F.; Schneewind, O.; Cossart, P. Inactivation of the srtA gene in Listeria monocytogenes inhibits anchoring of surface proteins and affects virulence. Mol. Microbiol. 2002, 43, 869–881. [Google Scholar] [CrossRef]
- Weiss, W.J.; Lenoy, E.; Murphy, T.; Tardio, L.A.; Burgio, P.; Projan, S.J.; Schneewind, O.; Alksne, L. Effect of srtA and srtB gene expression on the virulence of Staphylococcus aureus in animal models of infection. J. Antimicrob. Chemother. 2004, 53, 480–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolken, T.C.; Franke, C.A.; Jones, K.F.; Zeller, G.O.; Jones, C.H.; Dutton, E.K.; Hruby, D.E. Inactivation of the srtA gene in Streptococcus gordonii inhibits cell wall anchoring of surface proteins and decreases in vitro and in vivo adhesion. Infect. Immun. 2001, 69, 75–80. [Google Scholar] [CrossRef] [Green Version]
- Kharat, A.S.; Tomasz, A. Inactivation of the srtA gene affects localization of surface proteins and decreases adhesion of Streptococcus pneumoniae to human pharyngeal cells in vitro. Infect. Immun. 2003, 71, 2758–2765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanier, G.; Sekizaki, T.; Domínguez-Punaro, M.C.; Esgleas, M.; Osaki, M.; Takamatsu, D.; Segura, M.; Gottschalk, M. Disruption of srtA gene in Streptococcus suis results in decreased interactions with endothelial cells and extracellular matrix proteins. Vet. Microbiol. 2008, 127, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Paredes, J.G.; Larsson, P.; Thompson, K.D.; Penman, D.J.; Busse, H.J.; Öhrman, C.; Sjödin, A.; Soto, E.; Richards, R.H.; Adams, A.; et al. Reclassification of Francisella noatunensis subsp. orientalis ottem et al. 2009 as Francisella orientalis sp. nov., Francisella noatunensis subsp. chilensis subsp. nov. and emended description of Francisella noatunensis. Int. J. Syst. Evol. Microbiol. 2020, 70, 2034–2048. [Google Scholar] [CrossRef]
- Shoemaker, C.A.; Klesius, P.H.; Evans, J.J. Immunization of eyed channel catfish, Ictalurus punctatus, eggs with monovalent Flavobacterium columnare vaccine and bivalent F. columnare and Edwardsiella ictaluri vaccine. Vaccine 2007, 25, 1126–1131. [Google Scholar] [CrossRef] [PubMed]
- Klesius, P.H.; Shoemaker, C.A. Development and use of modified live Edwardsiella ictaluri vaccine against enteric septicemia of catfish. In Advances in Veterinary Medicine; Elsevier: Amsterdam, The Netherlands, 1999. [Google Scholar]
- Mohanty, B.R.; Sahoo, P.K. Edwardsiellosis in fish: A brief review. J. Biosci. 2007, 32, 1331–1344. [Google Scholar] [CrossRef] [PubMed]
- Criollo-Joaquin, M.; Motte, E.; Salvatierra, M.; Medina, J.; Diringer, B.; Sandoval, G.; Mialhe, E. Design and evaluation of the expression of a potential DNA vaccine against Tilapia lake virus (TiLV). Rev. Peru. Biol. 2019, 26, 301–310. [Google Scholar] [CrossRef]
- Mugimba, K.K.; Lamkhannat, M.; Dubey, S.; Mutoloki, S.; Munang’andu, H.M.; Evensen, Ø. Tilapia lake virus downplays innate immune responses during early stage of infection in Nile tilapia (Oreochromis niloticus). Sci. Rep. 2020. [Google Scholar] [CrossRef]
- Iversen, A.; Asche, F.; Hermansen, Ø.; Nystøyl, R. Production cost and competitiveness in major salmon farming countries 2003–2018. Aquaculture 2020, 522, 735089. [Google Scholar] [CrossRef]
- Boerlage, A.S.; Ashby, A.; Herrero, A.; Reeves, A.; Gunn, G.J.; Rodger, H.D. Epidemiology of marine gill diseases in Atlantic salmon (Salmo salar) aquaculture: A review. Rev. Aquac. 2020, 12, 2140–2159. [Google Scholar] [CrossRef]
- Gill Disease to Cost Salmon Farmers £30m. Available online: https://www.heraldscotland.com/business_hq/13088723.gill-disease-to-cost-salmon-farmers-30m/ (accessed on 4 February 2021).
- Stormoen, M.; Kristoffersen, A.B.; Jansen, P.A. Mortality related to pancreas disease in Norwegian farmed salmonid fish, Salmo salar L. and Oncorhynchus mykiss (Walbaum). J. Fish Dis. 2013, 36, 639–645. [Google Scholar] [CrossRef]
- Stokes, W.S.; Brown, K.; Kulpa-Eddy, J.; Srinivas, G.; Halder, M.; Draayer, H.; Galvin, J.; Claassen, I.; Gifford, G.; Woodland, R.; et al. Improving Animal Welfare and Reducing Animal Use for Veterinary Vaccine Potency Testing: State-Of-The-Science and Future Directions. Procedia Vaccinol. 2011, 5, 84–105. [Google Scholar] [CrossRef]
- Chang, C.J.; Sun, B.; Robertsen, B. Adjuvant activity of fish type I interferon shown in a virus DNA vaccination model. Vaccine 2015, 33, 2442–2448. [Google Scholar] [CrossRef] [Green Version]
- Ballesteros, N.A.; Alonso, M.; Saint-Jean, S.R.; Perez-Prieto, S.I. An oral DNA vaccine against infectious haematopoietic necrosis virus (IHNV) encapsulated in alginate microspheres induces dose-dependent immune responses and significant protection in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2015, 45, 877–888. [Google Scholar] [CrossRef] [Green Version]
- Woo, P.T.K. Cryptobia (Trypanoplasma) salmositica and salmonid cryptobiosis. J. Fish Dis. 2003, 26, 627–646. [Google Scholar] [CrossRef]
- Li, S.; Woo, P.T.K. Efficacy of a live Cryptobia salmositica vaccine, and the mechanism of protection in vaccinated rainbow trout, Oncorhynchus mykiss, against cryptobiosis. Vet. Immunol. Immunopathol. 1995, 48, 343–353. [Google Scholar] [CrossRef]
- Valdenegro-Vega, V.A.; Crosbie, P.B.B.; Cook, M.T.; Vincent, B.N.; Nowak, B.F. Administration of recombinant attachment protein (r22C03) of Neoparamoeba perurans induces humoral immune response against the parasite in Atlantic salmon (Salmo salar). Fish Shellfish Immunol. 2014, 38, 294–302. [Google Scholar] [CrossRef]
- Leal, Y.; Velazquez, J.; Hernandez, L.; Swain, J.K.; Rodríguez, A.R.; Martínez, R.; García, C.; Ramos, Y.; Estrada, M.P.; Carpio, Y. Promiscuous T cell epitopes boosts specific IgM immune response against a P0 peptide antigen from sea lice in different teleost species. Fish Shellfish Immunol. 2019, 92, 322–330. [Google Scholar] [CrossRef] [Green Version]
- Eichner, C.; Nilsen, F.; Grotmol, S.; Dalvin, S. A method for stable gene knock-down by RNA interference in larvae of the salmon louse (Lepeophtheirus salmonis). Exp. Parasitol. 2014, 140, 44–51. [Google Scholar] [CrossRef]
- Munang’andu, H.M.; Evensen, Ø. Correlates of protective immunity for fish vaccines. Fish Shellfish Immunol. 2019, 85, 132–140. [Google Scholar] [CrossRef]
- Wang, W.; Sun, J.; Liu, C.; Xue, Z. Application of immunostimulants in aquaculture: Current knowledge and future perspectives. Aquac. Res. 2017, 48, 1–23. [Google Scholar] [CrossRef]
- Tafalla, C.; Bøgwald, J.; Dalmo, R.A. Adjuvants and immunostimulants in fish vaccines: Current knowledge and future perspectives. Fish Shellfish Immunol. 2013, 35, 1740–1750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munang’andu, H.M.; Salinas, I.; Tafalla, C.; Dalmo, R.A. Editorial: Vaccines and Immunostimulants for Finfish. Front. Immunol. 2020, 11, 1–4. [Google Scholar] [CrossRef]
- Picchietti, S.; Miccoli, A.; Fausto, A.M. Gut immunity in European sea bass (Dicentrarchus labrax): A review. Fish Shellfish Immunol. 2021, 108, 94–108. [Google Scholar] [CrossRef]
- Parra, D.; Reyes-Lopez, F.E.; Tort, L. Mucosal Immunity and B Cells in Teleosts: Effect of Vaccination and Stress. Front. Immunol. 2015, 6, 354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koppang, E.O.; Kvellestad, A.; Fischer, U. Fish mucosal immunity: Gill. In Mucosal Health in Aquaculture; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 9780124171930. [Google Scholar]
- Xu, Z.; Parra, D.; Gómez, D.; Salinas, I.; Zhang, Y.A.; Von Gersdorff Jørgensen, L.; Heinecke, R.D.; Buchmann, K.; LaPatra, S.; Oriol Sunyer, J. Teleost skin, an ancient mucosal surface that elicits gut-like immune responses. Proc. Natl. Acad. Sci. USA 2013, 110, 13097–13102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.; Wang, Q.; Huang, Z.; Ding, L.; Xu, Z. Immunoglobulins, Mucosal Immunity and Vaccination in Teleost Fish. Front. Immunol. 2020, 11, 567941. [Google Scholar] [CrossRef]
- Das, P.K.; Salinas, I. Fish nasal immunity: From mucosal vaccines to neuroimmunology. Fish Shellfish Immunol. 2020, 104, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Cabillon, N.; Lazado, C. Mucosal Barrier Functions of Fish under Changing Environmental Conditions. Fishes 2019, 4, 2. [Google Scholar] [CrossRef] [Green Version]



| Pathogen | Vaccine Status | Adjuvant | Approx. Size (g) | Challenge | Ref. |
|---|---|---|---|---|---|
| Mycobacterium marinum | Experimental | MontanideTM ISA 760 VG | 50 | Yes | [20] |
| M. marinum | Experimental | No | 20 | Yes | [21] |
| Tenacibaculum maritimum | Experimental | No | 30 | Yes | [22] |
| T. maritimum | Experimental | No | 5 | No | [23] |
| Vibrio anguillarum + Vibrio ordalii | Commercial (AquaVac Vibrio Oral) | rTNFα | 30 | Yes | [24] |
| V. anguillarum + Photobacterium damselae | Commercial (AlphaJect 2000™ and AquaVac™ Vibrio-Pasteurella) | Non-mineral | 35 | Yes | [25] |
| Betanodavirus | Experimental | No | 2 and 6 | Yes (only one exp. group) | [26] |
| Betanodavirus | Experimental | No | 11 | Yes | [27] |
| Betanodavirus | Experimental | No | 6 | Yes | [28] |
| Betanodavirus | Experimental | No | 11 | Yes | [29] |
| Betanodavirus | Experimental | No | 30 | Yes | [30] |
| Betanodavirus | Experimental | No | 6 | Yes | [31] |
| Pathogen | Vaccine Status | Adjuvant | Approx. Size (g) | Challenge | Ref. |
|---|---|---|---|---|---|
| Streptococcus iniae | Experimental | No | 10 | Yes (homologous and heterologous) | [32] |
| S. iniae | Experimental | No | 40 | Yes | [33] |
| S. iniae | Experimental | No | 5 | Yes (heterologous) | [34] |
| S. iniae | Experimental | Oralject™ | 13 | Yes | [35] |
| S. iniae | Experimental | No | 25 | Yes | [36] |
| S. iniae | Experimental | No | 3 and 16 | Yes | [37] |
| Streptococcus agalactiae | Experimental | No | 100 | Yes | [38] |
| S. agalactiae | Experimental | No | 30 | Yes (heterologous) | [39] |
| S. agalactiae | Experimental | No | 30 | Yes | [40] |
| Polyvalent (S. agalactiae, S. iniae, Lactococcus garvieae and Enterococcus faecalis) | Commercial (Mevac Aquastrept) | MontanideTM IMS 1312 VG | 500 and 1-month-old fry | Yes | [41] |
| Francisella orientalis | Experimental | MontanideTM ISA 736A VG | 10 | Yes | [42] |
| F. orientalis | Experimental | MontanideTM (oil-based) | 15 | Yes (heterologous) | [43] |
| F. orientalis | Experimental | MontanideTM ISA 736A VG | 35 | Yes | [44] |
| Aeromonas hydrophila | Experimental | No | 55 | Yes | [45] |
| A. Hydrophila | Experimental | No | 10 | Yes | [46] |
| Flavobacterium columnare | Experimental | No | 9 | Yes (heterologous) | [47] |
| Vibrio anguillarum | Experimental | No | 3.5 | Yes | [48] |
| Edwardsiella tarda | Experimental | MontanideTM ISA 763A VG | 102 | Yes | [49] |
| E. tarda | Experimental | No | 42 | Yes | [50] |
| Caligus rogercresseyi | Experimental | MontanideTM 888 VG | 80 | No | [51] |
| Pathogen | Vaccine Status | Adjuvant | Approx. Size (g) | Challenge | Ref. |
|---|---|---|---|---|---|
| Tenacibaculum finnmarkense | Experimental | Mineral oil | 26 | Yes (homologous and heterologous) | [52] |
| Yersinia ruckeri | Experimental | No | 9 | Yes | [53] |
| Flavobacterium psychrophilum | Experimental | Squalene/alum or MontanideTM ISA 760 VG | 23 | Yes | [54] |
| Polyvalent | Commercial (Aquavac® PD7) | Paraffin | 40 | No | [55] |
| ISAV | Experimental | No | 40 | Yes | [56] |
| ISAV and Piscirickettsia salmonis | Commercial (Virbac-Centrovet) | Oil | 40 | No | [57] |
| ISAV | Experimental | IFNa- or IFNc | 40 | No | [58] |
| IPNV | Experimental | No | 0.5 and 20 | Yes | [59] |
| IHNV | NA | No | 5 g | Yes (heterologous) | [60] |
| SAV | NA | No | Post-smolt | Yes (heterologous) | [61] |
| PRV | Experimental and commercial (ALPHA JECT micro® 6) | Paraffin | 55 | Yes | [62] |
| PRV | Experimental | No | 35 | Yes | [63] |
| SAV | Experimental and commercial (Norvax® Compact PD) | Montanide ISA 763A VG (only in the latter) | 30 | Yes | [64] |
| Cryptobia salmositica | Experimental | No | 300 | No | [65] |
| Caligus rogercressey | Experimental | MontanideTM 888 VG | 80 | Yes | [66] |
| Neoparamoeba perurans | Experimental | FCA (first immunization) and FIA (booster) | 100 | Yes (two, 5-week apart) | [67] |
| Lepeophtheirus salmonis | Experimental | MontanideTM ISA50 V2 | 90 | Yes | [68] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miccoli, A.; Manni, M.; Picchietti, S.; Scapigliati, G. State-of-the-Art Vaccine Research for Aquaculture Use: The Case of Three Economically Relevant Fish Species. Vaccines 2021, 9, 140. https://doi.org/10.3390/vaccines9020140
Miccoli A, Manni M, Picchietti S, Scapigliati G. State-of-the-Art Vaccine Research for Aquaculture Use: The Case of Three Economically Relevant Fish Species. Vaccines. 2021; 9(2):140. https://doi.org/10.3390/vaccines9020140
Chicago/Turabian StyleMiccoli, Andrea, Matteo Manni, Simona Picchietti, and Giuseppe Scapigliati. 2021. "State-of-the-Art Vaccine Research for Aquaculture Use: The Case of Three Economically Relevant Fish Species" Vaccines 9, no. 2: 140. https://doi.org/10.3390/vaccines9020140
APA StyleMiccoli, A., Manni, M., Picchietti, S., & Scapigliati, G. (2021). State-of-the-Art Vaccine Research for Aquaculture Use: The Case of Three Economically Relevant Fish Species. Vaccines, 9(2), 140. https://doi.org/10.3390/vaccines9020140