The Use of Nanobiotechnology in Immunology and Vaccination
Abstract
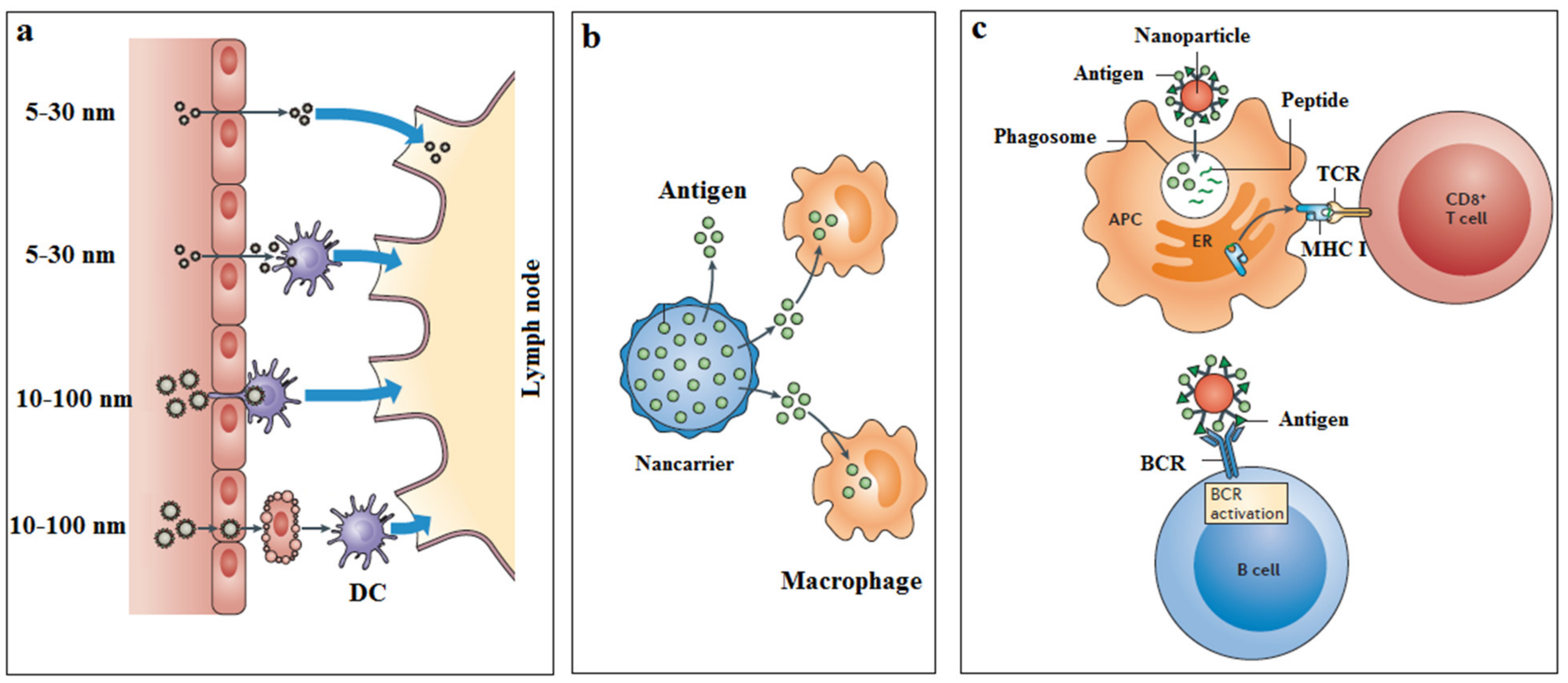
:1. Introduction
2. VLP-Based Vaccines
3. Artificial VLP
4. Nanoparticle-Based Vaccine Carrier
5. Self Assemble Peptide Nanoparticles (SAPNs)
6. Cationic Liposomes
7. Nano-Emulsion
8. Other Nanoparticles
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Jebali, A.; Nayeri, E.K.; Roohana, S.; Aghaei, S.; Ghaffari, M.; Daliri, K.; Fuente, G. Nano-carbohydrates: Synthesis and application in genetics, biotechnology, and medicine. Adv. Colloid Interface Sci. 2017, 240, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Reth, M. Matching cellular dimensions with molecular sizes. Nat. Immunol. 2013, 14, 765–767. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Encapsulation, protection, and delivery of bioactive proteins and peptides using nanoparticle and microparticle systems: A review. Adv. Colloid Interface Sci. 2018, 253, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.M.; Simon, J.K.; Baker, J.R., Jr. Applications of nanotechnology for immunology. Nat. Rev. Immunol. 2013, 13, 592–605. [Google Scholar] [CrossRef]
- Elsayed, H.; Nabi, G.; McKinstry, W.J.; Khoo, K.K.; Mak, J.; Salazar, A.M.; Tenbusch, M.; Temchura, V.; Überla, K. Intrastructural help: Harnessing T helper cells induced by licensed vaccines for improvement of HIV Env antibody responses to virus-like particle vaccines. J. Virol. 2018, 92. [Google Scholar] [CrossRef] [Green Version]
- Jennings, G.T.; Bachmann, M.F. Immunodrugs: Therapeutic VLP-based vaccines for chronic diseases. Annu. Rev. Pharmacol. Toxicol. 2009, 49, 303–326. [Google Scholar] [CrossRef] [PubMed]
- Noad, R.; Roy, P. Virus-like particles as immunogens. Trends Microbiol. 2003, 11, 438–444. [Google Scholar] [CrossRef]
- Boato, F.; Thomas, R.M.; Ghasparian, A.; Freund-Renard, A.; Moehle, K.; Robinson, J.A. Synthetic virus-like particles from self-assembling coiled-coil lipopeptides and their use in antigen display to the immune system. Angew. Chem. 2007, 46, 9015–9018. [Google Scholar] [CrossRef] [PubMed]
- Zeltins, A. Construction and characterization of virus-like particles: A review. Mol. Biotechnol. 2013, 53, 92–107. [Google Scholar] [CrossRef]
- Kushnir, N.; Streatfield, S.J.; Yusibov, V. Virus-like particles as a highly efficient vaccine platform: Diversity of targets and production systems and advances in clinical development. Vaccine 2012, 31, 58–83. [Google Scholar] [CrossRef]
- Buonaguro, L.; Tagliamonte, M.; Tornesello, M.L.; Buonaguro, F.M. Developments in virus-like particle-based vaccines for infectious diseases and cancer. Expert Rev. Vaccines 2011, 10, 1569–1583. [Google Scholar] [CrossRef] [PubMed]
- Goldinger, S.M.; Dummer, R.; Baumgaertner, P.; Mihic-Probst, D.; Schwarz, K.; Hammann-Haenni, A.; Willers, J.; Geldhof, C.; Prior, J.O.; Kündig, T.M. Nano-particle vaccination combined with TLR-7 and-9 ligands triggers memory and effector CD 8+ T-cell responses in melanoma patients. Eur. J. Immunol. 2012, 42, 3049–3061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cubas, R.; Zhang, S.; Kwon, S.; Sevick-Muraca, E.M.; Li, M.; Chen, C.; Yao, Q. Virus-like particle (VLP) lymphatic trafficking and immune response generation after immunization by different routes. J. Immunother. 2009, 32, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manolova, V.; Flace, A.; Bauer, M.; Schwarz, K.; Saudan, P.; Bachmann, M.F. Nanoparticles target distinct dendritic cell populations according to their size. Eur. J. Immunol. 2008, 38, 1404–1413. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.T.; Van Der Vlies, A.J.; Simeoni, E.; Angeli, V.; Randolph, G.J.; O’Neil, C.P.; Lee, L.K.; Swartz, M.A.; Hubbell, J.A. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat. Biotechnol. 2007, 25, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Douglas, R.G. The heritage of hepatitis B vaccine. JAMA 1996, 276, 1796–1798. [Google Scholar] [CrossRef]
- Hilleman, M.; McAleer, W.; Buynak, E.; McLean, A. The preparation and safety of hepatitis B vaccine. J. Infect. 1983, 7, 3–8. [Google Scholar] [CrossRef]
- Saslow, D.; Castle, P.E.; Cox, J.T.; Davey, D.D.; Einstein, M.H.; Ferris, D.G.; Goldie, S.J.; Harper, D.M.; Kinney, W.; Moscicki, A.B. American Cancer Society Guideline for human papillomavirus (HPV) vaccine use to prevent cervical cancer and its precursors. CA Cancer J. Clin. 2007, 57, 7–28. [Google Scholar] [CrossRef] [Green Version]
- Kool, M.; Fierens, K.; Lambrecht, B.N. Alum adjuvant: Some of the tricks of the oldest adjuvant. J. Med Microbiol. 2012, 61, 927–934. [Google Scholar] [CrossRef] [Green Version]
- Azimi, A.; Heidarian, S.; Zamani, H.; Taleghani, N.; Dehghani, M.; Seyedjafari, E. Optimized dose of synthetic analogues of Monophosphoryl lipid A as an effective alternative for formulating recombinant human papillomavirus vaccine. Biologicals 2020, 68, 60–64. [Google Scholar] [CrossRef]
- Dai, S.; Wang, H.; Deng, F. Advances and challenges in enveloped virus-like particle (VLP)-based vaccines. J. Immunol. Sci. 2018, 2, 36–41. [Google Scholar]
- Chang, L.-J.; Dowd, K.A.; Mendoza, F.H.; Saunders, J.G.; Sitar, S.; Plummer, S.H.; Yamshchikov, G.; Sarwar, U.N.; Hu, Z.; Enama, M.E. Safety and tolerability of chikungunya virus-like particle vaccine in healthy adults: A phase 1 dose-escalation trial. Lancet 2014, 384, 2046–2052. [Google Scholar] [CrossRef]
- Mohsen, M.O.; Zha, L.; Cabral-Miranda, G.; Bachmann, M.F. Major findings and recent advances in virus–like particle (VLP)-based vaccines. In Seminars in Immunology; Academic Press: Cambridge, MA, USA, 2017; Volume 34, pp. 123–132. [Google Scholar]
- Purwar, M.; Pokorski, J.K.; Singh, P.; Bhattacharyya, S.; Arendt, H.; DeStefano, J.; La Branche, C.C.; Montefiori, D.C.; Finn, M.; Varadarajan, R. Design, display and immunogenicity of HIV1 gp120 fragment immunogens on virus-like particles. Vaccine 2018, 36, 6345–6353. [Google Scholar] [CrossRef]
- Wang, B.-Z.; Liu, W.; Kang, S.-M.; Alam, M.; Huang, C.; Ye, L.; Sun, Y.; Li, Y.; Kothe, D.L.; Pushko, P. Incorporation of high levels of chimeric human immunodeficiency virus envelope glycoproteins into virus-like particles. J. Virol. 2007, 81, 10869–10878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karpenko, L.I.; Nekrasova, N.A.; Ilyichev, A.A.; Lebedev, L.R.; Ignatyev, G.M.; Agafonov, A.P.; Zaitsev, B.N.; Belavin, P.A.; Seregin, S.V.; Danilyuk, N.K. Comparative analysis using a mouse model of the immunogenicity of artificial VLP and attenuated Salmonella strain carrying a DNA-vaccine encoding HIV-1 polyepitope CTL-immunogen. Vaccine 2004, 22, 1692–1699. [Google Scholar] [CrossRef]
- Johansen, P.; Men, Y.; Merkle, H.P.; Gander, B. Revisiting PLA/PLGA microspheres: An analysis of their potential in parenteral vaccination. Eur. J. Pharm. Biopharm. 2000, 50, 129–146. [Google Scholar] [CrossRef]
- Ferreira, S.A.; Gama, F.M.; Vilanova, M. Polymeric nanogels as vaccine delivery systems. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 159–173. [Google Scholar] [CrossRef] [Green Version]
- Christensen, D.; Korsholm, K.S.; Rosenkrands, I.; Lindenstrøm, T.; Andersen, P.; Agger, E.M. Cationic liposomes as vaccine adjuvants. Expert Rev. Vaccines 2007, 6, 785–796. [Google Scholar] [CrossRef]
- Uenaka, A.; Wada, H.; Isobe, M.; Saika, T.; Tsuji, K.; Sato, E.; Sato, S.; Noguchi, Y.; Kawabata, R.; Yasuda, T. T cell immunomonitoring and tumor responses in patients immunized with a complex of cholesterol-bearing hydrophobized pullulan (CHP) and NY-ESO-1 protein. Cancer Immun. Arch. 2007, 7, 7–10. [Google Scholar]
- Letchford, K.; Burt, H. A review of the formation and classification of amphiphilic block copolymer nanoparticulate structures: Micelles, nanospheres, nanocapsules and polymersomes. Eur. J. Pharm. Biopharm. 2007, 65, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Lü, J.-M.; Wang, X.; Marin-Muller, C.; Wang, H.; Lin, P.H.; Yao, Q.; Chen, C. Current advances in research and clinical applications of PLGA-based nanotechnology. Expert Rev. Mol. Diagn. 2009, 9, 325–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, L.; Qi, X.R.; Zhou, X.J.; Maitani, Y.; Wang, S.C.; Jiang, Y.; Nagai, T. Pharmaceutical and immunological evaluation of a single-dose hepatitis B vaccine using PLGA microspheres. J. Control. Release 2006, 112, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-M.; Lee, S.J.; Kim, Y.S.; Lee, M.H.; Cha, G.S.; Jung, I.D.; Kang, T.H.; Han, H.D. Nanoparticle-based vaccine delivery for cancer immunotherapy. Immune Netw. 2013, 13, 177–183. [Google Scholar] [CrossRef] [Green Version]
- Kreuter, J. Nanoparticle-based dmg delivery systems. J. Control. Release 1991, 16, 169–176. [Google Scholar] [CrossRef]
- Marasini, N.; Skwarczynski, M.; Toth, I. Oral delivery of nanoparticle-based vaccines. Expert Rev. Vaccines 2014, 13, 1361–1376. [Google Scholar] [CrossRef] [PubMed]
- Negahdaripour, M.; Golkar, N.; Hajighahramani, N.; Kianpour, S.; Nezafat, N.; Ghasemi, Y. Harnessing self-assembled peptide nanoparticles in epitope vaccine design. Biotechnol. Adv. 2017, 35, 575–596. [Google Scholar] [CrossRef]
- Raman, S.; Machaidze, G.; Lustig, A.; Aebi, U.; Burkhard, P. Structure-based design of peptides that self-assemble into regular polyhedral nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2006, 2, 95–102. [Google Scholar] [CrossRef]
- He, B.; Ma, S.; Peng, G.; He, D. TAT-modified self-assembled cationic peptide nanoparticles as an efficient antibacterial agent. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 365–372. [Google Scholar] [CrossRef]
- Doll, T.A.; Neef, T.; Duong, N.; Lanar, D.E.; Ringler, P.; Müller, S.A.; Burkhard, P. Optimizing the design of protein nanoparticles as carriers for vaccine applications. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1705–1713. [Google Scholar] [CrossRef]
- Ishii, N.; Fukushima, J.; Kaneko, T.; Okada, E.; Tani, K.; Tanaka, S.-I.; Hamajima, K.; Xin, K.-Q.; Kawamoto, S.; Koff, W. Cationic liposomes are a strong adjuvant for a DNA vaccine of human immunodeficiency virus type 1. Aids Res. Hum. Retrovir. 1997, 13, 1421–1428. [Google Scholar] [CrossRef]
- O’Hagan, D.T. MF59 is a safe and potent vaccine adjuvant that enhances protection against influenza virus infection. Expert Rev. Vaccines 2007, 6, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Sahly, H.E. MF59™ as a vaccine adjuvant: A review of safety and immunogenicity. Expert Rev. Vaccines 2010, 9, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Vogel, F.R.; Caillet, C.; Kusters, I.C.; Haensler, J. Emulsion-based adjuvants for influenza vaccines. Expert Rev. Vaccines 2009, 8, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Podda, A. The adjuvanted influenza vaccines with novel adjuvants: Experience with the MF59-adjuvanted vaccine. Vaccine 2001, 19, 2673–2680. [Google Scholar] [CrossRef]
- Podda, A.; Del Giudice, G. MF59-adjuvanted vaccines: Increased immunogenicity with an optimal safety profile. Expert Rev. Vaccines 2003, 2, 197–204. [Google Scholar] [CrossRef]
- Suleiman, E.; Damm, D.; Batzoni, M.; Temchura, V.; Wagner, A.; Überla, K.; Vorauer-Uhl, K. Electrostatically Driven Encapsulation of Hydrophilic, Non-Conformational Peptide Epitopes into Liposomes. Pharmaceutics 2019, 11, 619. [Google Scholar] [CrossRef] [Green Version]
- Stanberry, L.; Simon, J.; Johnson, C.; Robinson, P.; Morry, J.; Flack, M.; Gracon, S.; Myc, A.; Hamouda, T.; Baker, J., Jr. Safety and immunogenicity of a novel nanoemulsion mucosal adjuvant W805EC combined with approved seasonal influenza antigens. Vaccine 2012, 30, 307–316. [Google Scholar] [CrossRef]
- Das, S.C.; Hatta, M.; Wilker, P.R.; Myc, A.; Hamouda, T.; Neumann, G.; Baker, J.R., Jr.; Kawaoka, Y. Nanoemulsion W805EC improves immune responses upon intranasal delivery of an inactivated pandemic H1N1 influenza vaccine. Vaccine 2012, 30, 6871–6877. [Google Scholar] [CrossRef] [Green Version]
- Damm, D.; Rojas-Sánchez, L.; Theobald, H.; Sokolova, V.; Wyatt, R.T.; Überla, K.; Epple, M.; Temchura, V. Calcium phosphate nanoparticle-based vaccines as a platform for improvement of HIV-1 Env antibody responses by intrastructural help. Nanomaterials 2019, 9, 1389. [Google Scholar] [CrossRef] [Green Version]
- Tannig, P.; Peter, A.S.; Lapuente, D.; Klessing, S.; Damm, D.; Tenbusch, M.; Überla, K.; Temchura, V. Modulation of Vaccine-Induced HIV-1-Specific Immune Responses by Co-Electroporation of PD-L1 Encoding DNA. Vaccines 2020, 8, 27. [Google Scholar] [CrossRef] [Green Version]
- Rashidi, M.; Jebali, A. Liposomal prodigiosin and plasmid encoding serial GCA nucleotides reduce inflammation in microglial and astrocyte cells by ATM/ATR signaling. J. Neuroimmunol. 2019, 326, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Zilker, C.; Kozlova, D.; Sokolova, V.; Yan, H.; Epple, M.; Überla, K.; Temchura, V. Nanoparticle-based B-cell targeting vaccines: Tailoring of humoral immune responses by functionalization with different TLR-ligands. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Bafghi, A.F.; Daghighi, M.; Daliri, K.; Jebali, A. Magnesium oxide nanoparticles coated with glucose can silence important genes of Leishmania major at sub-toxic concentrations. Colloids Surf. B Biointerfaces 2015, 136, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Bafghi, A.F.; Jebali, A.; Daliri, K. Silica nanowire conjugated with loop-shaped oligonucleotides: A new structure to silence cysteine proteinase gene in Leishmania tropica. Colloids Surf. B Biointerfaces 2015, 136, 323–328. [Google Scholar] [CrossRef]
- Ayatollahi, M.; Ayatollahi, G.; Rashidi, M.; Hekmatimoghaddam, S.; Mosshafi, M.; Jebali, A.; Iman, M.; Sardo, H.S. Prodigiosin-Conjugated Aptamer for Attachment to the Surface of Brain Cancer Cells Mediated by Glutamate Receptor. Colloid Interface Sci. Commun. 2018, 24, 45–48. [Google Scholar] [CrossRef]
- Barnowski, C.; Kadzioch, N.; Damm, D.; Yan, H.; Temchura, V. Advantages and Limitations of Integrated Flagellin Adjuvants for HIV-Based Nanoparticle B-Cell Vaccines. Pharmaceutics 2019, 11, 204. [Google Scholar] [CrossRef] [Green Version]
- Van der Pol, L.; Stork, M.; van der Ley, P. Outer membrane vesicles as platform vaccine technology. Biotechnol. J. 2015, 10, 1689–1706. [Google Scholar] [CrossRef]
- Shehata, M.M.; Mostafa, A.; Teubner, L.; Mahmoud, S.H.; Kandeil, A.; Elshesheny, R.; Boubak, T.A.; Frantz, R.; Pietra, L.L.; Pleschka, S. Bacterial outer membrane vesicles (omvs)-based dual vaccine for influenza a h1n1 virus and mers-cov. Vaccines 2019, 7, 46. [Google Scholar] [CrossRef] [Green Version]
- Zolnik, B.S.; González-Fernández, Á.; Sadrieh, N.; Dobrovolskaia, M.A. Minireview: Nanoparticles and the immune system. Endocrinology 2010, 151, 458–465. [Google Scholar] [CrossRef]
- Ngobili, T.A.; Daniele, M.A. Nanoparticles and direct immunosuppression. Exp. Biol. Med. 2016, 241, 1064–1073. [Google Scholar] [CrossRef] [Green Version]
- Ilinskaya, A.; Dobrovolskaia, M. Immunosuppressive and Anti-Inflammatory Properties of Engineered Nanomaterials. In Handbook of Immunological Properties of Engineered Nanomaterials: Volume 3: Engineered Nanomaterials and the Immune Cell Function; World Scientific: Singapore, 2016; pp. 139–163. [Google Scholar]
| Immunological Structure | Size (nm) |
|---|---|
| Complement | 1–5 |
| Toll-like receptor (TLR) | 2–10 |
| T cell receptor (TCR) | 2–10 |
| The cluster of differentiation (CD) markers | 2–10 |
| Antibody | 10–15 |
| T cells | 7000–12,000 |
| B cells | 7000–12,000 |
| Neutrophils | 10,000–12,000 |
| Dendritic cells | 10,000–22,000 |
| Macrophages | 10,000–22,000 |
| Nanoparticles | Size | Bioactivity |
|---|---|---|
| Dendrimer | <5 nm | Partition like small molecules and filter through the kidney |
| Polymer | 10–20 nm | Escape the vasculature, infiltrate the tissues and lymphatics like proteins |
| DNA polyplex | 50–100 nm | Penetrate the mucosal membranes and the skin and are taken up into cells |
| Liposome | >100 nm | Taken up mainly into phagocytic cells |
| Nano-Based Vaccines | Size Range | Mechanisms |
|---|---|---|
| Virus-like particles | 15–30 nm | Repetitive antigen display, structural or molecular mimicry of the virus, particle size-dependent tissue penetration and trafficking to lymphatics, and TLR activation |
| MF59 a | 150–200 nm | Neutrophil, monocyte, and DC recruitment, antigen uptake, and the induction of humoral and TH1-type immune responses |
| W805EC b | 200–400 nm | Antigen uptake by and activation of epithelial cells and DCs, TLR2 and TLR4 activation, local cytokine production, mucosal antibody responses, and TH1, TH2, and TH17 cell responses |
| PLGA c | 100–200 nm | Encapsulation for sustained local antigens and co-mediator release |
| Nanogel | 30–40 nm | Antigen entrapment in a hydrated nanogel matrix for slow release, delivery to APCs, and induction of tumor-specific T cells and antibody responses |
| Cationic liposomes | 200–1000 nm | Encapsulation and targeted antigen delivery or uptake by APCs, and recruitment of monocytes to the injection site |
| Nanoparticles | Size Range | Mechanisms | Medical Application | Current Use |
|---|---|---|---|---|
| Fullerenes | 0.5–1 nm | Suppression of mast cell and basophil degranulation | Allergy | In mice and in vitro |
| SWCNT a | 1–4 nm diameter; 1000–3000 nm length | Suppression of DC function | Inhalation exposure | In mice |
| MWCNT b | 10–20 nm diameter; 5000–15000 nm length | Suppression of T cell proliferation and function | Inhalation exposure | In mice |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keikha, R.; Daliri, K.; Jebali, A. The Use of Nanobiotechnology in Immunology and Vaccination. Vaccines 2021, 9, 74. https://doi.org/10.3390/vaccines9020074
Keikha R, Daliri K, Jebali A. The Use of Nanobiotechnology in Immunology and Vaccination. Vaccines. 2021; 9(2):74. https://doi.org/10.3390/vaccines9020074
Chicago/Turabian StyleKeikha, Reza, Karim Daliri, and Ali Jebali. 2021. "The Use of Nanobiotechnology in Immunology and Vaccination" Vaccines 9, no. 2: 74. https://doi.org/10.3390/vaccines9020074
APA StyleKeikha, R., Daliri, K., & Jebali, A. (2021). The Use of Nanobiotechnology in Immunology and Vaccination. Vaccines, 9(2), 74. https://doi.org/10.3390/vaccines9020074





