Cord Blood-Based Approach to Assess Candidate Vaccine Adjuvants Designed for Neonates and Infants
Abstract
:1. Introduction
2. Infant Immune Development and Its Impact on Vaccine Response
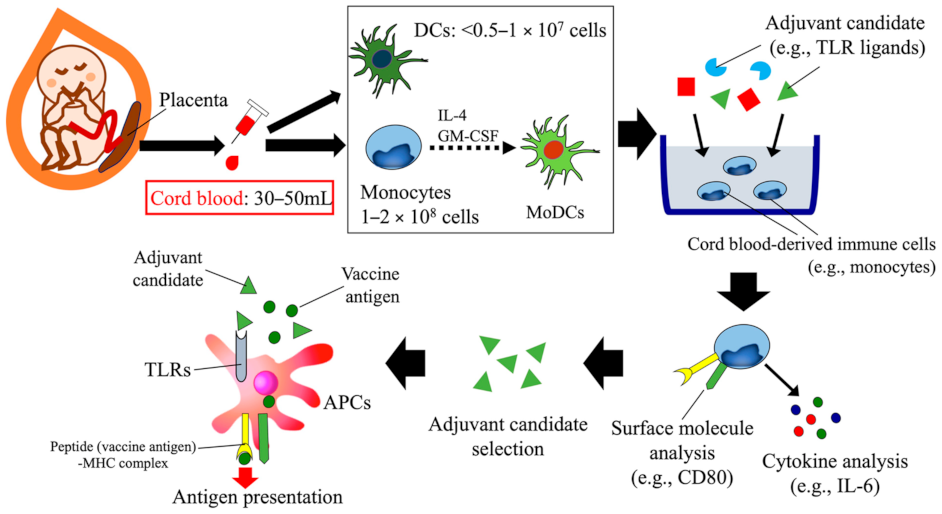
3. Human Cord Blood-Based Approach to Assess Candidate Vaccine Adjuvants Designed for Neonates and Infants
4. Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- E Lawn, J.; Cousens, S.; Zupan, J. 4 million neonatal deaths: When? Where? Why? Lancet 2005, 365, 891–900. [Google Scholar] [CrossRef]
- Nair, H.; Nokes, D.J.; Gessner, B.D.; Dherani, M.; Madhi, S.A.; Singleton, R.J.; O’Brien, K.L.; Roca, A.; Wright, P.F.; Bruce, N.; et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: A systematic review and meta-analysis. Lancet 2010, 375, 1545–1555. [Google Scholar] [CrossRef] [Green Version]
- Tokuhara, D. Challenges in developing mucosal vaccines and antibodies against infectious diarrhea in children. Pediatr. Int. 2018, 60, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, D.I.; Glass, R.I.; Rodgers, G.; Davidson, B.L.; Sack, D.A. Evaluation of rhesus rotavirus monovalent and tetravalent reassortant vaccines in US children. US Rotavirus Vaccine Efficacy Group. JAMA 1995, 273, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Belshe, R.B.; Mendelman, P.M.; Treanor, J.; King, J.; Gruber, W.C.; Piedra, P.; Bernstein, D.I.; Hayden, F.G.; Kotloff, K.; Zangwill, K.; et al. The Efficacy of Live Attenuated, Cold-Adapted, Trivalent, Intranasal Influenzavirus Vaccine in Children. N. Engl. J. Med. 1998, 338, 1405–1412. [Google Scholar] [CrossRef]
- Mendelman, P.M.; Cordova, J.; Cho, I. Safety, efficacy and effectiveness of the influenza virus vaccine, trivalent, types A and B, live, cold-adapted (CAIV-T) in healthy children and healthy adults. Vaccine 2001, 19, 2221–2226. [Google Scholar] [CrossRef]
- Hill, D.R.; Ford, L.; Lalloo, D.G. Oral cholera vaccines: Use in clinical practice. Lancet Infect. Dis. 2006, 6, 361–373. [Google Scholar] [CrossRef]
- Kapikian, A.Z.; Mitchell, R.H.; Chanock, R.M.; Shvedoff, R.A.; Stewart, C.E. An Epidemiologic Study of Altered Clinical Reactivity to Respiratory Syncytial (RS) Virus Infection in Children Previously Vaccinated with an Inactivated RS Virus Vaccine. Am. J. Epidemiol. 1969, 89, 405–421. [Google Scholar] [CrossRef]
- Kim, H.W.; Canchola, J.G.; Brandt, C.D.; Pyles, G.; Chanock, R.M.; Jensen, K.; Parrott, R.H. Respiratory Syncytial Virus Disease in Infants Despite Prior Administration of Antigenic Inactivated Vaccine. Am. J. Epidemiol. 1969, 89, 422–434. [Google Scholar] [CrossRef]
- Ciarlet, M.; Schödel, F. Development of a rotavirus vaccine: Clinical safety, immunogenicity, and efficacy of the pentavalent rotavirus vaccine, RotaTeq®. Vaccine 2009, 27, G72–G81. [Google Scholar] [CrossRef]
- O’Ryan, M.; Linhares, A.C. Update on Rotarix™: An oral human rotavirus vaccine. Expert Rev. Vac. 2009, 8, 1627–1641. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, L.; Viboud, C.; Elixhauser, A.; Taylor, R.J.; Kapikian, A.Z. More on RotaShield and Intussusception: The Role of Age at the Time of Vaccination. J. Infect. Dis. 2005, 192, S36–S43. [Google Scholar] [CrossRef] [PubMed]
- Takeyama, N.; Yuki, Y.; Tokuhara, D.; Oroku, K.; Mejima, M.; Kurokawa, S.; Kuroda, M.; Kodama, T.; Nagai, S.; Ueda, S.; et al. Oral rice-based vaccine induces passive and active immunity against enterotoxigenic E. coli-mediated diarrhea in pigs. Vaccine 2015, 33, 5204–5211. [Google Scholar] [CrossRef] [PubMed]
- Tokuhara, D.; Yuki, Y.; Nochi, T.; Kodama, T.; Mejima, M.; Kurokawa, S.; Takahashi, Y.; Nanno, M.; Nakanishi, U.; Takaiwa, F.; et al. Secretory IgA-mediated protection against V. cholerae and heat-labile enterotoxin-producing enterotoxigenic Escherichia coli by rice-based vaccine. Proc. Natl. Acad. Sci. USA 2010, 107, 8794–8799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nochi, T.; Yuki, Y.; Katakai, Y.; Shibata, H.; Tokuhara, D.; Mejima, M.; Kurokawa, S.; Takahashi, Y.; Nakanishi, U.; Ono, F.; et al. A Rice-Based Oral Cholera Vaccine Induces Macaque-Specific Systemic Neutralizing Antibodies but Does Not Influence Pre-Existing Intestinal Immunity. J. Immunol. 2009, 183, 6538–6544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuki, Y.; Nochi, T.; Harada, N.; Katakai, Y.; Shibata, H.; Mejima, M.; Kohda, T.; Tokuhara, D.; Kurokawa, S.; Takahashi, Y.; et al. In Vivo Molecular Imaging Analysis of a Nasal Vaccine That Induces Protective Immunity against Botulism in Nonhuman Primates. J. Immunol. 2010, 185, 5436–5443. [Google Scholar] [CrossRef]
- Skinner, J.M.; Indrawati, L.; Cannon, J.; Blue, J.; Winters, M.; Macnair, J.; Pujar, N.; Manger, W.; Zhang, Y.; Antonello, J.; et al. Pre-clinical evaluation of a 15-valent pneumococcal conjugate vaccine (PCV15-CRM197) in an infant-rhesus monkey immunogenicity model. Vaccine 2011, 29, 8870–8876. [Google Scholar] [CrossRef]
- Leroux-Roels, G.; Cramer, J.P.; Mendelman, P.M.; Sherwood, J.; Clemens, R.; Aerssens, A.; De Coster, I.; Borkowski, A.; Baehner, F.; Van Damme, P. Safety and Immunogenicity of Different Formulations of Norovirus Vaccine Candidate in Healthy Adults: A Randomized, Controlled, Double-Blind Clinical Trial. J. Infect. Dis. 2018, 217, 597–607. [Google Scholar] [CrossRef]
- Szmuness, W.; Stevens, C.E.; Zang, E.A.; Harley, E.J.; Kellner, A. A controlled clinical trial of the efficacy of the hepatitis B vaccine (heptavax B): A final report. Hepatology 1981, 1, 377–385. [Google Scholar] [CrossRef]
- Joensuu, J.; Koskenniemi, E.; Pang, X.-L.; Vesikari, T. Randomised placebo-controlled trial of rhesus-human reassortant rotavirus vaccine for prevention of severe rotavirus gastroenteritis. Lancet 1997, 350, 1205–1209. [Google Scholar] [CrossRef]
- Maupas, P.; Chiron, J.P.; Barin, F.; Coursaget, P.; Goudeau, A.; Perrin, J.; Denis, F.; Mar, I.D. Efficacy of Hepatitis B Vaccine in Prevention of Early HBsAg Carrier State in Children Controlled Trial in an Endemic Area (Senegal). Lancet 1981, 317, 289–292. [Google Scholar] [CrossRef]
- Gruber, W.C.; Darden, P.M.; Still, J.G.; Lohr, J.; Reed, G.; Wright, P.F. Evaluation of bivalent live attenuated influenza A vaccines in children 2 months to 3 years of age: Safety, immunogenicity and dose-response. Vaccine 1997, 15, 1379–1384. [Google Scholar] [CrossRef]
- Gans, H.A.; Arvin, A.M.; Galinus, J.; Logan, L.; DeHovitz, R.; Maldonado, Y. Deficiency of the Humoral Immune Response to Measles Vaccine in Infants Immunized at Age 6 Months. JAMA 1998, 280, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Wright, P.F.; Karron, R.A.; Belshe, R.B.; Thompson, J.; Crowe, J.J.E.; Boyce, T.G.; Halburnt, L.L.; Reed, G.W.; Whitehead, S.S.; Anderson, E.L.; et al. Evaluation of a Live, Cold-Passaged, Temperature-Sensitive, Respiratory Syncytial Virus Vaccine Candidate in Infancy. J. Infect. Dis. 2000, 182, 1331–1342. [Google Scholar] [CrossRef] [PubMed]
- Fujkuyama, Y.; Tokuhara, D.; Kataoka, K.; Gilbert, R.S.; McGhee, J.R.; Yuki, Y.; Kiyono, H.; Fujihashi, K. Novel vaccine development strategies for inducing mucosal immunity. Expert Rev. Vaccines 2012, 11, 367–379. [Google Scholar] [CrossRef] [Green Version]
- Kiyono, H.; Fukuyama, S. NALT- versus PEYER’S-patch-mediated mucosal immunity. Nat. Rev. Immunol. 2004, 4, 699–710. [Google Scholar] [CrossRef]
- Tokuhara, D.; Kurashima, Y.; Kamioka, M.; Nakayama, T.; Ernst, P.; Kiyono, H. A comprehensive understanding of the gut mucosal immune system in allergic inflammation. Allergol. Int. 2019, 68, 17–25. [Google Scholar] [CrossRef]
- Tokuhara, D.; Nochi, T.; Matsumura, A.; Mejima, M.; Takahashi, Y.; Kurokawa, S.; Kiyono, H.; Yuki, Y. Specific Expression of Apolipoprotein A-IV in the Follicle-Associated Epithelium of the Small Intestine. Dig. Dis. Sci. 2014, 59, 2682–2692. [Google Scholar] [CrossRef]
- Marshall-Clarke, S.; Reen, D.; Tasker, L.; Hassan, J. Neonatal immunity: How well has it grown up? Immunol. Today 2000, 21, 35–41. [Google Scholar] [CrossRef]
- Hamada, H.; Hiroi, T.; Nishiyama, Y.; Takahashi, H.; Masunaga, Y.; Hachimura, S.; Kaminogawa, S.; Takahashi-Iwanaga, H.; Iwanaga, T.; Kiyono, H.; et al. Identification of Multiple Isolated Lymphoid Follicles on the Antimesenteric Wall of the Mouse Small Intestine. J. Immunol. 2002, 168, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Rennert, P.D.; Browning, J.L.; Mebius, R.; Mackay, F.; Hochman, P.S. Surface lymphotoxin alpha/beta complex is required for the development of peripheral lymphoid organs. J. Exp. Med. 1996, 184, 1999–2006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spencer, J.; MacDonald, T.T.; Finn, T.; Isaacson, P.G. The development of gut associated lymphoid tissue in the terminal ileum of fetal human intestine. Clin. Exp. Immunol. 1986, 64, 536–543. [Google Scholar] [PubMed]
- Cornes, J.S. Number, size, and distribution of Peyer’s patches in the human small intestine: Part I The development of Peyer’s patches. Gut 1965, 6, 225–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eugster, H.-P.; Muller, M.; Karrer, U.; Car, B.D.; Schnyder, B.; Eng, V.M.; Woerly, G.; Le Hir, M.; Di Padova, F.; Aguet, M.; et al. Multiple immune abnormalities in tumor necrosis factor and lymphotoxin-α double-deficient mice. Int. Immunol. 1996, 8, 23–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, M.; Kweon, M.-N.; Rennert, P.D.; Hiroi, T.; Fujihashi, K.; McGhee, J.R.; Kiyono, H. Role of Gut-Associated Lymphoreticular Tissues in Antigen-Specific Intestinal IgA Immunity. J. Immunol. 2004, 173, 762–769. [Google Scholar] [CrossRef] [Green Version]
- Rijkers, G.; Sanders, E.; Breukels, M.; Zegers, B. Infant B cell responses to polysaccharide determinants. Vaccine 1998, 16, 1396–1400. [Google Scholar] [CrossRef]
- Gans, H.A.; Maldonado, Y.; Yasukawa, L.L.; Beeler, J.; Audet, S.; Rinki, M.M.; DeHovitz, R.; Arvin, A.M. IL-12, IFN-gamma, and T cell proliferation to measles in immunized infants. J. Immunol. 1999, 162, 5569–5575. [Google Scholar]
- Vekemans, J.; Ota, M.O.C.; Wang, E.C.Y.; Kidd, M.; Borysiewicz, L.K.; Whittle, H.C.; McAdam, K.P.W.J.; Morgan, G.; Marchant, A. T cell responses to vaccines in infants: Defective IFNγ production after oral polio vaccination. Clin. Exp. Immunol. 2002, 127, 495–498. [Google Scholar] [CrossRef]
- Upham, J.W.; Rate, A.; Rowe, J.; Kusel, M.; Sly, P.D.; Holt, P.G. Dendritic Cell Immaturity during Infancy Restricts the Capacity To Express Vaccine-Specific T-Cell Memory. Infect. Immun. 2006, 74, 1106–1112. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, M.; Leuridan, E.; Zhang, T.; De Wit, D.; Willems, F.; Van Damme, P.; Goldman, M.; Goriely, S. Acquisition of Adult-Like TLR4 and TLR9 Responses during the First Year of Life. PLoS ONE 2010, 5, e10407. [Google Scholar] [CrossRef]
- Vono, M.; Eberhardt, C.S.; Auderset, F.; Mastelic-Gavillet, B.; Lemeille, S.; Christensen, D.; Andersen, P.; Lambert, P.-H.; Siegrist, C.-A. Maternal Antibodies Inhibit Neonatal and Infant Responses to Vaccination by Shaping the Early-Life B Cell Repertoire within Germinal Centers. Cell Rep. 2019, 28, 1773–1784.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malloy, A.M.W.; Ruckwardt, T.J.; Morabito, K.M.; Lau-Kilby, A.W.; Graham, B.S. Pulmonary Dendritic Cell Subsets Shape the Respiratory Syncytial Virus–Specific CD8+T Cell Immunodominance Hierarchy in Neonates. J. Immunol. 2017, 198, 394–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima, L.; Molina, M.D.G.F.; Pereira, B.S.; Nadaf, M.L.A.; Nadaf, M.I.V.; Takano, O.A.; Carneiro-Sampaio, M.; Palmeira, P. Acquisition of specific antibodies and their influence on cell-mediated immune response in neonatal cord blood after maternal pertussis vaccination during pregnancy. Vaccine 2019, 37, 2569–2579. [Google Scholar] [CrossRef]
- Kandasamy, S.; Chattha, K.S.; Vlasova, A.N.; Saif, L.J. Prenatal vitamin A deficiency impairs adaptive immune responses to pentavalent rotavirus vaccine (RotaTeq®) in a neonatal gnotobiotic pig model. Vaccine 2014, 32, 816–824. [Google Scholar] [CrossRef]
- Wiedermann, U.; A Hanson, L.; Holmgren, J.; Kahu, H.; I Dahlgren, U. Impaired mucosal antibody response to cholera toxin in vitamin A-deficient rats immunized with oral cholera vaccine. Infect. Immun. 1993, 61, 3952–3957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Surman, S.L.; Rudraraju, R.; Sealy, R.; Jones, B.; Hurwitz, J.L. Vitamin A Deficiency Disrupts Vaccine-Induced Antibody-Forming Cells and the Balance of IgA/IgG Isotypes in the Upper and Lower Respiratory Tract. Viral Immunol. 2012, 25, 341–344. [Google Scholar] [CrossRef]
- Enioutina, E.Y.; Bareyan, D.; Daynes, R.A. TLR-Induced Local Metabolism of Vitamin D3Plays an Important Role in the Diversification of Adaptive Immune Responses. J. Immunol. 2009, 182, 4296–4305. [Google Scholar] [CrossRef] [Green Version]
- Iwata, M.; Hirakiyama, A.; Eshima, Y.; Kagechika, H.; Kato, C.; Song, S.-Y. Retinoic Acid Imprints Gut-Homing Specificity on T Cells. Immunity 2004, 21, 527–538. [Google Scholar] [CrossRef] [Green Version]
- Mora, J.R.; Iwata, M.; Eksteen, B.; Song, S.-Y.; Junt, T.; Senman, B.; Otipoby, K.L.; Yokota, A.; Takeuchi, H.; Ricciardi-Castagnoli, P.; et al. Generation of Gut-Homing IgA-Secreting B Cells by Intestinal Dendritic Cells. Science 2006, 314, 1157–1160. [Google Scholar] [CrossRef] [Green Version]
- Madhi, S.A.; Cunliffe, N.A.; Steele, D.; Witte, D.; Kirsten, M.; Louw, C.; Ngwira, B.; Victor, J.C.; Gillard, P.H.; Cheuvart, B.B.; et al. Effect of Human Rotavirus Vaccine on Severe Diarrhea in African Infants. New Engl. J. Med. 2010, 362, 289–298. [Google Scholar] [CrossRef] [Green Version]
- E Armah, G.; O Sow, S.; Breiman, R.F.; Dallas, M.J.; Tapia, M.D.; Feikin, D.R.; Binka, F.N.; Steele, A.D.; Laserson, K.F.; A Ansah, N.; et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: A randomised, double-blind, placebo-controlled trial. Lancet 2010, 376, 606–614. [Google Scholar] [CrossRef]
- Chattha, K.S.; Kandasamy, S.; Vlasova, A.N.; Saif, L.J. Vitamin A Deficiency Impairs Adaptive B and T Cell Responses to a Prototype Monovalent Attenuated Human Rotavirus Vaccine and Virulent Human Rotavirus Challenge in a Gnotobiotic Piglet Model. PLoS ONE 2013, 8, e82966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bucardo, F.; Nordgren, J.; Reyes, Y.; Gonzalez, F.; Sharma, S.; Svensson, L. The Lewis A phenotype is a restriction factor for Rotateq and Rotarix vaccine-take in Nicaraguan children. Sci. Rep. 2018, 8, 8. [Google Scholar] [CrossRef] [Green Version]
- Naylor, C.; Lu, M.; Haque, R.; Mondal, D.; Buonomo, E.L.; Nayak, U.; Mychaleckyj, J.C.; Kirkpatrick, B.D.; Colgate, R.; Carmolli, M.P.; et al. Environmental Enteropathy, Oral Vaccine Failure and Growth Faltering in Infants in Bangladesh. EBioMedicine 2015, 2, 1759–1766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klinge, J.; Lugauer, S.; Korn, K.; Heininger, U.; Stehr, K. Comparison of immunogenicity and reactogenicity of a measles, mumps and rubella (MMR) vaccine in German children vaccinated at 9–11, 12–14 or 15–17 months of age☆. (☆ Results of this study were presented at the annual meetings of the German Society for Paediatrics and Adolescent Medicine, September 1997, Vienna (Austria) and the German Society for Paediatric Infectious Diseases, November 1997, Berlin (Germany)). Vaccine 2000, 18, 3134–3140. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, J.M.; Greenberg, D.P.; Wong, V.K.; Partridge, S.; Chang, S.-J.; Chiu, C.-Y.; Ward, J.I. Effect of neonatal immunization with diphtheria and tetanus toxoids on antibody responses to Haemophilus influenzae type b conjugate vaccines. J. Pediatr. 1995, 126, 198–205. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—a new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [Green Version]
- Garmory, H.S.; Brown, K.A.; Titball, R.W. DNA vaccines: Improving expression of antigens. Genet. Vaccines Ther. 2003, 1, 2. [Google Scholar] [CrossRef] [Green Version]
- Asanuma, H.; Zamri, N.B.; Sekine, S.-I.; Fukuyama, Y.; Tokuhara, D.; Gilbert, R.S.; Fukuiwa, T.; Fujihashi, K.; Sata, T.; Tashiro, M.; et al. A novel combined adjuvant for nasal delivery elicits mucosal immunity to influenza in aging. Vaccine 2012, 30, 803–812. [Google Scholar] [CrossRef] [Green Version]
- Fukuyama, Y.; Tokuhara, D.; Sekine, S.; Aso, K.; Kataoka, K.; Davydova, J.; Yamamoto, M.; Gilbert, R.S.; Tokuhara, Y.; Fujihashi, K.; et al. Potential Roles of CCR5+ CCR6+ Dendritic Cells Induced by Nasal Ovalbumin plus Flt3 Ligand Expressing Adenovirus for Mucosal IgA Responses. PLoS ONE 2013, 8, e60453. [Google Scholar] [CrossRef] [Green Version]
- Fukuyama, Y.; Tokuhara, D.; Sekine, S.; Kataoka, K.; Markham, J.D.; Irwin, A.R.; Moon, G.H.; Tokuhara, Y.; Fujihashi, K.; Davydova, J.; et al. Notch-ligand expression by NALT dendritic cells regulates mucosal Th1- and Th2-type responses. Biochem. Biophys. Res. Commun. 2012, 418, 6–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, Y.; Fan, Q.; Hao, D.; Wu, J.; Ma, G.; Su, Z. Chitosan-based mucosal adjuvants: Sunrise on the ocean. Vaccine 2015, 33, 5997–6010. [Google Scholar] [CrossRef] [PubMed]
- Neimert-Andersson, T.; Binnmyr, J.; Enoksson, M.; Langebäck, J.; Zettergren, L.; Hällgren, A.-C.; Franzen, H.; Enoksson, S.L.; Lafolie, P.; Lindberg, A.; et al. Evaluation of safety and efficacy as an adjuvant for the chitosan-based vaccine delivery vehicle ViscoGel in a single-blind randomised Phase I/IIa clinical trial. Vaccine 2014, 32, 5967–5974. [Google Scholar] [CrossRef] [Green Version]
- Zhu, F.-C.; Meng, F.-Y.; Li, J.; Li, X.-L.; Mao, Q.-Y.; Tao, H.; Zhang, Y.-T.; Yao, X.; Chu, K.; Chen, Q.-H.; et al. Efficacy, safety, and immunology of an inactivated alum-adjuvant enterovirus 71 vaccine in children in China: A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2013, 381, 2024–2032. [Google Scholar] [CrossRef]
- O’Hagan, D.; Rappuoli, R.; De Gregorio, E.; Tsai, T.F.; Del Giudice, G. MF59 adjuvant: The best insurance against influenza strain diversity. Expert Rev. Vaccines 2011, 10, 447–462. [Google Scholar] [CrossRef]
- Patel, M.M.; Davis, W.; Beacham, L.; Spencer, S.; Campbell, A.P.; Lafond, K.; A Rolfes, M.; Levine, M.Z.; Azziz-Baumgartner, E.; Thompson, M.G.; et al. Priming with MF59 adjuvanted versus nonadjuvanted seasonal influenza vaccines in children–A systematic review and a meta-analysis. Vaccine 2020, 38, 608–619. [Google Scholar] [CrossRef]
- Esposito, S.; Fling, J.; Chokephaibulkit, K.; De Bruijn, M.; Oberye, J.; Zhang, B.; Vossen, J.; Heijnen, E.; Smolenov, I. Immunogenicity and Safety of an MF59-adjuvanted Quadrivalent Seasonal Influenza Vaccine in Young Children at High Risk of Influenza-associated Complications: A Phase III, Randomized, Observer-blind, Multicenter Clinical Trial. Pediatr. Infect. Dis. J. 2020, 39, e185–e191. [Google Scholar] [CrossRef]
- Dowling, D.J.; Scott, E.A.; Scheid, A.; Bergelson, I.; Joshi, S.; Pietrasanta, C.; Brightman, S.; Sanchez-Schmitz, G.; Van Haren, S.D.; Ninković, J.; et al. Toll-like receptor 8 agonist nanoparticles mimic immunomodulating effects of the live BCG vaccine and enhance neonatal innate and adaptive immune responses. J. Allergy Clin. Immunol. 2017, 140, 1339–1350. [Google Scholar] [CrossRef] [Green Version]
- Nohmi, K.; Tokuhara, D.; Tachibana, D.; Saito, M.; Sakashita, Y.; Nakano, A.; Terada, H.; Katayama, H.; Koyama, M.; Shintaku, H. Zymosan Induces Immune Responses Comparable with Those of Adults in Monocytes, Dendritic Cells, and Monocyte-Derived Dendritic Cells from Cord Blood. J. Pediatr. 2015, 167, 155–162.e2. [Google Scholar] [CrossRef]
- Hikita, N.; Cho, Y.; Tachibana, D.; Hamazaki, T.; Koyama, M.; TokuharaKenji, D. Cell surface antigens of neonatal monocytes are selectively impaired in basal expression, but hyperresponsive to lipopolysaccharide and zymosan. J. Reprod. Immunol. 2019, 136, 102614. [Google Scholar] [CrossRef]
- Yanai, S.; Tokuhara, D.; Tachibana, D.; Saito, M.; Sakashita, Y.; Shintaku, H.; Koyama, M. Diabetic pregnancy activates the innate immune response through TLR5 or TLR1/2 on neonatal monocyte. J. Reprod. Immunol. 2016, 117, 17–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levy, O.; Zarember, K.A.; Roy, R.; Cywes, C.; Godowski, P.J.; Wessels, M.R. Selective Impairment of TLR-Mediated Innate Immunity in Human Newborns: Neonatal Blood Plasma Reduces Monocyte TNF-α Induction by Bacterial Lipopeptides, Lipopolysaccharide, and Imiquimod, but Preserves the Response to R-848. J. Immunol. 2004, 173, 4627–4634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kollmann, T.R.; Crabtree, J.; Rein-Weston, A.; Blimkie, D.; Thommai, F.; Wang, X.Y.; Lavoie, P.M.; Furlong, J.; Fortuno, E.S.; Hajjar, A.M.; et al. Neonatal Innate TLR-Mediated Responses Are Distinct from Those of Adults. J. Immunol. 2009, 183, 7150–7160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Wit, D.; Tonon, S.; Olislagers, V.; Goriely, S.; Boutriaux, M.; Goldman, M.; Willems, F. Impaired responses to toll-like receptor 4 and toll-like receptor 3 ligands in human cord blood. J. Autoimmun. 2003, 21, 277–281. [Google Scholar] [CrossRef]
- Iwasaki, A.; Medzhitov, R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 2004, 5, 987–995. [Google Scholar] [CrossRef]
- Marodi, L. Neonatal Innate Immunity to Infectious Agents. Infect. Immun. 2006, 74, 1999–2006. [Google Scholar] [CrossRef] [Green Version]
- Levy, O. Innate immunity of the newborn: Basic mechanisms and clinical correlates. Nat. Rev. Immunol. 2007, 7, 379–390. [Google Scholar] [CrossRef]
- Medzhitov, R.; Janeway, C. Innate Immunity. New Engl. J. Med. 2000, 343, 338–344. [Google Scholar] [CrossRef]
- Rehli, M. Of mice and men: Species variations of Toll-like receptor expression. Trends Immunol. 2002, 23, 375–378. [Google Scholar] [CrossRef]
- Nahori, M.-A.; Fournié-Amazouz, E.; Que-Gewirth, N.S.; Balloy, V.; Chignard, M.; Raetz, C.R.H.; Girons, I.S.; Werts, C. Differential TLR Recognition of Leptospiral Lipid A and Lipopolysaccharide in Murine and Human Cells. J. Immunol. 2005, 175, 6022–6031. [Google Scholar] [CrossRef] [Green Version]
- Lien, E.; Means, T.K.; Heine, H.; Yoshimura, A.; Kusumoto, S.; Fukase, K.; Fenton, M.J.; Oikawa, M.; Qureshi, N.; Monks, B.; et al. Toll-like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide. J. Clin. Investig. 2000, 105, 497–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen-Nissen, E.; Smith, K.D.; Bonneau, R.; Strong, R.K.; Aderem, A. A conserved surface on Toll-like receptor 5 recognizes bacterial flagellin. J. Exp. Med. 2007, 204, 393–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.-Y.; Herman, M.; Ciancanelli, M.J.; De Diego, R.P.; Sancho-Shimizu, V.; Abel, L.; Casanova, J.-L. TLR3 immunity to infection in mice and humans. Curr. Opin. Immunol. 2013, 25, 19–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schüller, S.S.; Sadeghi, K.; Wisgrill, L.; Dangl, A.; Diesner, S.C.; Prusa, A.-R.; Klebermasz-Schrehof, K.; Greber-Platzer, S.; Neumüller, J.; Helmer, H.; et al. Preterm neonates display altered plasmacytoid dendritic cell function and morphology. J. Leukoc. Biol. 2013, 93, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Namakula, R.; De Bree, L.C.J.; Tvedt, T.H.A.; Netea, M.G.; Cose, S.; Hanevik, K. Monocytes from neonates and adults have a similar capacity to adapt their cytokine production after previous exposure to BCG and β-glucan. PLoS ONE 2020, 15, e0229287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hornsby, E.; Pfeffer, P.E.; Laranjo, N.; Cruikshank, W.; Tuzova, M.; Litonjua, A.A.; Weiss, S.T.; Carey, V.; O’Connor, G.; Hawrylowicz, C.M. Vitamin D supplementation during pregnancy: Effect on the neonatal immune system in a randomized controlled trial. J. Allergy Clin. Immunol. 2018, 141, 269–278.e1. [Google Scholar] [CrossRef] [Green Version]
- Atègbo, J.-M.; Grissa, O.; Yessoufou, A.; Hichami, A.; Dramane, K.L.; Moutairou, K.; Miled, A.; Grissa, A.; Jerbi, M.; Tabka, Z.; et al. Modulation of Adipokines and Cytokines in Gestational Diabetes and Macrosomia. J. Clin. Endocrinol. Metab. 2006, 91, 4137–4143. [Google Scholar] [CrossRef]
- Gauthier, T.W.; Drews-Botsch, C.; Falek, A.; Coles, C.; Yeligar, S.M. Maternal Alcohol Abuse and Neonatal Infection. Alcohol. Clin. Exp. Res. 2005, 29, 1035–1043. [Google Scholar] [CrossRef]
- Belderbos, M.E.; Houben, M.L.; Wilbrink, B.; Lentjes, E.; Bloemen, E.M.; Kimpen, J.L.L.; Rovers, M.; Bont, L. Cord Blood Vitamin D Deficiency Is Associated With Respiratory Syncytial Virus Bronchiolitis. Pediatrics 2011, 127, e1513–e1520. [Google Scholar] [CrossRef]
- Noakes, P.S.; Hale, J.; Thomas, R.; Lane, C.; Devadason, S.G.; Prescott, S.L. Maternal smoking is associated with impaired neonatal toll-like-receptor-mediated immune responses. Eur. Respir. J. 2006, 28, 721–729. [Google Scholar] [CrossRef] [Green Version]
- Ara, Y.; Saito, T.; Takagi, T.; Hagiwara, E.; Miyagi, Y.; Sugiyama, M.; Kawamoto, S.; Ishii, N.; Yoshida, T.; Hanashi, D.; et al. Zymosan enhances the immune response to DNA vaccine for human immunodeficiency virus type-1 through the activation of complement system. Immunology 2001, 103, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Ainai, A.; Ichinohe, T.; Tamura, S.-I.; Kurata, T.; Sata, T.; Tashiro, M.; Hasegawa, H. Zymosan enhances the mucosal adjuvant activity of poly(I:C) in a nasal influenza vaccine. J. Med Virol. 2010, 82, 476–484. [Google Scholar] [CrossRef] [PubMed]
- ElAzab, M.F.A.; Inoue, Y.; Kamei, H.; Horiuchi, H.; Furusawa, S. Zymosan A enhances humoral immune responses to soluble protein in chickens. J. Veter. Med. Sci. 2017, 79, 1335–1341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.-H.; You, S.-H.; Ko, M.-K.; Jo, H.E.; Shin, S.H.; Jo, H.; Lee, M.J.; Kim, S.-M.; Kim, B.; Lee, J.-S.; et al. Improved immune responses and safety of foot-and-mouth disease vaccine containing immunostimulating components in pigs. J. Veter. Sci. 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Azuma, M.; Hatsugai, R.; Fujimoto, Y.; Hashimoto, M.; Fukase, K.; Matsumoto, M.; Seya, T. The second and third amino acids of Pam2 lipopeptides are key for the proliferation of cytotoxic T cells. Innate Immun. 2018, 24, 323–331. [Google Scholar] [CrossRef] [Green Version]
- Halliday, A.; Turner, J.D.; Guimaraes, A.F.; A Bates, P.; Taylor, M.J. The TLR2/6 ligand PAM2CSK4 is a Th2 polarizing adjuvant in Leishmania major and Brugia malayi murine vaccine models. Parasites Vectors 2016, 9, 96. [Google Scholar] [CrossRef] [Green Version]
- Tokuhara, D. Comparison of zymosan- and MALP2-mediated innate immune response in human cord blood. manuscript in preparation.
- Dillon, S.; Agrawal, S.; Banerjee, K.; Letterio, J.; Denning, T.L.; Oswald-Richter, K.; Kasprowicz, D.J.; Kellar, K.; Pare, J.; Van Dyke, T.; et al. Yeast zymosan, a stimulus for TLR2 and dectin-1, induces regulatory antigen-presenting cells and immunological tolerance. J. Clin. Investig. 2006, 116, 916–928. [Google Scholar] [CrossRef]
- Underhill, D.M.; Ozinsky, A.; Hajjar, A.M.; Stevens, A.; Wilson, C.B.; Bassetti, M.; Aderem, A. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nat. Cell Biol. 1999, 401, 811–815. [Google Scholar] [CrossRef]
- Hoebe, K.; Georgel, P.T.; Rutschmann, S.; Du, X.; Mudd, S.; Crozat, K.; Sovath, S.; Shamel, L.; Hartung, T.; Zähringer, U.; et al. CD36 is a sensor of diacylglycerides. Nat. Cell Biol. 2005, 433, 523–527. [Google Scholar] [CrossRef]
- Kobayashi, M.; Yoshiki, R.; Sakabe, J.; Kabashima, K.; Nakamura, M.; Tokura, Y. Expression of toll-like receptor 2, NOD2 and dectin-1 and stimulatory effects of their ligands and histamine in normal human keratinocytes. Br. J. Dermatol. 2009, 160, 297–304. [Google Scholar] [CrossRef]
- Nagai, Y.; Kobayashi, T.; Motoi, Y.; Ishiguro, K.; Akashi, S.; Saitoh, S.-I.; Kusumoto, Y.; Kaisho, T.; Akira, S.; Matsumoto, M.; et al. The Radioprotective 105/MD-1 Complex Links TLR2 and TLR4/MD-2 in Antibody Response to Microbial Membranes. J. Immunol. 2005, 174, 7043–7049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dominguez, P.M.; Ardavín, C. Differentiation and function of mouse monocyte-derived dendritic cells in steady state and inflammation. Immunol. Rev. 2010, 234, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Terry, R.L.; Getts, D.R.; Deffrasnes, C.; Van Vreden, C.; Campbell, I.L.; King, N.J.C. Inflammatory monocytes and the pathogenesis of viral encephalitis. J. Neuroinflamm. 2012, 9, 270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, Z.; Da, Y.; Xue, Z.; Zhang, K.; Zhuang, H.; Peng, M.; Li, Y.; Li, W.; Simard, A.; Hao, J.; et al. Vorinostat, a histone deacetylase inhibitor, suppresses dendritic cell function and ameliorates experimental autoimmune encephalomyelitis. Exp. Neurol. 2013, 241, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.-J.; Brady, J.L.; Ryg-Cornejo, V.; Hansen, D.S.; Vremec, D.; Shortman, K.; Zhan, Y.; Lew, A.M. GM-CSF–Responsive Monocyte-Derived Dendritic Cells Are Pivotal in Th17 Pathogenesis. J. Immunol. 2014, 192, 2202–2209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemoine, S.; Jaron, B.; Tabka, S.; Ettreiki, C.; Deriaud, E.; Zhivaki, D.; Le Ray, C.; Launay, O.; Majlessi, L.; Tissieres, P.; et al. Dectin-1 activation unlocks IL12A expression and reveals the TH1 potency of neonatal dendritic cells. J. Allerg. Clin. Immunol. 2015, 136, 1355–1368.e15. [Google Scholar] [CrossRef] [PubMed]
- Openshaw, P.J.; Culley, F.J.; Olszewska, W. Immunopathogenesis of vaccine-enhanced RSV disease. Vaccine 2001, 20, S27–S31. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P. Men and mice: Relating their ages. Life Sci. 2016, 152, 244–248. [Google Scholar] [CrossRef]
- Sengupta, P. The Laboratory Rat: Relating Its Age with Human’s. Int. J. Prev. Med. 2013, 4, 624–630. [Google Scholar]
- Colson, V.; Orgeur, P.; Foury, A.; Mormède, P. Consequences of weaning piglets at 21 and 28 days on growth, behaviour and hormonal responses. Appl. Anim. Behav. Sci. 2006, 98, 70–88. [Google Scholar] [CrossRef]
- Thompson, E.A.; Loré, K. Non-human primates as a model for understanding the mechanism of action of toll-like receptor-based vaccine adjuvants. Curr. Opin. Immunol. 2017, 47, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Reitsema, L.J.; Partrick, K.A.; Muir, A.B. Inter-individual variation in weaning among rhesus macaques (Macaca mulatta): Serum stable isotope indicators of suckling duration and lactation. Am. J. Primatol. 2015, 78, 1113–1134. [Google Scholar] [CrossRef] [PubMed]
- De Waal, L.; Power, U.F.; Yüksel, S.; Van Amerongen, G.; Nguyen, T.N.; Niesters, H.G.M.; De Swart, R.L.; Osterhaus, A.D.M.E. Evaluation of BBG2Na in infant macaques: Specific immune responses after vaccination and RSV challenge. Vaccine 2004, 22, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.E.; Lager, K.; Splichal, I.; Francis, D.; Kacskovics, I.; Sinkora, M.; Wertz, N.; Sun, J.; Zhao, Y.; Brown, W.; et al. The piglet as a model for B cell and immune system development. Vet. Immunol. Immunopathol. 2009, 128, 147–170. [Google Scholar] [CrossRef] [Green Version]
- Trivedi, H.N.; HayGlass, K.T.; Gangur, V.; Allardice, J.G.; E Embree, J.; A Plummer, F. Analysis of Neonatal T Cell and Antigen Presenting Cell Functions. Hum. Immunol. 1997, 57, 69–79. [Google Scholar] [CrossRef]
- Barman, N.N.; Bianchi, A.T.J.; Zwart, R.J.; Pabst, R.; Rothkötter, H.J. Jejunal and ileal Peyer’s patches in pigs differ in their postnatal development. Brain Struct. Funct. 1996, 195, 41–50. [Google Scholar] [CrossRef]
- Mestas, J.; Hughes, C.C.W. Of mice and not men: Differences between mouse and human immunology. J. Immunol. 2004, 172, 2731–2738. [Google Scholar] [CrossRef] [Green Version]
- Biswas, B.; Narmadha, G.; Choudhary, M.; French, F.S.; Hall, S.H.; Yenugu, S. ORIGINAL ARTICLE: Identification of Toll-Like Receptors in the Rat (Rattus norvegicus): Messenger RNA Expression in the Male Reproductive Tract Under Conditions of Androgen Variation. Am. J. Reprod. Immunol. 2009, 62, 243–252. [Google Scholar] [CrossRef]
- Uddin, M.J.; Kaewmala, K.; Tesfaye, D.; Tholen, E.; Looft, C.; Hoelker, M.; Schellander, K.; Cinar, M.U. Expression patterns of porcine Toll-like receptors family set of genes (TLR1-10) in gut-associated lymphoid tissues alter with age. Res. Vet. Sci. 2013, 95, 92–102. [Google Scholar] [CrossRef]
- Shao, L.; Fischer, D.D.; Kandasamy, S.; Saif, L.; Vlasova, A.N. Tissue-specific mRNA expression profiles of porcine Toll-like receptors at different ages in germ-free and conventional pigs. Vet. Immunol. Immunopathol. 2016, 171, 7–16. [Google Scholar] [CrossRef] [Green Version]
- Werling, D.; Jann, O.C.; Offord, V.; Glass, E.J.; Coffey, T.J. Variation matters: TLR structure and species-specific pathogen recognition. Trends Immunol. 2009, 30, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Vaure, C.; Liu, Y. A Comparative Review of Toll-Like Receptor 4 Expression and Functionality in Different Animal Species. Front. Immunol. 2014, 5, 316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elahi, S.; Buchanan, R.M.; Babiuk, L.A.; Gerdts, V. Maternal Immunity Provides Protection against Pertussis in Newborn Piglets. Infect. Immun. 2006, 74, 2619–2627. [Google Scholar] [CrossRef] [Green Version]
- Hurley, W.L.; Theil, P.K. Perspectives on Immunoglobulins in Colostrum and Milk. Nutrition 2011, 3, 442–474. [Google Scholar] [CrossRef] [PubMed]

| Major similarities | Overall reduced expressions of surface molecules (e.g., MHC class antigens, CD80, CD86 and/or CD40) and low induction of cytokines (e.g., IL−6 and IL−12) on APCs [40,42,69,70,115]. Reduced induction of IFNγ on T cells [37,116] |
| Immaturity of immune organs (e.g., PPs and MLNs) [26,27,28,115,117] | |
| Major differences | Species-specific TLR expressions [118,119,120,121] (e.g., human, TLRs 1–10; mouse and rat, TLRs 1–9 and 11–13; piglet, TLRs 1–10) |
| Species-specific TLR responses [122,123] (e.g., LPS sensitivity, human > piglet > mouse and rat) | |
| Maternal antibodies [115,124,125] (e.g., human, placentally transferred serum IgG Ab and breast milk IgA Ab; mouse and rat, breast milk IgG and IgA Abs, and placentally transferred serum IgG Ab; piglet, breast milk IgG and IgA Abs) | |
| Weaning period [109,110,111] (e.g., human, 6 months; mouse, rat and piglet, 3–4 weeks) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tokuhara, D.; Hikita, N. Cord Blood-Based Approach to Assess Candidate Vaccine Adjuvants Designed for Neonates and Infants. Vaccines 2021, 9, 95. https://doi.org/10.3390/vaccines9020095
Tokuhara D, Hikita N. Cord Blood-Based Approach to Assess Candidate Vaccine Adjuvants Designed for Neonates and Infants. Vaccines. 2021; 9(2):95. https://doi.org/10.3390/vaccines9020095
Chicago/Turabian StyleTokuhara, Daisuke, and Norikatsu Hikita. 2021. "Cord Blood-Based Approach to Assess Candidate Vaccine Adjuvants Designed for Neonates and Infants" Vaccines 9, no. 2: 95. https://doi.org/10.3390/vaccines9020095







