Piscine Orthoreovirus (PRV)-3, but Not PRV-2, Cross-Protects against PRV-1 and Heart and Skeletal Muscle Inflammation in Atlantic Salmon
Abstract
:1. Introduction
2. Materials and Methods
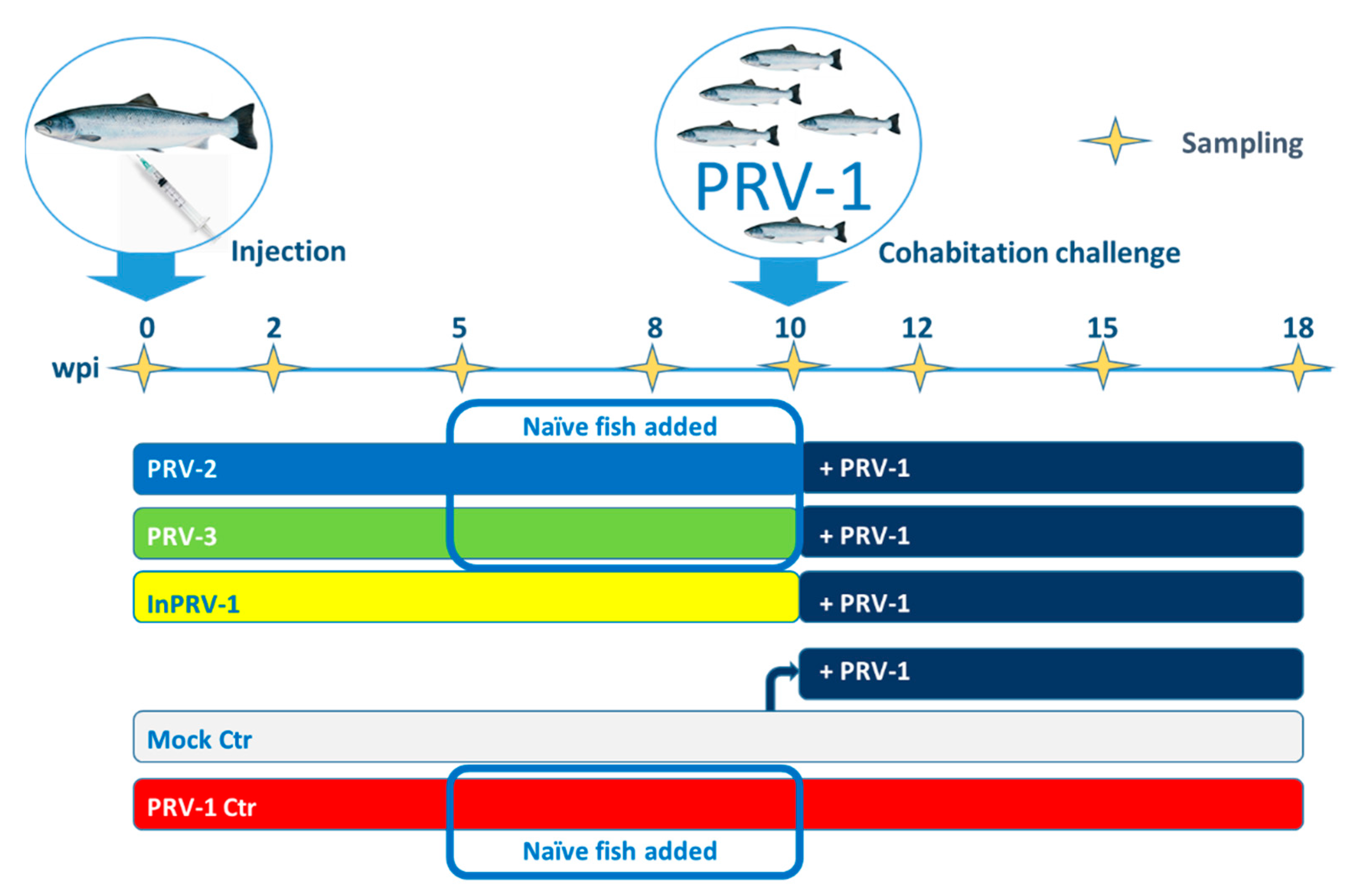
2.1. Experimental Trial and Sampling
2.2. RNA Preparation and RT-qPCR for Virus and Host Response Gene Analyses
2.3. Bead-Based Immunoassay
2.4. Histopathology
2.5. Statistical Analysis
3. Results
3.1. PRV Immunization Trial
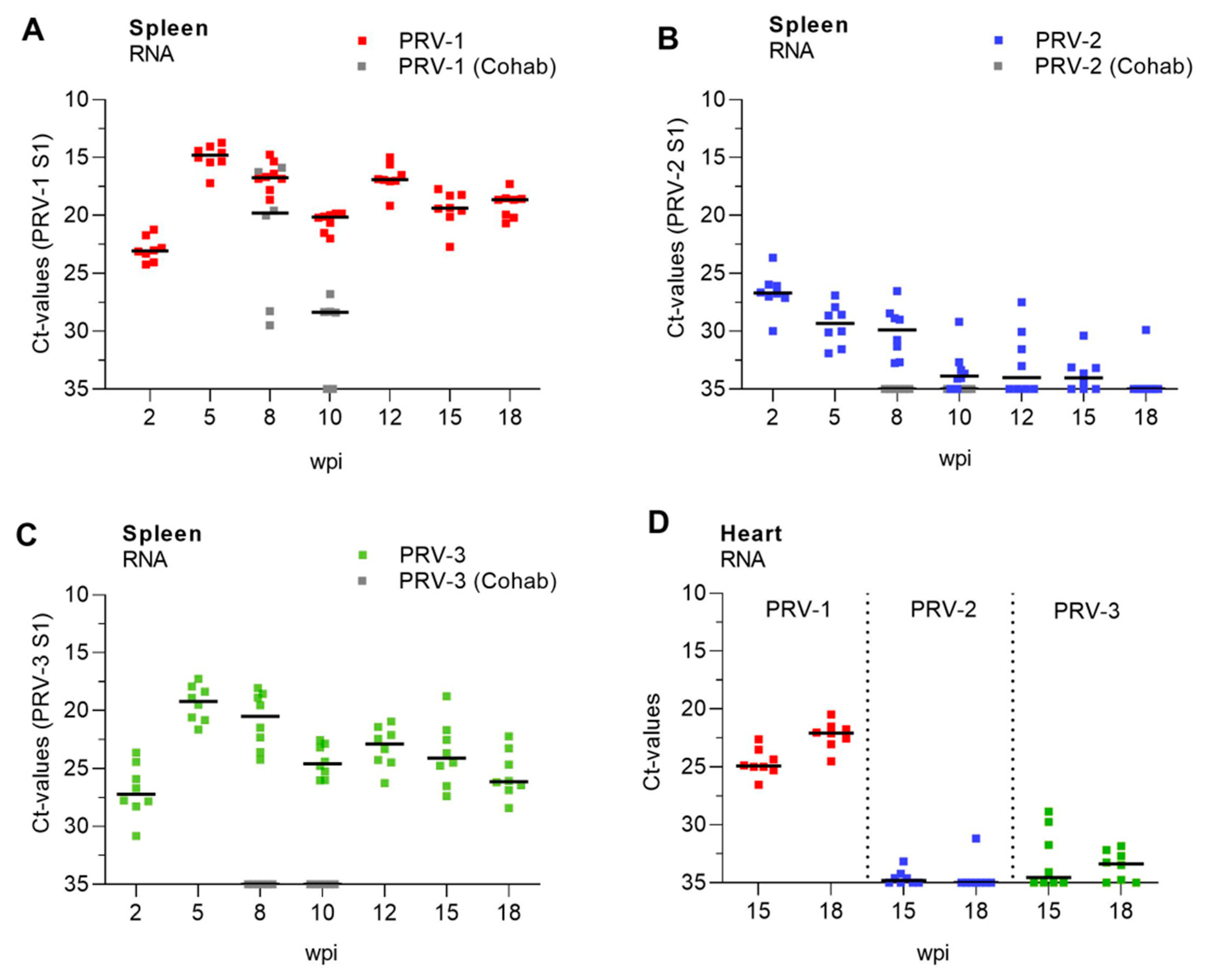
3.2. Replication and Transmission of PRV Genotypes in Atlantic Salmon
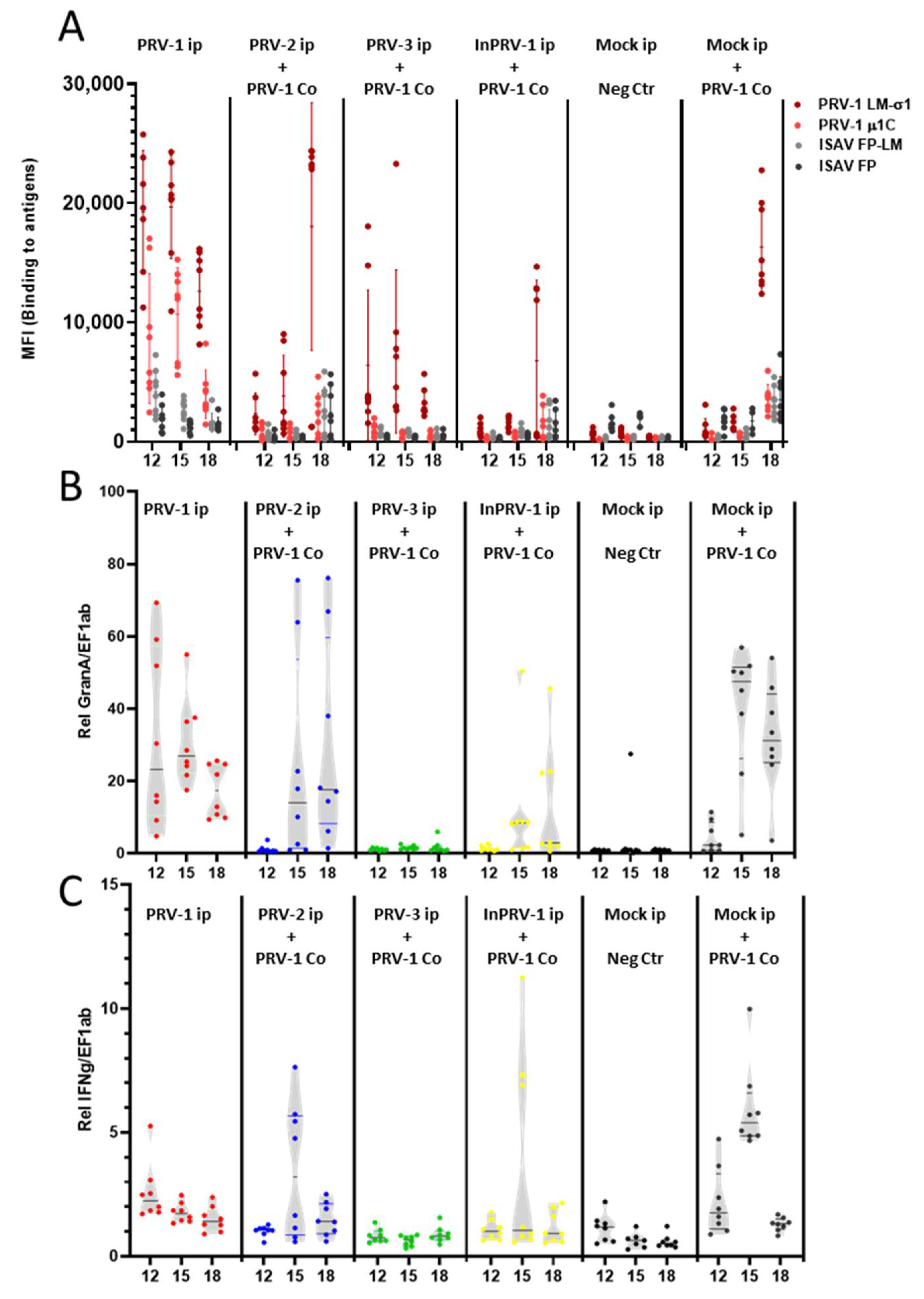
3.3. Production of Anti-PRV Antibodies
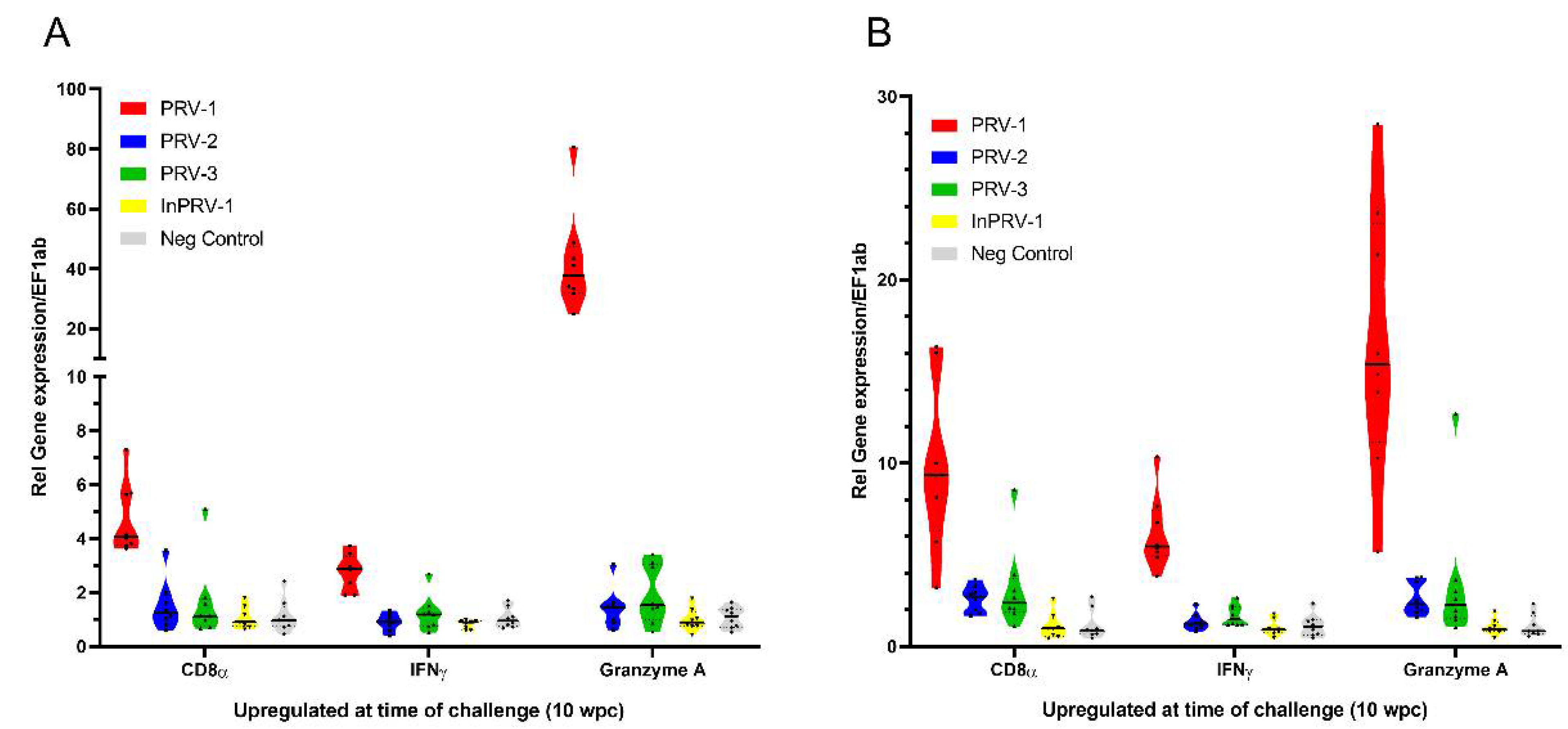
3.4. Innate and Cellular Immune Responses
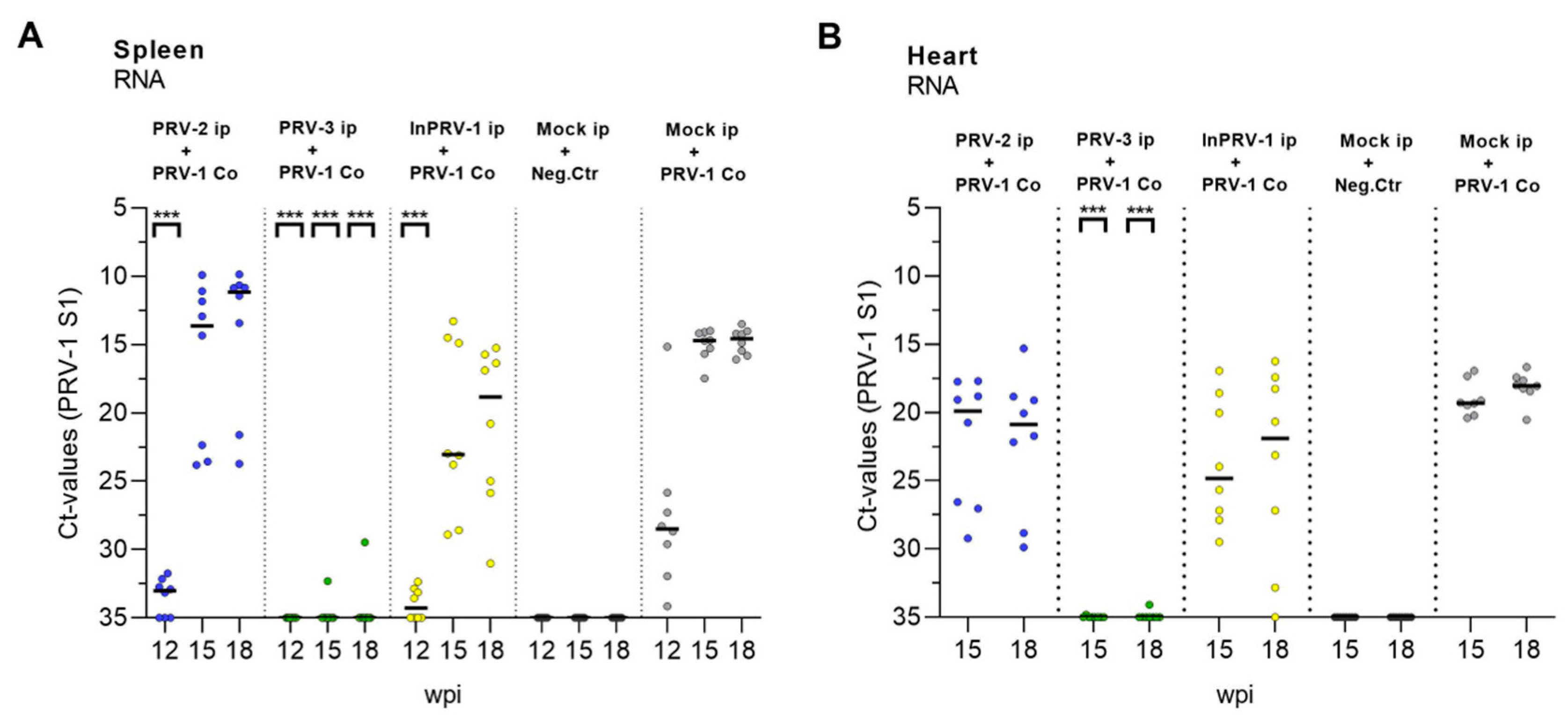
3.5. Protection from PRV Infection and HSMI
3.6. Immune Responses after Challenge of Immunized Salmon
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Byrne, K.A.; Loving, C.L.; McGill, J.L. Innate Immunomodulation in Food Animals: Evidence for Trained Immunity? Front. Immunol. 2020, 11, 1099. [Google Scholar] [CrossRef]
- Segner, H.; Sundh, H.; Buchmann, K.; Douxfils, J.; Sundell, K.S.; Mathieu, C.; Ruane, N.; Jutfelt, F.; Toften, H.; Vaughan, L. Health of farmed fish: Its relation to fish welfare and its utility as welfare indicator. Fish Physiol. Biochem. 2011, 38, 85–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, A. Progress, challenges and opportunities in fish vaccine development. Fish Shellfish Immunol. 2019, 90, 210–214. [Google Scholar] [CrossRef]
- Palacios, G.; Lovoll, M.; Tengs, T.; Hornig, M.; Hutchison, S.; Hui, J.; Kongtorp, R.-T.; Savji, N.; Bussetti, A.V.; Solovyov, A.; et al. Heart and Skeletal Muscle Inflammation of Farmed Salmon Is Associated with Infection with a Novel Reovirus. PLoS ONE 2010, 5, e11487. [Google Scholar] [CrossRef]
- Wessel, Ø.; Braaen, S.; Alarcón, M.; Haatveit, H.; Roos, N.; Markussen, T.; Tengs, T.; Dahle, M.K.; Rimstad, E. Infection with purified Piscine orthoreovirus demonstrates a causal relationship with heart and skeletal muscle inflammation in Atlantic salmon. PLoS ONE 2017, 12, e0183781. [Google Scholar] [CrossRef] [Green Version]
- Markussen, T.; Dahle, M.K.; Tengs, T.; Lovoll, M.; Finstad, O.W.; Wiik-Nielsen, C.R.; Grove, S.; Lauksund, S.; Robertsen, B.; Rimstad, E. Sequence analysis of the genome of piscine orthoreovirus (PRV) associated with heart and skeletal muscle inflammation (HSMI) in Atlantic salmon (Salmo salar). PLoS ONE 2013, 8, e70075. [Google Scholar] [CrossRef]
- Dhamotharan, K.; Tengs, T.; Wessel, Ø.; Braaen, S.; Nyman, I.B.; Hansen, E.F.; Christiansen, D.H.; Dahle, M.K.; Rimstad, E.; Markussen, T. Evolution of the Piscine orthoreovirus Genome Linked to Emergence of Heart and Skeletal Muscle Inflammation in Farmed Atlantic Salmon (Salmo salar). Viruses 2019, 11, 465. [Google Scholar] [CrossRef] [Green Version]
- Løvoll, M.; Alarcón, M.; Jensen, B.B.; Taksdal, T.; Kristoffersen, A.B.; Tengs, T. Quantification of piscine reovirus (PRV) at different stages of Atlantic salmon Salmo salar production. Dis. Aquat. Org. 2012, 99, 7–12. [Google Scholar] [CrossRef] [Green Version]
- Garseth, A.H.; Fritsvold, C.; Opheim, M.; Skjerve, E.; Biering, E. Piscine reovirus (PRV) in wild Atlantic salmon, Salmo salar L., and sea-trout, Salmo trutta L., in Norway. J. Fish Dis. 2013, 36, 483–493. [Google Scholar] [CrossRef]
- Madhun, A.S.; Isachsen, C.H.; Omdal, L.M.; Einen, A.C.B.; Maehle, S.; Wennevik, V.; Niemela, E.; Svasand, T.; Karlsbakk, E. Prevalence of piscine orthoreovirus and salmonid alphavirus in sea-caught returning adult Atlantic salmon (Salmo salar L.) in northern Norway. J. Fish Dis. 2018, 41, 797–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kongtorp, R.T.; Taksdal, T.; Lyngøy, A. Pathology of heart and skeletal muscle inflammation (HSMI) in farmed Atlantic salmon Salmo salar. Dis. Aquat. Org. 2004, 59, 217–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferguson, H.W.; Kongtorp, R.T.; Taksdal, T.; Graham, D.; Falk, K. An outbreak of disease resembling heart and skeletal muscle inflammation in Scottish farmed salmon, Salmo salar L., with observations on myocardial regeneration. J. Fish Dis. 2005, 28, 119–123. [Google Scholar] [CrossRef]
- Hauge, H.; Dahle, M.; Moldal, T.; Thoen, E.; Gjevre, A.-G.; Weli, S.; Alarcón, M.; Grove, S. Piscine orthoreovirus can infect and shed through the intestine in experimentally challenged Atlantic salmon (Salmo salar L.). Vet. Res. 2016, 47, 1–12. [Google Scholar] [CrossRef]
- Wessel, O.; Olsen, C.M.; Rimstad, E.; Dahle, M.K. Piscine orthoreovirus (PRV) replicates in Atlantic salmon (Salmo salar L.) eryth-rocytes ex vivo. Vet. Res. 2015, 46, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wessel, Ø.; Hansen, E.F.; Dahle, M.K.; Alarcon, M.; Vatne, N.A.; Nyman, I.B.; Soleim, K.B.; Dhamotharan, K.; Timmerhaus, G.; Markussen, T.; et al. Piscine Orthoreovirus-1 Isolates Differ in Their Ability to Induce Heart and Skeletal Muscle Inflammation in Atlantic Salmon (Salmo salar). Pathogens 2020, 9, 1050. [Google Scholar] [CrossRef]
- Mikalsen, A.B.; Haugland, O.; Rode, M.; Solbakk, I.T.; Evensen, O. Atlantic Salmon Reovirus Infection Causes a CD8 T Cell Myocarditis in Atlantic Salmon (Salmo salar L.). PLoS ONE 2012, 7, e37269. [Google Scholar] [CrossRef]
- Johansen, L.-H.; Thim, H.L.; Jørgensen, S.M.; Afanasyev, S.; Strandskog, G.; Taksdal, T.; Fremmerlid, K.; McLoughlin, M.; Jørgensen, J.B.; Krasnov, A. Comparison of transcriptomic responses to pancreas disease (PD) and heart and skeletal muscle inflammation (HSMI) in heart of Atlantic salmon (Salmo salar L). Fish Shellfish Immunol. 2015, 46, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Kibenge, M.J.T.; Wang, Y.; Gayeski, N.; Morton, A.; Beardslee, K.; McMillan, B.; Kibenge, F.S.B. Piscine orthoreovirus sequences in escaped farmed Atlantic salmon in Washington and British Columbia. Virol. J. 2019, 16, 41. [Google Scholar] [CrossRef] [Green Version]
- Bjørgen, H.; Wessel, Ø.; Fjelldal, P.G.; Hansen, T.; Sveier, H.; Sæbø, H.R.; Enger, K.B.; Monsen, E.; Kvellestad, A.; Rimstad, E.; et al. Piscine orthoreovirus (PRV) in red and melanised foci in white muscle of Atlantic salmon (Salmo salar). Vet. Res. 2015, 46, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Krasnov, A.; Moghadam, H.; Larsson, T.; Afanasyev, S.; Morkore, T. Gene expression profiling in melanised sites of Atlantic salmon fillets. Fish Shellfish Immunol. 2016, 55, 56–63. [Google Scholar] [CrossRef]
- Di Cicco, E.; Ferguson, H.W.; Kaukinen, K.H.; Schultze, A.D.; Li, S.; Tabata, A.; Günther, O.P.; Mordecai, G.; Suttle, C.A.; Miller, K.M. The same strain of Piscine orthoreovirus (PRV-1) is involved in the development of different, but related, diseases in Atlantic and Pacific Salmon in British Columbia. FACETS 2018, 3. [Google Scholar] [CrossRef] [Green Version]
- Polinski, M.P.; Marty, G.D.; Snyman, H.N.; Garver, K.A. Piscine orthoreovirus demonstrates high infectivity but low virulence in Atlantic salmon of Pacific Canada. Sci. Rep. 2019, 9, 3297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garver, K.A.; Johnson, S.C.; Polinski, M.P.; Bradshaw, J.C.; Marty, G.D.; Snyman, H.N.; Morrison, D.B.; Richard, J. Piscine Orthoreovirus from Western North America Is Transmissible to Atlantic Salmon and Sockeye Salmon but Fails to Cause Heart and Skeletal Muscle Inflammation. PLoS ONE 2016, 11, e0146229. [Google Scholar] [CrossRef] [Green Version]
- Di Cicco, E.; Ferguson, H.W.; Schulze, A.D.; Kaukinen, K.H.; Li, S.; Vanderstichel, R.; Wessel, O.; Rimstad, E.; Gardner, I.A.; Hammell, K.L.; et al. Heart and skeletal muscle inflammation (HSMI) disease diagnosed on a British Columbia salmon farm through a longitudinal farm study. PLoS ONE 2017, 12, e0171471. [Google Scholar] [CrossRef] [Green Version]
- Purcell, M.K.; Powers, R.L.; Taksdal, T.; McKenney, D.; Conway, C.M.; Elliott, D.G.; Polinski, M.; Garver, K.; Winton, J. Consequences of Piscine orthoreovirus genotype 1 (PRV-1) infections in Chinook salmon (Oncorhynchus tshawytscha), coho salmon (O. kisutch) and rainbow trout (O. mykiss). J. Fish Dis. 2020, 43, 719–728. [Google Scholar] [CrossRef]
- Takano, T.; Nawata, A.; Sakai, T.; Matsuyama, T.; Ito, T.; Kurita, J.; Terashima, S.; Yasuike, M.; Nakamura, Y.; Fujiwara, A.; et al. Full-Genome Sequencing and Confirmation of the Causative Agent of Erythrocytic Inclusion Body Syndrome in Coho Salmon Identifies a New Type of Piscine Orthoreovirus. PLoS ONE 2016, 11, e0165424. [Google Scholar] [CrossRef] [Green Version]
- Garseth, Å.H.; Moldal, T.; Gåsnes, S.K.; Hjortaas, M.J.; Sollien, V.P.; Gjevre, A.-G. Piscine orthoreovirus-3 is prevalent in wild seatrout (Salmo trutta L.) in Norway. J. Fish Dis. 2019, 42, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Olsen, A.B.; Hjortaas, M.; Tengs, T.; Hellberg, H.; Johansen, R. First Description of a New Disease in Rainbow Trout (Oncorhynchus mykiss (Walbaum)) Similar to Heart and Skeletal Muscle Inflammation (HSMI) and Detection of a Gene Sequence Related to Piscine Orthoreovirus (PRV). PLoS ONE 2015, 10, e0131638. [Google Scholar] [CrossRef]
- Hauge, H.; Vendramin, N.; Taksdal, T.; Olsen, A.B.; Wessel, O.; Mikkelsen, S.S.; Alencar, A.L.F.; Olesen, N.J.; Dahle, M.K. Infection ex-periments with novel Piscine orthoreovirus from rainbow trout (Oncorhynchus mykiss) in salmonids. PLoS ONE 2017, 12, e0180293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vendramin, N.; Kannimuthu, D.; Olsen, A.B.; Cuenca, A.; Teige, L.H.; Wessel, Ø.; Iburg, T.M.; Dahle, M.K.; Rimstad, E.; Olesen, N.J. Piscine orthoreovirus subtype 3 (PRV-3) causes heart inflammation in rainbow trout (Oncorhynchus mykiss). Vet. Res. 2019, 50, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhamotharan, K.; Vendramin, N.; Markussen, T.; Wessel, Ø.; Cuenca, A.; Nyman, I.B.; Olsen, A.B.; Tengs, T.; Dahle, M.K.; Rimstad, E. Molecular and Antigenic Characterization of Piscine orthoreovirus (PRV) from Rainbow Trout (Oncorhynchus mykiss). Viruses 2018, 10, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polinski, M.P.; Vendramin, N.; Cuenca, A.; Garver, K.A. Piscine orthoreovirus: Biology and distribution in farmed and wild fish. J. Fish Dis. 2020, 43, 43. [Google Scholar] [CrossRef]
- Wessel, Ø.; Haugland, Ø.; Rode, M.; Fredriksen, B.N.; Dahle, M.K.; Rimstad, E. Inactivated Piscine orthoreovirus vaccine protects against heart and skeletal muscle inflammation in Atlantic salmon. J. Fish Dis. 2018, 41, 1411–1419. [Google Scholar] [CrossRef] [PubMed]
- Pham, P.H.; Misk, E.; Papazotos, F.; Jones, G.; Polinski, M.P.; Contador, E.; Russell, S.; Garver, K.A.; Lumsden, J.S.; Bols, N.C. Screening of Fish Cell Lines for Piscine Orthoreovirus-1 (PRV-1) Amplification: Identification of the Non-Supportive PRV-1 Invitrome. Pathogens 2020, 9, 833. [Google Scholar] [CrossRef] [PubMed]
- Haatveit, H.M.; Hodneland, K.; Braaen, S.; Hansen, E.F.; Nyman, I.B.; Dahle, M.K.; Frost, P.; Rimstad, E. DNA vaccine expressing the non-structural proteins of Piscine orthoreovirus delay the kinetics of PRV infection and induces moderate protection against heart -and skeletal muscle inflammation in Atlantic salmon (Salmo salar). Vaccine 2018, 36, 7599–7608. [Google Scholar] [CrossRef] [PubMed]
- Dahle, M.K.; Wessel, Ø.; Timmerhaus, G.; Nyman, I.B.; Jørgensen, S.M.; Rimstad, E.; Krasnov, A. Transcriptome analyses of Atlantic salmon (Salmo salar L.) erythrocytes infected with piscine orthoreovirus (PRV). Fish Shellfish Immunol. 2015, 45, 780–790. [Google Scholar] [CrossRef]
- Johansen, L.-H.; Dahle, M.K.; Wessel, Ø.; Timmerhaus, G.; Løvoll, M.; Røsæg, M.; Jørgensen, S.M.; Rimstad, E.; Krasnov, A. Differences in gene expression in Atlantic salmon parr and smolt after challenge with Piscine orthoreovirus (PRV). Mol. Immunol. 2016, 73, 138–150. [Google Scholar] [CrossRef]
- Teige, L.H.; Lund, M.; Haatveit, H.M.; Rosaeg, M.V.; Wessel, O.; Dahle, M.K.; Storset, A.K. A bead based multiplex immunoassay detects Piscine orthoreovirus specific antibodies in Atlantic salmon (Salmo salar). Fish Shellfish Immunol. 2017, 63, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Teige, L.H.; Kumar, S.; Johansen, G.M.; Wessel, Ø.; Vendramin, N.; Lund, M.; Rimstad, E.; Boysen, P.; Dahle, M.K. Detection of Salmonid IgM Specific to the Piscine Orthoreovirus Outer Capsid Spike Protein Sigma 1 Using Lipid-Modified Antigens in a Bead-Based Antibody Detection Assay. Front. Immunol. 2019, 10, 2119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahle, M.K.; Jorgensen, J.B. Antiviral defense in salmonids—Mission made possible? Fish Shellfish Immunol. 2019, 87, 421–437. [Google Scholar] [CrossRef]
- Finstad, Ø.W.; Falk, K.; Løvoll, M.; Evensen, Ø.; Rimstad, E. Immunohistochemical detection of piscine reovirus (PRV) in hearts of Atlantic salmon coincide with the course of heart and skeletal muscle inflammation (HSMI). Vet. Res. 2012, 43, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finstad, O.W.; Dahle, M.K.; Lindholm, T.H.; Nyman, I.B.; Lovoll, M.; Wallace, C.; Olsen, C.M.; Storset, A.K.; Rimstad, E. Piscine orthoreo-virus (PRV) infects Atlantic salmon erythrocytes. Vet. Res. 2014, 45, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Sampedro, L.; Perdiguero, B.; Mejías-Pérez, E.; García-Arriaza, J.; Di Pilato, M.; Esteban, M. The Evolution of Poxvirus Vaccines. Viruses 2015, 7, 1726–1803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lund, M.; Dahle, M.K.; Timmerhaus, G.; Alarcon, M.; Powell, M.; Aspehaug, V.; Rimstad, E.; Jørgensen, S.M. Hypoxia tolerance and responses to hypoxic stress during heart and skeletal muscle inflammation in Atlantic salmon (Salmo salar). PLoS ONE 2017, 12, e0181109. [Google Scholar] [CrossRef] [PubMed]
- Key, T.; Read, J.; Nibert, M.L.; Duncan, R. Piscine reovirus encodes a cytotoxic, non-fusogenic, integral membrane protein and previously unrecognized virion outer-capsid proteins. J. Gen. Virol. 2013, 94, 1039–1050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wessel, O.; Nyman, I.B.; Markussen, T.; Dahle, M.K.; Rimstad, E. Piscine orthoreovirus (PRV) o3 protein binds dsRNA. Virus Res. 2015, 198, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.; Shatkin, A.J. Double-Stranded RNA-Dependent Protein Kinase (PKR) Is Regulated by Reovirus Structural Proteins. Virology 1997, 234, 364–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malik, M.S.; Bjørgen, H.; Dhamotharan, K.; Wessel, Ø.; Koppang, E.O.; Di Cicco, E.; Hansen, E.F.; Dahle, M.K.; Rimstad, E. Erythroid Progenitor Cells in Atlantic Salmon (Salmo salar) May Be Persistently and Productively Infected with Piscine Orthoreovirus (PRV). Viruses 2019, 11, 824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhamotharan, K.; Bjørgen, H.; Malik, M.S.; Nyman, I.B.; Markussen, T.; Dahle, M.K.; Koppang, E.O.; Wessel, Ø.; Rimstad, E. Dissemination of Piscine orthoreovirus-1 (PRV-1) in Atlantic Salmon (Salmo salar) during the Early and Regenerating Phases of Infection. Pathogens 2020, 9, 143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wessel, Ø.; Krasnov, A.; Timmerhaus, G.; Rimstad, E.; Dahle, M.K. Antiviral Responses and Biological Concequences of Piscine orthoreovirus Infection in Salmonid Erythrocytes. Front. Immunol. 2019, 9, 3182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veenstra, K.A.; Hodneland, K.; Fischer, S.; Takehana, K.; Belmonte, R.; Fischer, U. Cellular Immune Responses in Rainbow Trout (Onchorhynchus mykiss) Following Vaccination and Challenge Against Salmonid Alphavirus (SAV). Vaccines 2020, 8, 725. [Google Scholar] [CrossRef] [PubMed]
- McDonald, S.M.; Nelson, M.I.; Turner, P.E.; Patton, J.T. Reassortment in segmented RNA viruses: Mechanisms and outcomes. Nat. Rev. Genet. 2016, 14, 448–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malik, M.S.; Teige, L.H.; Braaen, S.; Olsen, A.B.; Nordberg, M.; Amundsen, M.M.; Dhamotharan, K.; Svenning, S.; Edholm, E.S.; Takano, T.; et al. Piscine Orthoreovirus (PRV)-3, but Not PRV-2, Cross-Protects against PRV-1 and Heart and Skeletal Muscle Inflammation in Atlantic Salmon. Vaccines 2021, 9, 230. https://doi.org/10.3390/vaccines9030230
Malik MS, Teige LH, Braaen S, Olsen AB, Nordberg M, Amundsen MM, Dhamotharan K, Svenning S, Edholm ES, Takano T, et al. Piscine Orthoreovirus (PRV)-3, but Not PRV-2, Cross-Protects against PRV-1 and Heart and Skeletal Muscle Inflammation in Atlantic Salmon. Vaccines. 2021; 9(3):230. https://doi.org/10.3390/vaccines9030230
Chicago/Turabian StyleMalik, Muhammad Salman, Lena H. Teige, Stine Braaen, Anne Berit Olsen, Monica Nordberg, Marit M. Amundsen, Kannimuthu Dhamotharan, Steingrim Svenning, Eva Stina Edholm, Tomokazu Takano, and et al. 2021. "Piscine Orthoreovirus (PRV)-3, but Not PRV-2, Cross-Protects against PRV-1 and Heart and Skeletal Muscle Inflammation in Atlantic Salmon" Vaccines 9, no. 3: 230. https://doi.org/10.3390/vaccines9030230
APA StyleMalik, M. S., Teige, L. H., Braaen, S., Olsen, A. B., Nordberg, M., Amundsen, M. M., Dhamotharan, K., Svenning, S., Edholm, E. S., Takano, T., Jørgensen, J. B., Wessel, Ø., Rimstad, E., & Dahle, M. K. (2021). Piscine Orthoreovirus (PRV)-3, but Not PRV-2, Cross-Protects against PRV-1 and Heart and Skeletal Muscle Inflammation in Atlantic Salmon. Vaccines, 9(3), 230. https://doi.org/10.3390/vaccines9030230






