Monoclonal Antibodies Targeting an Opisthorchis viverrini Extracellular Vesicle Tetraspanin Protect Hamsters against Challenge Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Preparation of Recombinant Ov-TSP-2-LEL Antigen
2.3. Mouse Immunization
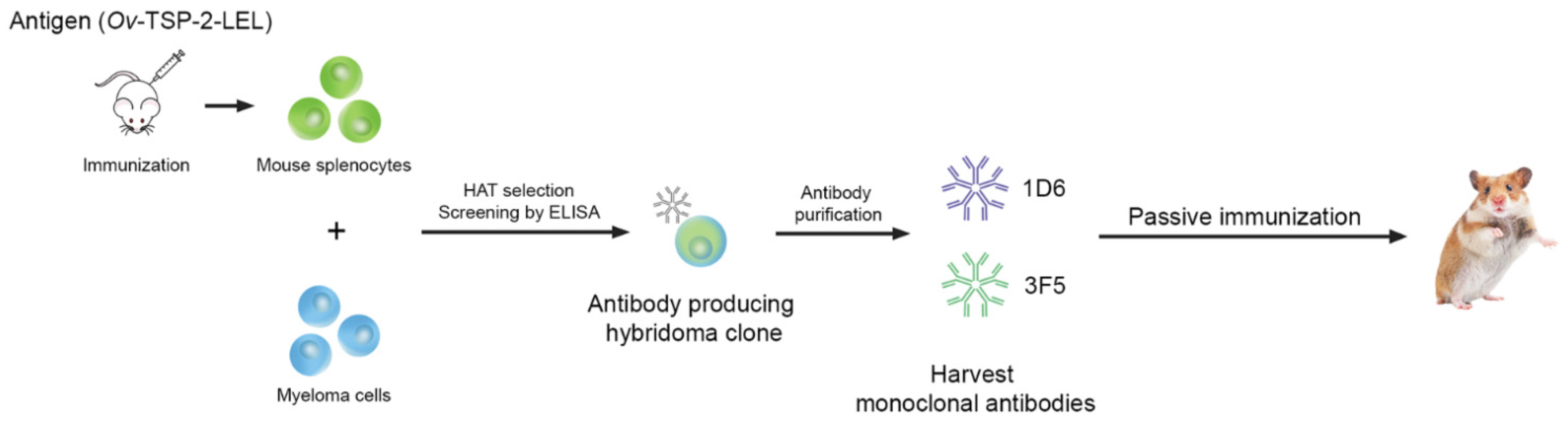
2.4. Hybridoma Generation
2.5. Expansion and Production of Monoclonal Antibodies
2.6. Purification of Monoclonal Antibodies
2.7. Screening of Hybridoma Cells by Indirect ELISA
2.8. Preparation of O. viverrini Metacercariae
2.9. Passive Immunization, Challenge, and Specimen Collection
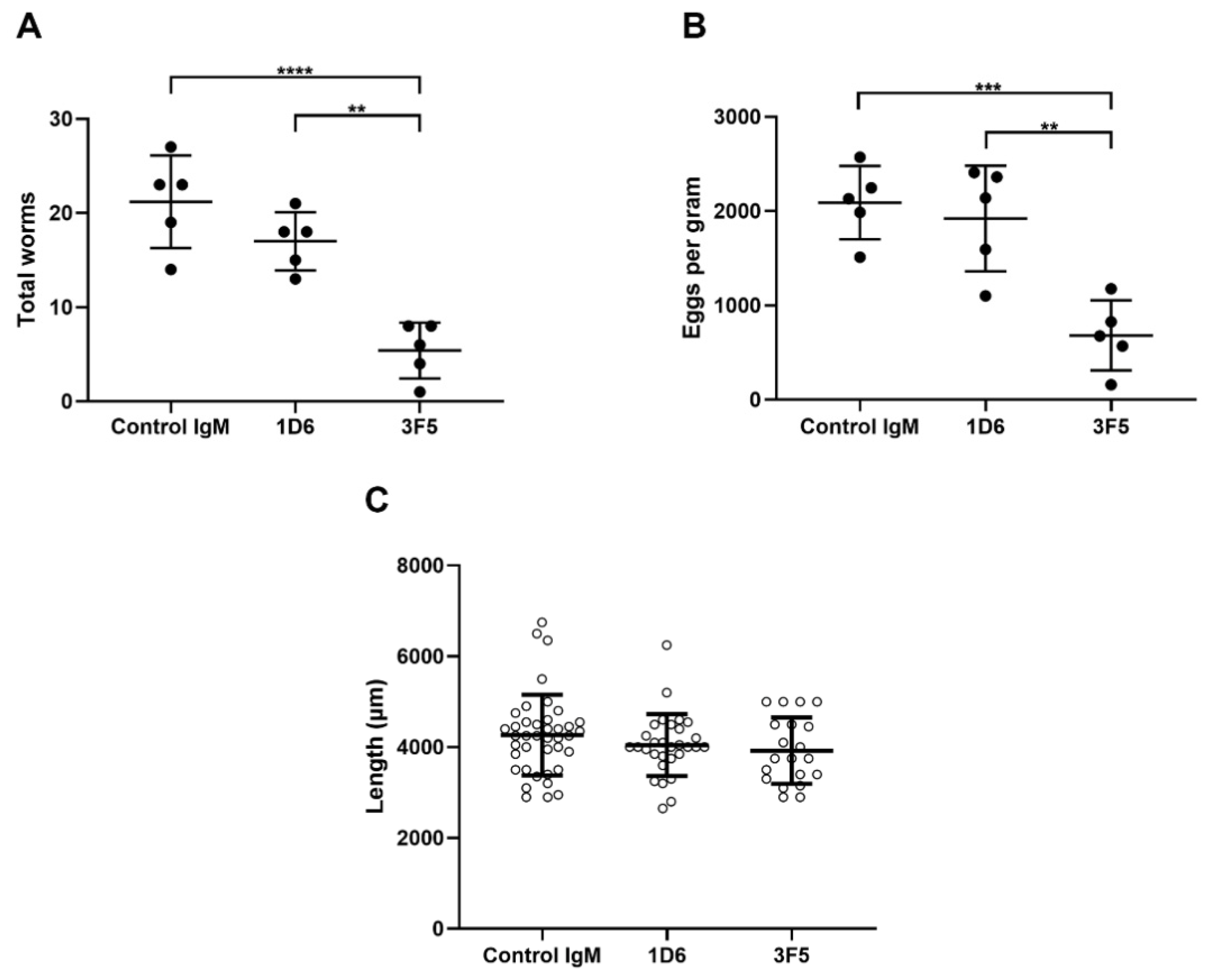
2.10. Fecal Egg Counts and Worm Recovery
2.11. Detection of Hamster Anti-Ov-TSP-2-LEL-Specific IgG, IgM, and IgA
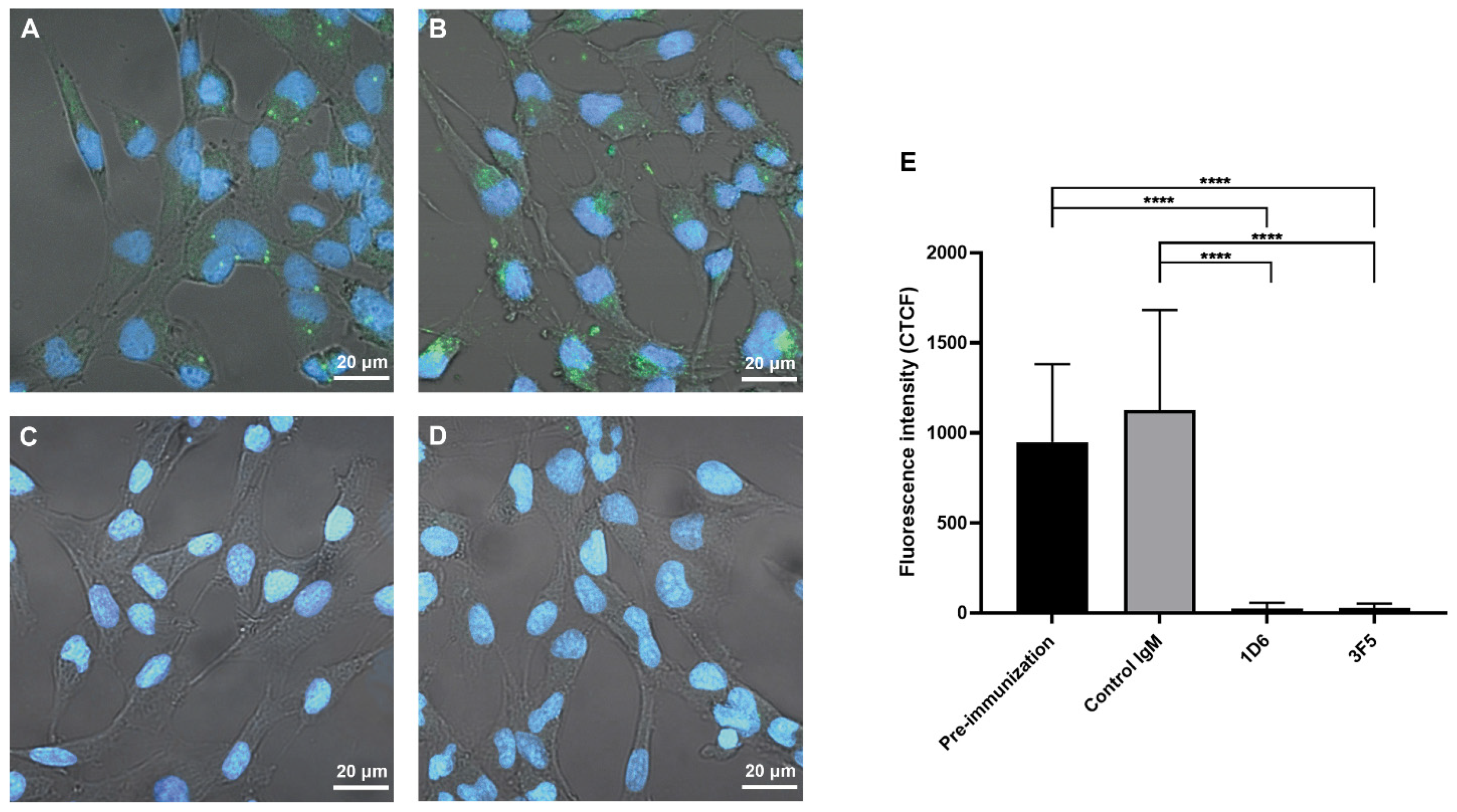
2.12. O. viverrini EV Internalization by Human Biliary Epithelial Cells
2.13. Statistical Analysis
3. Results
3.1. Generation and Characterization of Anti-Ov-TSP-2-LEL mAbs
3.2. Passive Immunization with Anti-Ov-TSP-2-LEL IgM mAbs and Detection in Serum
3.3. Passive Immunization with Anti-Ov-TSP-2-LEL IgM mAbs Induced Partial Protection Against O. viverrini Infection
3.4. Antibodies from Passively Immunized Hamsters Block Uptake of O. viverrini EVs by Human Cholangiocytes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sripa, B.; Brindley, P.J.; Mulvenna, J.; Laha, T.; Smout, M.J.; Mairiang, E.; Bethony, J.M.; Loukas, A. The tumorigenic liver fluke Opisthorchis viverrini—Multiple pathways to cancer. Trends Parasitol. 2012, 28, 395–407. [Google Scholar] [CrossRef] [Green Version]
- Sithithaworn, P.; Ziegler, A.D.; Grundy-Warr, C.; Andrews, R.H.; Petney, T.N. Changes to the life cycle of liver flukes: Dams, roads, and ponds. Lancet Infect. Dis. 2012, 12, 588. [Google Scholar] [CrossRef]
- Sripa, B.; Pairojkul, C. Cholangiocarcinoma: Lessons from Thailand. Curr. Opin. Gastroenterol. 2008, 24, 349–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaiyadet, S.; Sotillo, J.; Krueajampa, W.; Thongsen, S.; Brindley, P.J.; Sripa, B.; Loukas, A.; Laha, T. Vaccination of hamsters with Opisthorchis viverrini extracellular vesicles and vesicle-derived recombinant tetraspanins induces antibodies that block vesicle uptake by cholangiocytes and reduce parasite burden after challenge infection. PLoS Negl. Trop. Dis. 2019, 13, e0007450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phumrattanaprapin, W.; Chaiyadet, S.; Loukas, A.; Brindley, P.J.; Sotillo, J.; Laha, T. Surface display on Bacillus subtilis spores and vaccine potential of a tetraspanin from carcinogenic liver fluke, Opisthorchis viverrini. Southeast Asian J. Trop. Med. 2018, 49, 933–948. [Google Scholar]
- Phung, L.T.; Chaiyadet, S.; Hongsrichan, N.; Sotillo, J.; Dieu, H.D.T.; Tran, C.Q.; Brindley, P.J.; Loukas, A.; Laha, T. Recombinant Opisthorchis viverrini tetraspanin expressed in Pichia pastoris as a potential vaccine candidate for opisthorchiasis. Parasitol. Res. 2019, 118, 3419–3427. [Google Scholar] [CrossRef]
- Boucheix, C.; Rubinstein, E. Tetraspanins. Cell. Mol. Life Sci. CMLS 2001, 58, 1189–1205. [Google Scholar] [CrossRef] [PubMed]
- Chaiyadet, S.; Krueajampa, W.; Hipkaeo, W.; Plosan, Y.; Piratae, S.; Sotillo, J.; Smout, M.; Sripa, B.; Brindley, P.J.; Loukas, A.; et al. Suppression of mRNAs encoding CD63 family tetraspanins from the carcinogenic liver fluke Opisthorchis viverrini results in distinct tegument phenotypes. Sci. Rep. 2017, 7, 14342. [Google Scholar] [CrossRef]
- Chaiyadet, S.; Sotillo, J.; Smout, M.; Cantacessi, C.; Jones, M.K.; Johnson, M.S.; Turnbull, L.; Whitchurch, C.B.; Potriquet, J.; Laohaviroj, M.; et al. Carcinogenic Liver Fluke Secretes Extracellular Vesicles That Promote Cholangiocytes to Adopt a Tumorigenic Phenotype. J. Infect. Dis. 2015, 212, 1636–1645. [Google Scholar] [CrossRef]
- Phumrattanaprapin, W.; Chaiyadet, S.; Brindley, P.J.; Pearson, M.; Smout, M.J.; Loukas, A.; Laha, T. Orally administered Bacillus spores expressing an extracellular vesicle-derived tetraspanin protect hamsters against challenge infection with carcinogenic human liver fluke. J. Infect. Dis. 2020, 223, 1445–1455. [Google Scholar] [CrossRef]
- Kearney, B.J.; Voorhees, M.A.; Williams, P.L.; Olschner, S.P.; Rossi, C.A.; Schoepp, R.J. Corning HYPERFlask((R)) for viral amplification and production of diagnostic reagents. J. Virol. Methods 2017, 242, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Hiramoto, E.; Tsutsumi, A.; Suzuki, R.; Matsuoka, S.; Arai, S.; Kikkawa, M.; Miyazaki, T. The IgM pentamer is an asymmetric pentagon with an open groove that binds the AIM protein. Sci. Adv. 2018, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grubman, S.A.; Perrone, R.D.; Lee, D.W.; Murray, S.L.; Rogers, L.C.; Wolkoff, L.I.; Mulberg, A.E.; Cherington, V.; Jefferson, D.M. Regulation of intracellular pH by immortalized human intrahepatic biliary epithelial cell lines. Am. J. Physiol. 1994, 266, G1060–G1070. [Google Scholar] [CrossRef] [PubMed]
- Kaplon, H.; Reichert, J.M. Antibodies to watch in 2019. mAbs 2019, 11, 219–238. [Google Scholar] [CrossRef]
- Lu, R.M.; Hwang, Y.C.; Liu, I.J.; Lee, C.C.; Tsai, H.Z.; Li, H.J.; Wu, H.C. Development of therapeutic antibodies for the treatment of diseases. J. Biomed. Sci. 2020, 27, 1. [Google Scholar] [CrossRef]
- Marston, H.D.; Paules, C.I.; Fauci, A.S. Monoclonal antibodies for emerging infectious diseases—Borrowing from history. N. Engl. J. Med. 2018, 378, 1469–1472. [Google Scholar] [CrossRef]
- Wagner, E.K.; Maynard, J.A. Engineering therapeutic antibodies to combat infectious diseases. Curr. Opin. Chem. Eng. 2018, 19, 131–141. [Google Scholar] [CrossRef]
- Forthal, D.N. Functions of antibodies. Microbiol. Spectr. 2014, 2, 2–4. [Google Scholar] [CrossRef] [Green Version]
- Brigandi, R.A.; Rotman, H.L.; Yutanawiboonchai, W.; Leon, O.; Nolan, T.J.; Schad, G.A.; Abraham, D. Strongyloides stercoralis: Role of antibody and complement in immunity to the third stage of larvae in BALB/cByJ mice. Exp. Parasitol. 1996, 82, 279–289. [Google Scholar] [CrossRef]
- Kopf, M.; Brombacher, F.; Hodgkin, P.D.; Ramsay, A.J.; Milbourne, E.A.; Dai, W.J.; Ovington, K.S.; Behm, C.A.; Kohler, G.; Young, I.G.; et al. IL-5-deficient mice have a developmental defect in CD5+ B-1 cells and lack eosinophilia but have normal antibody and cytotoxic T cell responses. Immunity 1996, 4, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Herbert, D.R.; Lee, J.J.; Lee, N.A.; Nolan, T.J.; Schad, G.A.; Abraham, D. Role of IL-5 in innate and adaptive immunity to larval Strongyloides stercoralis in mice. J. Immunol. 2000, 165, 4544–4551. [Google Scholar] [CrossRef] [Green Version]
- Parab, P.B.; Rajasekariah, G.R.; Chandrashekar, R.; Alkan, S.S.; Braun, D.G.; Subrahmanyam, D. Characterization of a monoclonal antibody against infective larvae of Brugia malayi. Immunology 1988, 64, 169–174. [Google Scholar] [PubMed]
- Xu, C.B.; Verwaerde, C.; Grzych, J.M.; Fontaine, J.; Capron, A. A monoclonal antibody blocking the Schistosoma mansoni 28-kDa glutathione S-transferase activity reduces female worm fecundity and egg viability. Eur. J. Immunol. 1991, 21, 1801–1807. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.K.; Wu, G.L.; Zhao, W.X. Production and characterization of a murine protective monoclonal antibody against Schistosoma japonicum schistosomula. J. Parasitol. Parasit. Dis. 1992, 10, 22–25. [Google Scholar]
- Marcet, R.; Diaz, A.; Arteaga, E.; Finlay, C.M.; Sarracent, J. Passive protection against fasciolosis in mice by immunization with a monoclonal antibody (ES-78 MoAb). Parasite Immunol. 2002, 24, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Amornpunt, S.; Sarasombath, S.; Sirisinha, S. Production and characterization of monoclonal antibodies against the excretory-secretory antigen of the liver fluke (Opisthorchis viverrini). Int. J. Parasitol. 1991, 21, 421–428. [Google Scholar] [CrossRef]
- Arimatsu, Y.; Teimoori, S.; Surapaitoon, A.; Sripa, B. Production and characterization of monoclonal antibodies against highly immunogenic Opisthorchis viverrini proteins and development of coproantigen detection. Mol. Biochem. Parasitol. 2020, 240, 111323. [Google Scholar] [CrossRef]
- Sirisinha, S.; Chawengkirttikul, R.; Haswell-Elkins, M.R.; Elkins, D.B.; Kaewkes, S.; Sithithaworn, P. Evaluation of a monoclonal antibody-based enzyme linked immunosorbent assay for the diagnosis of Opisthorchis viverrini infection in an endemic area. Am. J. Trop Med. Hyg. 1995, 52, 521–524. [Google Scholar] [CrossRef]
- Worasith, C.; Wangboon, C.; Duenngai, K.; Kiatsopit, N.; Kopolrat, K.; Techasen, A.; Sithithaworn, J.; Khuntikeo, N.; Loilome, W.; Namwat, N.; et al. Comparing the performance of urine and copro-antigen detection in evaluating Opisthorchis viverrini infection in communities with different transmission levels in Northeast Thailand. PLoS Negl. Trop. Dis. 2019, 13, e0007186. [Google Scholar] [CrossRef]
- Sirisinha, S.; Rattanasiriwilai, W.; Puengtomwatanakul, S.; Sobhon, P.; Saitongdee, P.; Koonchornboon, T. Complement-mediated killing of Opisthorchis viverrini via activation of the alternative pathway. Int. J. Parasitol. 1986, 16, 341–346. [Google Scholar] [CrossRef]
- Meningher, T.; Lerman, G.; Regev-Rudzki, N.; Gold, D.; Ben-Dov, I.Z.; Sidi, Y.; Avni, D.; Schwartz, E. Schistosomal MicroRNAs isolated from extracellular vesicles in sera of infected patients: A new tool for diagnosis and follow-up of human schistosomiasis. J. Infect. Dis. 2017, 215, 378–386. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phumrattanaprapin, W.; Pearson, M.; Pickering, D.; Tedla, B.; Smout, M.; Chaiyadet, S.; Brindley, P.J.; Loukas, A.; Laha, T. Monoclonal Antibodies Targeting an Opisthorchis viverrini Extracellular Vesicle Tetraspanin Protect Hamsters against Challenge Infection. Vaccines 2021, 9, 740. https://doi.org/10.3390/vaccines9070740
Phumrattanaprapin W, Pearson M, Pickering D, Tedla B, Smout M, Chaiyadet S, Brindley PJ, Loukas A, Laha T. Monoclonal Antibodies Targeting an Opisthorchis viverrini Extracellular Vesicle Tetraspanin Protect Hamsters against Challenge Infection. Vaccines. 2021; 9(7):740. https://doi.org/10.3390/vaccines9070740
Chicago/Turabian StylePhumrattanaprapin, Wuttipong, Mark Pearson, Darren Pickering, Bemnet Tedla, Michael Smout, Sujittra Chaiyadet, Paul J. Brindley, Alex Loukas, and Thewarach Laha. 2021. "Monoclonal Antibodies Targeting an Opisthorchis viverrini Extracellular Vesicle Tetraspanin Protect Hamsters against Challenge Infection" Vaccines 9, no. 7: 740. https://doi.org/10.3390/vaccines9070740
APA StylePhumrattanaprapin, W., Pearson, M., Pickering, D., Tedla, B., Smout, M., Chaiyadet, S., Brindley, P. J., Loukas, A., & Laha, T. (2021). Monoclonal Antibodies Targeting an Opisthorchis viverrini Extracellular Vesicle Tetraspanin Protect Hamsters against Challenge Infection. Vaccines, 9(7), 740. https://doi.org/10.3390/vaccines9070740







