Comparative Immunogenicity of BNT162b2 mRNA Vaccine with Natural SARS-CoV-2 Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Vaccination for HCWs
2.2. Natural Infection Group
2.3. Serological Tests
2.4. Statistical Analysis
3. Results
3.1. Vaccinated HCWs
3.2. Natural Infection
3.3. Comparison of Anti-RBD Levels in Vaccinated HCWs and in Individuals with Natural Infection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sahin, U.; Muik, A.; Vogler, I.; Derhovanessian, E.; Kranz, L.M.; Vormehr, M.; Quandt, J.; Bidmon, N.; Ulges, A.; Baum, A.; et al. BNT162b2 induces SARS-CoV-2-neutralising antibodies and poly-specific T cells in humans. Nature 2021, 595, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Dagan, N.; Barda, N.; Kepten, E.; Miron, O.; Perchik, S.; Katz, M.A.; Hernan, M.A.; Lipsitch, M.; Reis, B.; Balicer, R.D. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N. Engl. J. Med. 2021, 384, 1412–1423. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef]
- Wajnberg, A.; Amanat, F.; Firpo, A.; Altman, D.R.; Bailey, M.J.; Mansour, M.; McMahon, M.; Meade, P.; Mendu, D.R.; Muellers, K.; et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science 2020, 370, 1227–1230. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.; Wagstaffe, H.R.; Gilmour, K.C.; Mai, A.L.; Lewis, J.; Hunt, A.; Sirr, J.; Bengt, C.; Grandjean, L.; Goldblatt, D. Evaluation of a novel multiplexed assay for determining IgG levels and functional activity to SARS-CoV-2. J. Clin. Virol. 2020, 130, 104572. [Google Scholar] [CrossRef]
- Perkmann, T.; Perkmann-Nagele, N.; Koller, T.; Mucher, P.; Radakovics, A.; Marculescu, R.; Wolzt, M.; Wagner, O.F.; Binder, C.J.; Haslacher, H. Anti-Spike protein assays to determine post-vaccination antibody levels: A head-to-head comparison of five quantitative assays. medRxiv 2021, preprint. [Google Scholar] [CrossRef]
- Irsara, C.; Egger, A.E.; Prokop, W.; Nairz, M.; Loacker, L.; Sahanic, S.; Pizzini, A.; Sonnweber, T.; Holzer, B.; Mayer, W.; et al. Clinical validation of the quantitative Siemens SARS-CoV-2 spike IgG assay (sCOVG) reveals improved sensitivity and a good correlation with virus neutralization titers. medRxiv 2021, preprint. [Google Scholar] [CrossRef]
- Marinaki, S.; Adamopoulos, S.; Degiannis, D.; Roussos, S.; Pavlopoulou, I.D.; Hatzakis, A.; Boletis, I.N. Immunogenicity of SARS-CoV-2 BNT162b2 vaccine in solid organ transplant recipients. Am. J. Transplant 2021, 21, 2913–2915. [Google Scholar] [CrossRef]
- Huang, A.T.; Garcia-Carreras, B.; Hitchings, M.D.T.; Yang, B.; Katzelnick, L.C.; Rattigan, S.M.; Borgert, B.A.; Moreno, C.A.; Solomon, B.D.; Trimmer-Smith, L.; et al. A systematic review of antibody mediated immunity to coronaviruses: Kinetics, correlates of protection, and association with severity. Nat. Commun. 2020, 11, 4704. [Google Scholar] [CrossRef] [PubMed]
- Post, N.; Eddy, D.; Huntley, C.; van Schalkwyk, M.C.I.; Shrotri, M.; Leeman, D.; Rigby, S.I.; Williams, S.V.; Bermingham, W.H.; Kellam, P.; et al. Antibody response to SARS-CoV-2 infection in humans: A systematic review. PLoS ONE 2020, 15, e0244126. [Google Scholar] [CrossRef]
- Lau, E.H.Y.; Tsang, O.T.Y.; Hui, D.S.C.; Kwan, M.Y.W.; Chan, W.; Chiu, S.S.; Ko, R.L.W.; Chan, K.H.; Cheng, S.M.S.; Perera, R.A.P.M.; et al. Neutralizing antibody titres in SARS-CoV-2 infections. Nat. Commun. 2021, 12, 63. [Google Scholar] [CrossRef] [PubMed]
- Hall, V.J.; Foulkes, S.; Charlett, A.; Atti, A.; Monk, E.J.M.; Simmons, R.; Wellington, E.; Cole, M.J.; Saei, A.; Oguti, B.; et al. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: A large, multicentre, prospective cohort study (SIREN). Lancet 2021, 397, 1459–1469. [Google Scholar] [CrossRef]
- Lucas, C.; Klein, J.; Sundaram, M.; Liu, F.; Wong, P.; Silva, J.; Mao, T.; Tokuyama, M.; Lu, P.; Yale IMPACT Research Team. Kinetics of antibody responses dictate COVID-19 outcome. medRxiv 2020. preprint. [Google Scholar] [CrossRef]
- Lynch, K.L.; Whitman, J.D.; Lacanienta, N.P.; Beckerdite, E.W.; Kastner, S.A.; Shy, B.R.; Goldgof, G.M.; Levine, A.G.; Bapat, S.P.; Stramer, S.L.; et al. Magnitude and Kinetics of Anti–Severe Acute Respiratory Syndrome Coronavirus 2 Antibody Responses and Their Relationship to Disease Severity. Clin. Infect. Dis. 2020, 72, 301–308. [Google Scholar] [CrossRef]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An mRNA Vaccine against SARS-CoV-2—Preliminary Report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef]
- Keech, C.; Albert, G.; Cho, I.; Robertson, A.; Reed, P.; Neal, S.; Plested, J.S.; Zhu, M.; Cloney-Clark, S.; Zhou, H.; et al. Phase 1–2 Trial of a SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine. N. Engl. J. Med. 2020, 383, 2320–2332. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Clinical Management: Living Guidance. WHO, 25 January 2021. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-1 (accessed on 15 June 2021).
- SARS-CoV-2 Immunoassay. Available online: https://www.corelaboratory.abbott/int/en/offerings/segments/infectious-disease/sars-cov-2 (accessed on 15 June 2021).
- Chandrashekar, A.; Liu, J.; Martinot, A.J.; McMahan, K.; Mercado, N.B.; Peter, L.; Tostanoski, L.H.; Yu, J.; Maliga, Z.; Nekorchuk, M.; et al. SARS-CoV-2 infection protects against rechallenge in rhesus macaques. Science 2020, 369, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Corbett, K.S.; Flynn, B.; Foulds, K.E.; Francica, J.R.; Boyoglu-Barnum, S.; Werner, A.P.; Flach, B.; O’Connell, S.; Bock, K.W.; Minai, M.; et al. Evaluation of the mRNA-1273 Vaccine against SARS-CoV-2 in Nonhuman Primates. N. Engl. J. Med. 2020, 383, 1544–1555. [Google Scholar] [CrossRef]
- Mercado, N.B.; Zahn, R.; Wegmann, F.; Loos, C.; Chandrashekar, A.; Yu, J.; Liu, J.; Peter, L.; McMahan, K.; Tostanoski, L.H.; et al. Single-shot AD26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature 2020, 586, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Addetia, A.; Crawford, K.H.D.; Dingens, A.; Zhue, H.; Roychoudhury, P.; Huang, M.L.; Jerome, K.R.; Bloom, J.D.; Greninger, A.L. Neutralizing Antibodies Correlate with Protection from SARS-CoV-2 in Humans during a Fishery Vessel Outbreak with a High Attack Rate. J. Clin. Microbiol. 2020, 58, e02107-20. [Google Scholar] [CrossRef] [PubMed]
- Letizia, A.G.; Ge, Y.; Vangeti, S.; Goforth, C.; Weir, D.L.; Kuzmira, N.A.; Balinsky, C.A.; Chen, H.W.; Ewing, D.; Soares-Schanoski, A.; et al. SARS-CoV-2 seropositivity and subsequent infection risk in healthy young adults: A prospective cohort study. Lancet Respir. Med. 2021, 9, 712–720. [Google Scholar] [CrossRef]
- Feng, S.; Phillips, D.J.; White, T.; Sayal, H.; Aley, P.K.; Bibi, S.; Dold, C.; Fuskova, M.; Gilbert, S.C.; Hirsch, I.; et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. medRxiv 2021, preprint. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, G.; Pan, H.; Li, C.; Hu, Y.; Han, W.; Chen, Z.; Tang, R.; Yin, W.; Chen, X.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2020, 21, 181–192. [Google Scholar] [CrossRef]
- Wang, Z.; Schmidt, F.; Weisblum, Y.; Muecksch, F.; Barnes, C.O.; Finkin, S.; Schaefer-Babajew, D.; Cipolla, M.; Gaebler, C.; Lieberman, J.A.; et al. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature 2021, 592, 616–622. [Google Scholar] [CrossRef]
- Anderson, E.J.; Rouphael, N.G.; Widge, A.T.; Jackson, L.A.; Roberts, P.C.; Makhene, M.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A.J.; et al. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults. N. Engl. J. Med. 2020, 383, 2427–2438. [Google Scholar] [CrossRef]
- Ebinger, J.E.; Fert-Bober, J.; Printsev, I.; Wu, M.; Sun, N.; Prostko, J.C.; Frias, E.C.; Stewart, J.L.; Van Eyk, J.E.; Braun, J.G.; et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat. Med. 2021, 27, 981–984. [Google Scholar] [CrossRef]
- Corbett, K.S.; Nason, M.C.; Flach, B.; Gagne, M.; O’Connell, S.; Johnston, T.S.; Shah, S.N.; Edara, V.V.; Floyd, K.; McDanal, C.; et al. Immune correlates of protection by mRNA -1273 immunization against SARS-CoV-2 Infection in nonhuman primates. Science 2021. [Google Scholar] [CrossRef]
- Jabal, K.A.; Ben-Amram, H.; Beiruti, K.; Batheesh, Y.; Sussan, C.; Zarka, S.; Edelstein, M. Impact of age, ethnicity, sex and prior infection status on immunogenicity following a single dose of the BNT162b2 mRNA COVID-19 vaccine: Real-world evidence from healthcare workers, Israel, December 2020 to January 2021. Euro Surveill. 2021, 26, 2100096. [Google Scholar] [CrossRef]
- Collier, D.A.; Ferreira, I.A.T.M.; Kotagiri, P.; Datir, R.; Lim, E.; Touzier, E.; Meng, B.; Abdullahi, A.; The CITIID-NIHR BioResource COVID-19 Collaboration; Elmer, A.; et al. Age-related heterogeneity in immune responses to SARS-CoV-2 vaccine BNT162b2. medRxiv 2021. preprint. [Google Scholar] [CrossRef]
- Nabeer, P.; Jürjenson, V.; Adamson, A.; Sepp, E.; Tserel, L.; Kisand, K.; Peterson, P. Antibody response after COVID-19 mRNA vaccination in relation to age, sex, and side effects. medRxiv 2021, preprint. [Google Scholar] [CrossRef]
- Pellini, R.; Venuti, A.; Pimpinelli, F.; Abril, E.; Blandino, G.; Campo, F.; Conti, L.; De Virgilio, A.; De Marco, F.; Di Domenico, E.G.; et al. Obesity may hamper SARS-CoV-2 vaccine immunogenicity. medRxiv 2021, preprint. [Google Scholar] [CrossRef]
- Kremsner, P.; Mann, P.; Bosch, J.; Fendel, R.; Gabor, J.J.; Kreidenweiss, A.; Kroidl, A.; Leroux-Roels, G.; Schindler, C.; Schunk, M.; et al. Phase 1 Assessment of the Safety and Immunogenicity of an mRNA-Lipid Nanoparticle Vaccine Candidate Against SARS-CoV-2 in Human Volunteers. medRxiv 2020. preprint. [Google Scholar] [CrossRef]
- Dolgin, E. CureVac COVID vaccine let-down spotlights mRNA design challenges. Nature 2021, 594, 483. [Google Scholar] [CrossRef] [PubMed]
- Abu-Raddad, L.J.; Chemaitelly, H.; Butt, A.A. Effectiveness of the BNT162b2 Covid-19 Vaccine against the B.1.1.7 and B.1.351 Variants. N. Engl. J. Med. 2021, 385, 187–189. [Google Scholar] [CrossRef]
- Bernal, J.L.; Andrews, N.; Gower, C.; Gallagher, E.; Simmons, R.; Thelwall, S.; Stowe, J.; Tessier, E.; Groves, N.; Dabrera, G.; et al. Effectiveness of COVID-19 vaccines against the B.1.617.2 variant. medRxiv 2021, preprint. [Google Scholar] [CrossRef]
- Planas, D.; Veyer, D.; Baidaliuk, A.; Staropoli, I.; Guivel-Berhassine, F.; Rajah, M.M.; Planchais, C.; Porrot, F.; Robillard, N.; Puech, J.; et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature 2021, 596, 276–280. [Google Scholar] [CrossRef]
- Jin, P.; Li, J.; Pan, H.; Wu, Y.; Zhu, F. Immunological surrogate endpoints of COVID-2019 vaccines: The evidence we have versus the evidence we need. Signal Transduct. Target Ther. 2021, 6, 48. [Google Scholar] [CrossRef] [PubMed]

| N (%) | |
|---|---|
| Total | 871 (100.0) |
| Gender | |
| Male | 318 (36.5) |
| Female | 553 (63.5) |
| Age (y) | |
| Mean (SD) | 47.8 (10.3) |
| 18–34 | 113 (13.0) |
| 35–44 | 215 (24.7) |
| 45–54 | 315 (36.2) |
| 55–70 | 228 (26.2) |
| Country of Birth | |
| Greece | 804 (92.3) |
| Other | 67 (7.7) |
| Hospital | |
| 1 | 514 (59.0) |
| 2 | 357 (41.0) |
| Risk Factors for Severe COVID-19 | |
| Yes | 134 (15.7) |
| No | 721 (84.3) |
| Job title | |
| HCWs involved with the patient care | 709 (81.4) |
| HCWs not involved with the patient care | 162 (18.6) |
| History of SARS-CoV-2 infection | |
| Yes | 32 (3.7) |
| No | 839 (96.3) |
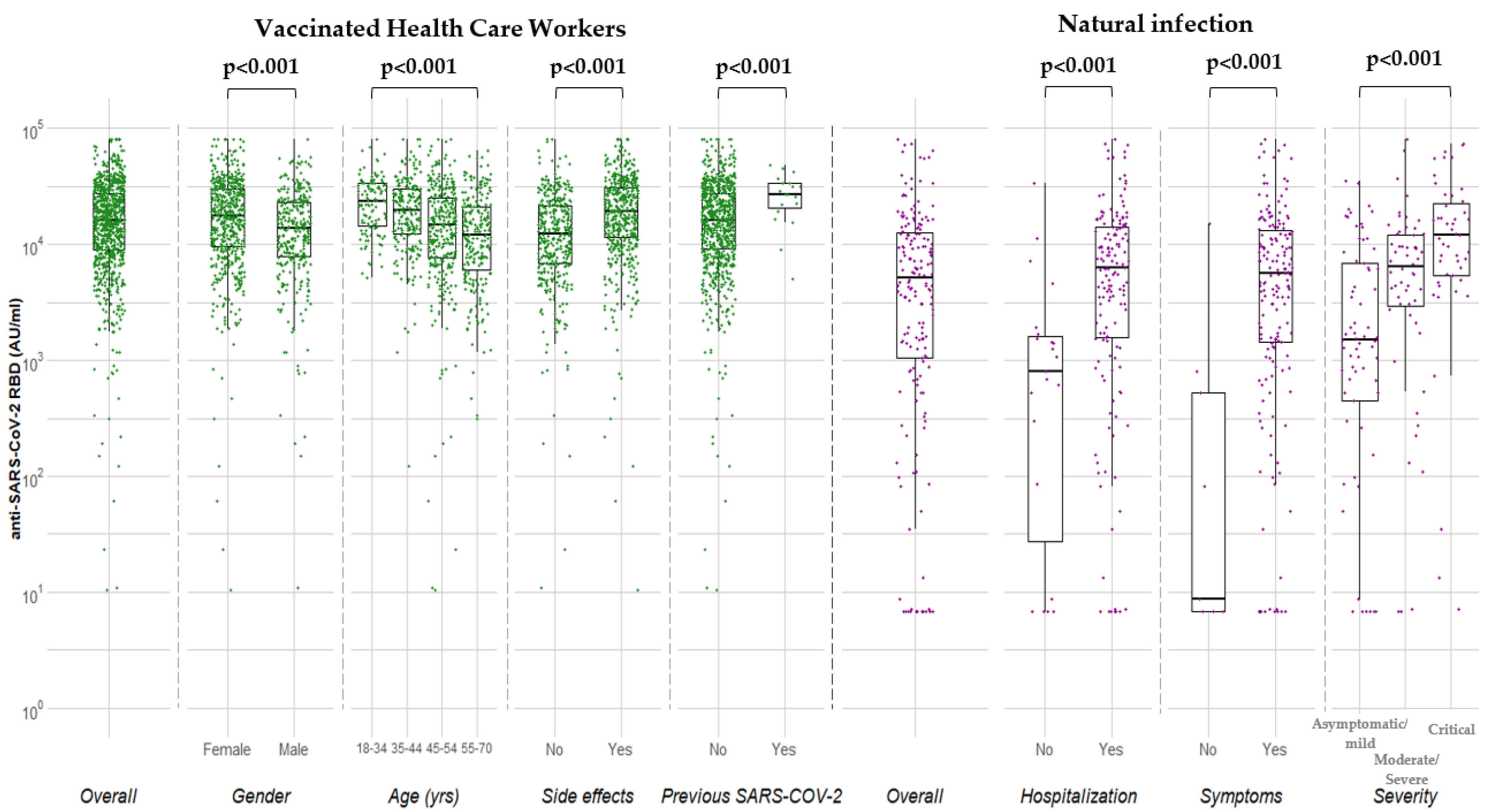
| Covariate | N (%) | Median (25th, 75th) (AU/mL) | p | β (95% CI) | p |
|---|---|---|---|---|---|
| Overall | 871 (100.0) | 15,877 (8854–27,355) | – | ||
| Gender | <0.001 | ||||
| Male | 318 (36.5) | 13,661 (7780–23,245) | Ref. | ||
| Female | 553 (63.5) | 17,711 (9678–29,726) | 2823 (859–4787) | 0.005 | |
| Age (y) | <0.001 | ||||
| 18–34 | 113 (13.0) | 23,248 (14,447–33,403) | Ref. | ||
| 35–44 | 215 (24.7) | 19,669 (12,210–29,683) | −2466 (−5583–651) | 0.121 | |
| 45–54 | 315 (36.2) | 14,748 (7636–25,363) | −6228 (−9203–−3254) | <0.001 | |
| 55–70 | 228 (26.2) | 11,977 (5993–21,101) | −7651 (−10,823–−4479) | <0.001 | |
| Country of birth | 0.524 | ||||
| Greece | 804 (92.3) | 15,612 (8785–26,994) | – | ||
| Other | 67 (7.7) | 17,293 (9569–28,664) | – | ||
| Risk factors for COVID-19 | 0.066 | ||||
| No | 721 (84.3) | 16,289 (9348–27,506) | Ref. | ||
| Yes | 134 (15.7) | 13,374 (7422–25,044) | −246 (−2800–2309) | 0.850 | |
| BMI (kg/m2) | 0.125 | ||||
| Under/Normal weight: <25 | 383 (44.0) | 16,692 (9597–29,375) | – | ||
| Overweight: 25–30 | 323 (37.1) | 14,823 (7931–24,804) | – | ||
| Obesity: ≥30 | 165 (18.9) | 15,525 (8326–25,606) | – | ||
| Side effects of vaccination | |||||
| No | 375 (43.1) | 12,210 (6848–21,298) | Ref. | Ref. | |
| Yes | 496 (56.9) | 19,196 (11,334–30,841) | <0.001 a | 5024 (3122–6926) | <0.001 |
| Fever | 128 (25.81) | 28,687 (16,207–39,100) | <0.0001 b | ||
| Fatigue | 287 (57.9) | 19,616 (12,137–31,255) | <0.0001 c | ||
| Allergic reactions | 14 (2.8) | 16,163 (9947–27,997) | 0.2792 d | ||
| Local | 198 (39.9) | 18,425 (10,086–30,300) | <0.0001 e | ||
| Other systematic | 248 (50.0) | 21,692 (12,229–33,381) | <0.0001 f | ||
| Previous SARS-COV-2 | <0.001 | ||||
| No | 839 (96.3) | 15,520 (8710–26,480) | Ref. | ||
| Yes | 32 (3.7) | 29,324 (17,751–41,821) g | 9971 (5158–14,783) | <0.001 | |
| PCR Positive (+) | 19 (59.4) | 26,986 (19,212–33,866) h | |||
| No history of PCR testing | 13 (40.6) | 33,950 (9947–49,915) i |
| Variable | N (%) |
|---|---|
| Total | 180 (100.0) |
| Gender | |
| Male | 126 (70.0) |
| Female | 54 (30.0) |
| Age (y) | |
| Mean (SD) | 59.6 (16.7) |
| ≤54 | 63 (36.4) |
| 55–64 | 42 (24.3) |
| ≥65 | 68 (39.3) |
| Hospitalization | |
| No | 23 (12.8) |
| Yes | 157 (87.2) |
| Symptoms | |
| Symptomatic | 171 (95.0) |
| Asymptomatic | 9 (5.0) |
| Severity of Symptoms | |
| Mild | 60 (36.8) |
| Moderate | 39 (23.9) |
| Severe | 17 (10.4) |
| Critical | 47 (28.8) |
| Covariate | N (%) | Median (25th–75th) (AU/mL) | p |
|---|---|---|---|
| Overall | 180 (100.0) | 5088 (1050–12,620) | |
| Gender | 0.2691 | ||
| Male | 126 (70.0) | 5632 (1273–13,837) | |
| Female | 54 (30.0) | 3743 (883–11,187) | |
| Age (y) a | 0.0603 | ||
| 18–44 | 33 (19.1) | 1297 (296–7519) | |
| 45–54 | 30 (17.3) | 6577 (1601–16,623) | |
| 55–64 | 42 (24.3) | 6868 (2433–11,975) | |
| 65+ | 68 (39.3) | 5814 (1553–13,110) | |
| Hospitalization | <0.001 | ||
| No | 23 (12.8) | 808 (9–1668) | |
| Yes | 157 (87.2) | 6271 (1583–14,121) | |
| Asymptomatic | 0.0005 | ||
| No | 171 (95.0) | 5547 (1415–13,325) | |
| Yes | 9 (5.0) | 9 (<6.8–520) | |
| Severity b | 0.0001 | ||
| Mild | 60 (36.8) | 1634 (751–7868) | |
| Moderate | 39 (23.9) | 6082 (2433–9579) | |
| Severe | 17 (10.4) | 6638 (3053–13,837) | |
| Critical | 47 (28.8) | 11,975 (5318–23,351) | |
| Time from symptoms onset (days) c | 0.8887 | ||
| 15–29 | 82 (52.9) | 6980 (1060–14,968) | |
| 30–44 | 48 (31.0) | 6122 (2169–13,925) | |
| 45–59 | 25 (16.1) | 5452 (3306–9579) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Psichogiou, M.; Karabinis, A.; Poulakou, G.; Antoniadou, A.; Kotanidou, A.; Degiannis, D.; Pavlopoulou, I.D.; Chaidaroglou, A.; Roussos, S.; Mastrogianni, E.; et al. Comparative Immunogenicity of BNT162b2 mRNA Vaccine with Natural SARS-CoV-2 Infection. Vaccines 2021, 9, 1017. https://doi.org/10.3390/vaccines9091017
Psichogiou M, Karabinis A, Poulakou G, Antoniadou A, Kotanidou A, Degiannis D, Pavlopoulou ID, Chaidaroglou A, Roussos S, Mastrogianni E, et al. Comparative Immunogenicity of BNT162b2 mRNA Vaccine with Natural SARS-CoV-2 Infection. Vaccines. 2021; 9(9):1017. https://doi.org/10.3390/vaccines9091017
Chicago/Turabian StylePsichogiou, Mina, Andreas Karabinis, Garyphallia Poulakou, Anastasia Antoniadou, Anastasia Kotanidou, Dimitrios Degiannis, Ioanna D. Pavlopoulou, Antigoni Chaidaroglou, Sotirios Roussos, Elpida Mastrogianni, and et al. 2021. "Comparative Immunogenicity of BNT162b2 mRNA Vaccine with Natural SARS-CoV-2 Infection" Vaccines 9, no. 9: 1017. https://doi.org/10.3390/vaccines9091017
APA StylePsichogiou, M., Karabinis, A., Poulakou, G., Antoniadou, A., Kotanidou, A., Degiannis, D., Pavlopoulou, I. D., Chaidaroglou, A., Roussos, S., Mastrogianni, E., Eliadi, I., Basoulis, D., Petsios, K., Leontis, K., Kakalou, E., Protopapas, K., Jahaj, E., Pratikaki, M., Syrigos, K. N., ... Hatzakis, A. (2021). Comparative Immunogenicity of BNT162b2 mRNA Vaccine with Natural SARS-CoV-2 Infection. Vaccines, 9(9), 1017. https://doi.org/10.3390/vaccines9091017












