Abstract
Cellulose is a biopolymer that may be derived from a variety of agricultural wastes such as rice husks, wheat straw, banana, and so on. Cellulose fibril that is reduced in size, often known as nanocellulose (NC), is a bio-based polymer with nanometer-scale widths with a variety of unique properties. The use of NC as a reinforcing material for nanocomposites has become a popular research issue. This research paper focuses on the production of banana pseudostem cellulose nanofiber. Nano-sized fiber was obtained from banana pseudostem through several processes, namely, grinding, sieving, pre-treatment, bleaching, and acid hydrolysis. The product yield was found to be 40.5% and 21.8% for Musa acuminata and Musa balbisiana, respectively, by the weight of the raw fiber. The reduction in weight was due to the removal of hemicellulose and lignin during processing. Transmission electron microscopy (TEM) analysis showed that the average fiber size decreased from 180 µm to 80.3 ± 21.3 nm. Finally, FTIR analysis showed that the fibers experienced chemical changes after the treatment processes.
1. Introduction
Biodegradable polymers made from renewable resources have received a lot of interest as next-generation packaging materials. Because of their biodegradability and renewability, polysaccharides have the potential to replace petrochemical polymers. Cellulose is the most abundant biomass on the planet. Micro-fibrillated celluloses have been fragmented into micro/nano-sized fibers and used to make transparent packaging membranes [1]. Nanocellulose (NC) has various benefits over micro- and macro-cellulose composites. For example, NCs are cheap, biodegradable, and renewable with high surface reactivity as well as low density and low energy consumption. Likewise, NCs improve various properties when introduced as reinforcements in nanocomposites. NC composites are widely employed in the automotive, packaging, electronics, and biotechnology sectors, among others, due to their tolerability, style flexibility, and processability. However, there are significant drawbacks to using NCs as a reinforcing material including excessive wetness absorption, poor wettability, incompatibility with most chemical compound matrices, and process temperature limits [2]. These drawbacks have prompted researchers to address these issues through the introduction of numerous processing methods. For example, the NC or compound matrices have been developed or modified using entirely new process techniques, which could supply superior NC-reinforced composites with fascinating properties [2]. Plant cellulose has been used as a natural reinforcer in food packaging films because it is a natural polysaccharide polymer with good thermal stability, and numerous recent studies have shown that incorporating plant-derived celluloses into protein- or starch-based films can improve the mechanical properties [3]. In this study, a biofilm reinforced with NC was extracted from the inner and outer layers of the banana pseudostem.
In the banana plant, the pseudostem is responsible for supplying and transporting nutrients from the soil to the fruits. However, the pseudostem becomes waste biomass after the banana fruit has grown and been processed, which makes the plant unusable for the following harvest [4]. Furthermore, 10% of each tonne of banana fruit collected is discarded resulting in approximately four tonnes of biomass waste. The root, pseudostem, rotting fruit, peel, fruit-stem, and rhizome are all sources of biomass waste. This indicates that each phase of banana fruit production results in four times the amount of biomass waste [5]. Banana farms yield 220 tonnes of biomass waste per hectare [6]. The leftovers are considered garbage and must be handled properly, otherwise these can generate greenhouse gases if burnt or abandoned, thereby causing environmental issues [7]. However, crop wastes can be utilized for cellulose fibers [8].
Of all the banana species in the world, Abaca (Musa textilis) is the most widely recognized for its leaf-based fiber. Meanwhile, Musa acuminata is the most consumed banana [7]. The pseudostem of banana species has high tensile strength and rigidity, which indicates it is a promising fiber material [9]. The use of CNFs as matrix support components improves the thermo-mechanical characteristics, reduces polymer exposure to water, and maintains biodegradability [10]. The mixing of CNFs with polysaccharides (such as starch) improves the general matrix characteristics [11,12,13]. However, the extraction of CNFs from lignocellulosic sources requires several methods. Currently, the isolation of nanofibril cells is accomplished through chemical hydrolysis (e.g., acid therapy and catalytic oxidation), which separates cellulose fibers from the plant cell wall [14]. Other ways to obtain CNF involve high-intensity ultrasonication [15]. The primary goal of this research is to acquire nano-sized fibers from banana pseudostem through an acid hydrolysis process. Secondly, the structure of banana pseudostem-based CNFs will be investigated. Finally, the chemical and thermal characteristics of banana pseudostem CNF will be examined.
2. Materials and Methods
2.1. Grinding and Sieving
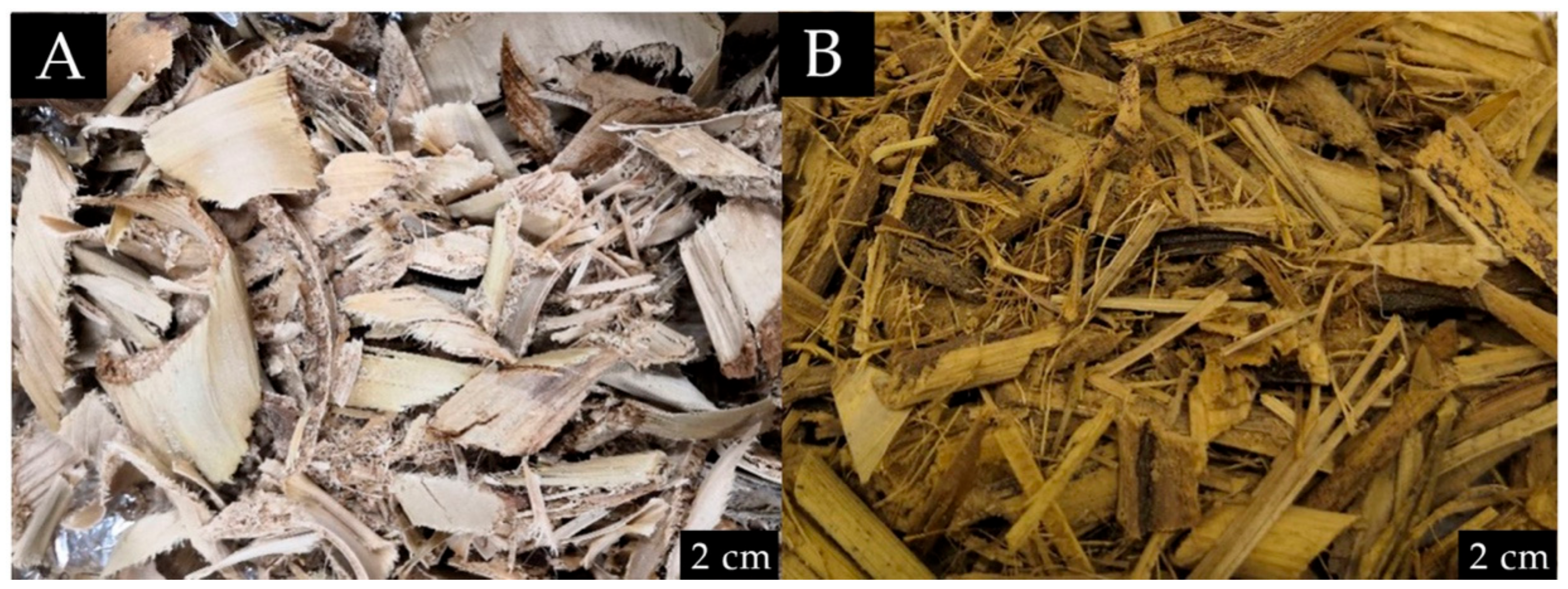
The isolation of CNFs from banana pseudostem requires several steps. First, the partially-dried banana trunks were obtained from Universiti Putra Malaysia Kampus Bintulu, Sarawak. Upon receipt, the banana trunks were dried in a pressure convection oven at 60 °C for 48 h. Figure 1 shows the differences between the dried Musa acuminata and Musa balbisiana. The dried M. balbisiana pseudostem appears darker when compared to M. acuminata, which may indicate higher lignin and cellulose content. A previous study indicates that the black-colored substances obtained from the pulping process is mostly due to lignin (46% of the total solid) and hemicellulose [16]. Another study also reported that the wood fiber from M. balbisiana has significantly higher lignin and hemicellulose contents [17].

Figure 1.
Difference between (A) dried M. acuminata; (B) dried M. balbisiana before grinding.
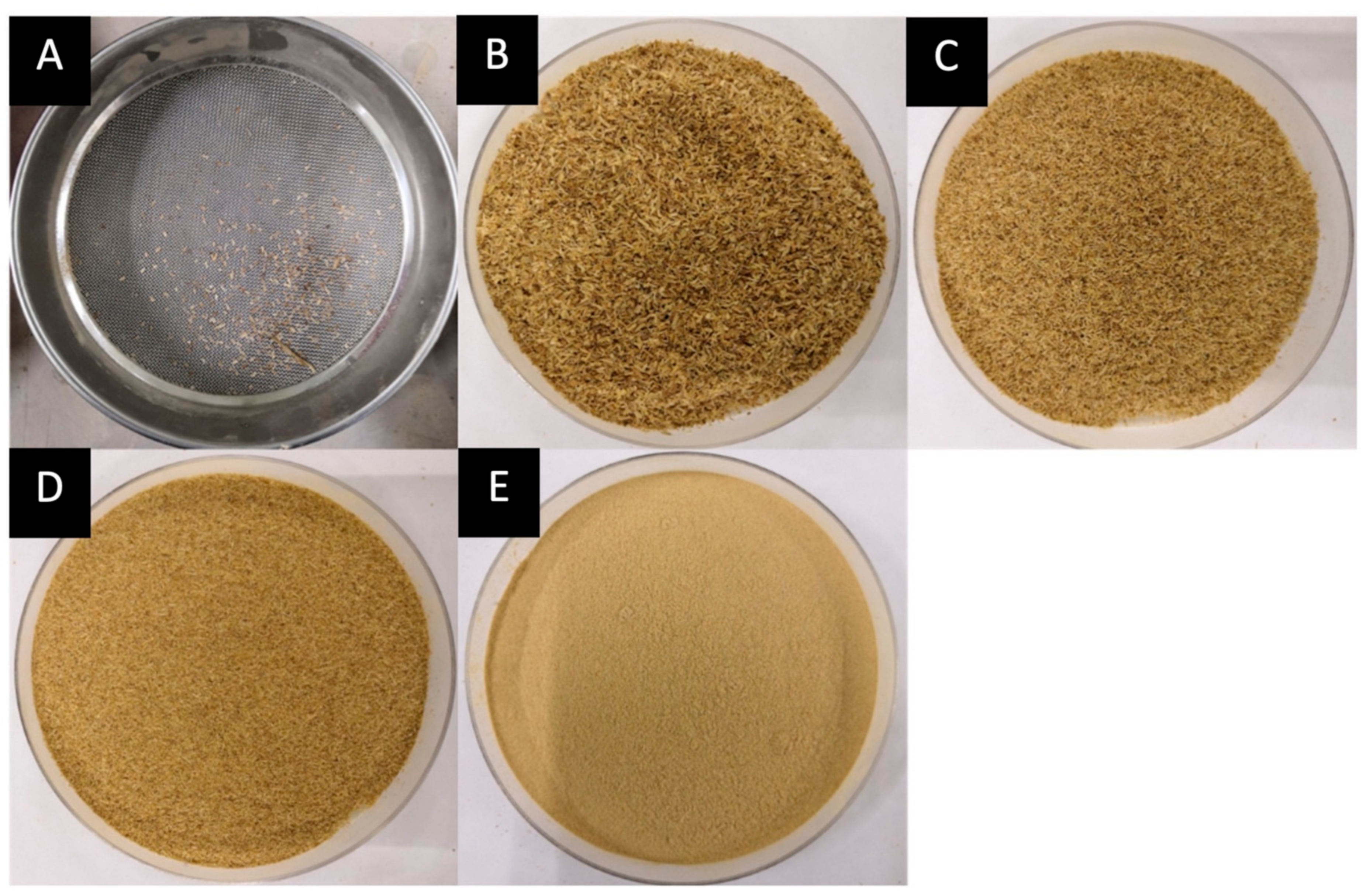
The banana pseudostem was ground in a household blender for 30 s with ten-second pauses in between. The ground banana pseudostem was then sieved using laboratory test sieve ASTM E11. Several sieves were arranged accordingly from larger to smaller mesh sizes; 1 mm, 500 µm, 355 µm, and 180 µm, and a collector. The sieves were put on a shaker for 2 min. Figure 2 shows the weight distribution according to each mesh size.
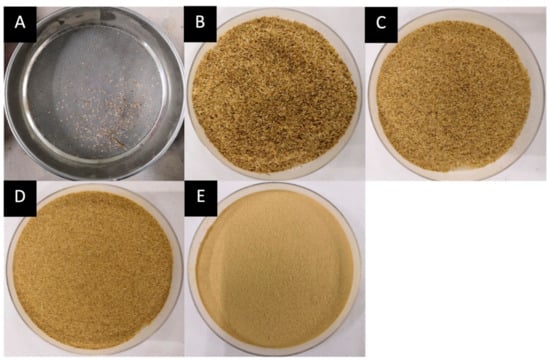
Figure 2.
Fiber weight distribution according to mesh size (M. acuminata) of (A) <1 mm = 0.6474 g, (B) 1 mm–500 µm = 26.5757 g, (C) 500 µm–355 µm = 44.7802 g, (D) 355 µm–180 µm = 48.8895 g, (E) <180 µm = 114.6674 g.
2.2. Pre-Treatment
The pre-treatment and bleaching processes eliminate hemicellulose, lignin, and other substances such as wax, which purifies the extracted cellulose fibers [18]. Next, 20 g of fiber with a size <180 µm was immersed in hot distilled water (dH2O), also called the retting process, at temperatures between 70 °C and 80 °C in a water bath. The cycle was completed in 12 h. This step was done to eliminate impurities and large particles including foreign materials [19]. The fibers were then washed with water and filtered using cotton mesh (soy filter mesh). It was then dried in the oven for 24 h at 60 °C. The fibers were then sterilized by autoclaving at 121 °C for 15 min and treated with 200 mL of 2.5 mol L−1 sodium hydroxide (NaOH). This approach, known as the steam explosion method, was developed to remove hemicelluloses [20]. At this point, filtration using filter paper or cotton mesh did not work as it caused clogging. Hence, the centrifugal technique was introduced to replace the previously reported separation method [21]. The NaOH solution was removed from the fiber by centrifuging for 15 min at 10,000 RPM before washing with water. The fiber was rewashed with distilled water after the water residue was removed. The treatment was carried out at least five times or until the pH level reached 7. The water was then evacuated before moving on to the next step.
2.3. Bleaching Process
After pre-treatment, the fiber was subjected to bleaching. This was accomplished by soaking 200 mL of sodium chlorite in NaClO2 solution at 70 °C under acidic conditions (pH 4–5) [22]. The acidic condition was adjusted by adding 2 mL of glacial acetic acid. The fiber became whiter due to the elimination of lignin and trace hemicellulose. The residue, on the other hand, turned yellowish in color. The residue of the bleaching chemical was centrifuged out of the fiber, and the fiber was well washed with distilled water. This technique was carried out again (5–7 times) until the pH of the treated fiber reached 7 [23]. Before the acid hydrolysis procedure began, the treated fiber was dried at 37 °C and stored.
2.4. Acid Hydrolysis Process
CNFs were isolated in order to produce nano-sized cellulose fibers, which can be obtained by hydrolysis and concentrated acid treatment [22]. Firstly, 64 wt% of 105 mL of H2SO4 solution was added with 10 g of treated cellulose fiber. The mixture was rapidly agitated at 45 °C for 90 min. The hydrolysis process was then terminated by the addition of 400 mL of chilled water. Later, the precipitate was obtained by diluting the suspension and centrifuging at 11,000 RPM for 10 min. The mixture was resuspended in water; this time with a lot of agitation. It was then placed in a Supelco dialysis tube and immersed in water for 7 days to remove any remaining acid. The water was changed every day. Finally, the CNF suspension was homogenized for 30 s using the homogenizer (Model: IKA Ultra Turra T-25, Staufen, Germany). The CNF was stored in the refrigerator prior to further analysis.
2.5. Composition of Hemicellulose, Cellulose, Lignin, and Extractives of Banana Pseudostem Fibre after Pre-Treatment, Bleaching and Acid Hydrolysis
The composition of three lignocellulosic components (hemicellulose, cellulose, and lignin) in the banana pseudostems was determined by the method proposed in the literature [24,25].
2.5.1. Determination of Extractives Content
In this step, 60 mL acetone was added to 1 g of banana pseudostem fiber to determine the composition of extractives in the biomass sample. The temperature was kept at 90 °C for 2 h using a heater. The sample was then dried in an oven at 105–110 °C for 2 h until it reached a consistent weight (B). The composition of extractives was computed using the formula below.
A − B = Extractives content (g)
2.5.2. Determination of Hemicellulose Content
In this step, 0.5 mol/L of NaOH solution was added to 1 g of the extractive-free banana pseudostem sample to assess the hemicellulose content in the biomass sample (B). The mixture was refluxed at 80 °C for 310 min. The sample was then rinsed in dH2O until it was clear of Na+. Using pH paper, the Na+ was detected and the measurement was reduced to 7. The sample was dried in an oven at 105–110 °C until it reached a consistent weight (C). The hemicellulose content was calculated using the formula below.
(B − C) = Hemicellulose content (g)
2.5.3. Determination of Lignin Content
Next, 1 g of the extractive-free banana pseudostem was treated with 30 mL of 98% H2SO4 (B). The sample was kept at room temperature for 24 h. It was then heated for 1 h at 100 °C. The solid residue was filtered and rinsed with dH2O until the sulphate ion was undetectable. The sulphate ion was detected by a titration procedure using a 10% solution of BaCl2. The sample was dried in an oven at 105–110 °C until it reached a consistent weight (D). The lignin content was calculated from the ultimate weight of the residue.
(D) = Amount of Lignin (g)
2.5.4. Determination of Cellulose Content
The total quantity of biomass sample utilized in the experiment was 1 g. The cellulose (E) content was determined by calculating the difference between the sample’s original weight and the three other component weights measured during the experimental phase.
(A − B) + (B − C) + D + E = 1 g
2.6. Analysis of Banana Pseudostem Cellulose Nanofibers
2.6.1. Thermogravimetric Analysis
Thermogravimetric analysis tests were performed in a TGA 4000 Thermogravimetric Analyzer, Perkin Elmer, Waltham, MA, USA and analyzed with Pyris™ software (Version 11.1.1.0492, Shelton, CT, USA). Approximately 5 mg specimens were weighed in aluminum pans and heated from 30 °C to 900 °C at a heating rate of 10 °C/min with nitrogen purging. The weight losses of the samples were measured as a function of temperature. Nitrogen was purged at a flow rate of 120 mL/min to provide an inert atmosphere for non-isothermal thermal degradation and to remove gaseous and condensable products, thus minimizing any secondary vapor-phase interactions.
2.6.2. X-ray Diffraction
The crystallinity index of both species’ banana pseudostem CNFs was determined using X-ray diffraction (Rigaku SmartLab Intelligent X-ray Diffraction System, Tokyo, Japan) under the following conditions: monochromatic Cu K radiation of 15.418 nm; 40 kV, 40 mA with a step size of 0.02° and time/step around 20 s from 3° to 100° (2). An empirical technique was used to calculate the crystallinity index (CrI) using the following equation [26]:
where I002 is the maximum intensity of the (002) lattice diffraction at 2θ = 22.5° and Iam is the intensity of the scattered diffraction by the amorphous part of the sample at 2θ = 18°.
2.6.3. Macroscopic Observation
First, the macroscopic observation of the fiber morphology was recorded using an Olympus SZ51-RT (Olympus, Tokyo, Japan) stereo zoom microscope, attached with an industrial microscope camera ToupTek S3CMOS, with a 5-megapixel sensor, 2.2 × 2.2 µm. The image was captured at 250 magnification of the original specimen size with an auto white balance and exposure setting. The captured images were analyzed using the image analysis software ToupView version 3.7. Secondly, the CNF morphology was also determined using high-resolution transmission electron microscopy (TEM) (Hitachi H-7700 Philips; 120 kV) (HRTEM). A drop of the liquid nanofiber suspension was added to the carbon-coated grid and left to dry at room temperature. The scale measurements of the cellulose nanofiber were carried out using the Image J scanner before and after the homogenization process. In total, ten nanofibers were randomly picked, measured and the result was stated as the mean value of the sample. Lastly, the chemical composition of the cellulose nanofiber was determined using Fourier transform infrared spectroscopy (FTIR) analysis. Dried CNF was dried overnight at 50–60 °C for 120 min before analysis. KBr pellet was used in sample preparation for FTIR analysis. The spectra recorder (FTIR Nicolet MAGNA-IR 860 spectrometer; Thermo Fisher Scientific, Inc.; Waltham, MA, USA) with absorbance mode, resolution: 4 cm−1, 64 scans were utilized to record the spectra.
3. Results and Discussion
3.1. Yield after Pre-Treatment and Acid Hydrolysis
The product yield after each process were recorded gravimetrically using a weighing balance. Based on the results, the yield of CNF obtained was 40.5% (M. acuminata) and 21.8% (M. balbisiana) per weight of raw fiber. Each CNF extraction process removed a specific composition of holocellulose, lignin, and ashes in the fiber [27]. Likewise, losses occurred during transferring, sieving, and washing throughout the treatments and processes as well as during the estimation of hemicellulose and lignin removal (in weight). Table 1 shows the CNF yields after each process for both M. acuminata and M. balbisiana.

Table 1.
Yield harvested after each process for M. acuminata and M. balbisiana.
3.2. Morphology
3.2.1. Banana Pseudostem Fibers after Pre-Treatment
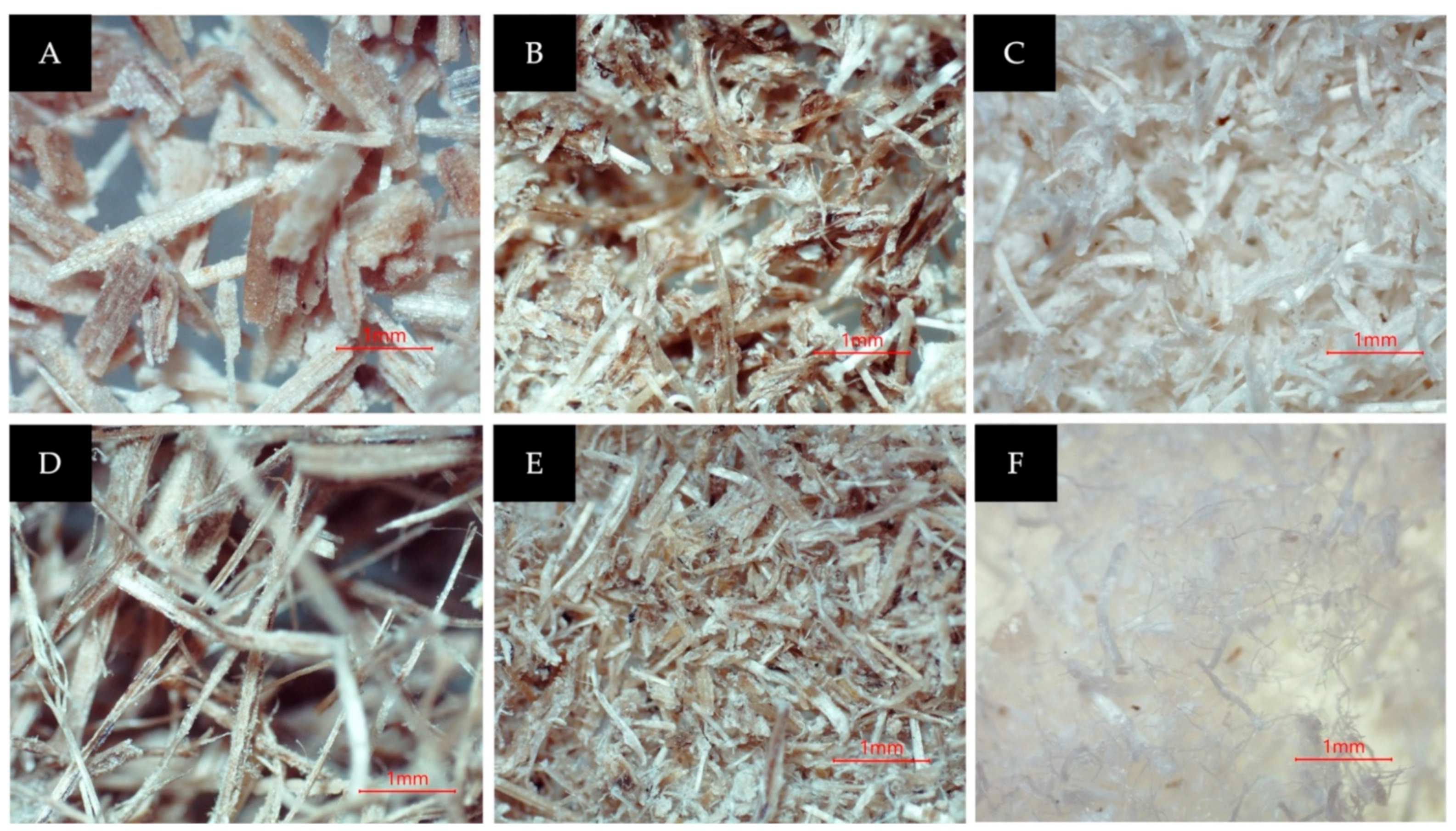
The initial diameter of the raw fiber was 355 µm (on average). The observations revealed that the physical improvements occurred in banana pseudo stem fiber due to the significant removal of hemicellulose and lignin fractions during the pre-treatment. After alkali treatment (with steam explosion), the fiber structure started to “unwind”. The fiber was distorted and started breaking down. Therefore, the pre-treatment process removes substantial portions of hemicellulose and lignin fiber materials, leaving cellulose unbound and discarded. A smaller strand started to appear. In comparison, the untreated fiber, still has a pecking structure (rigid form) with highly ordered fibrils since hemicellulose and lignin still bind the microfibrils together. The bleaching process caused the structure to further unwind, making the diameter of the cellulose strand smaller. Further removal of lignin also caused the fiber to become whiter. The images of the CNFs for each process are shown in Figure 3 below.
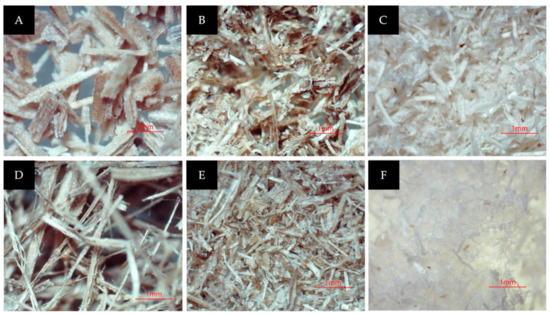
Figure 3.
(A) M. acuminata pseudostem after being dried and sieved; (B) after the steam explosion; (C) after the bleaching process; (D) M. balbisiana pseudostem after being dried and sieved; (E) after the steam explosion; (F) after the bleaching process.
3.2.2. Transmission Electron Micrograph (TEM) Analysis of Musa acuminata CNF
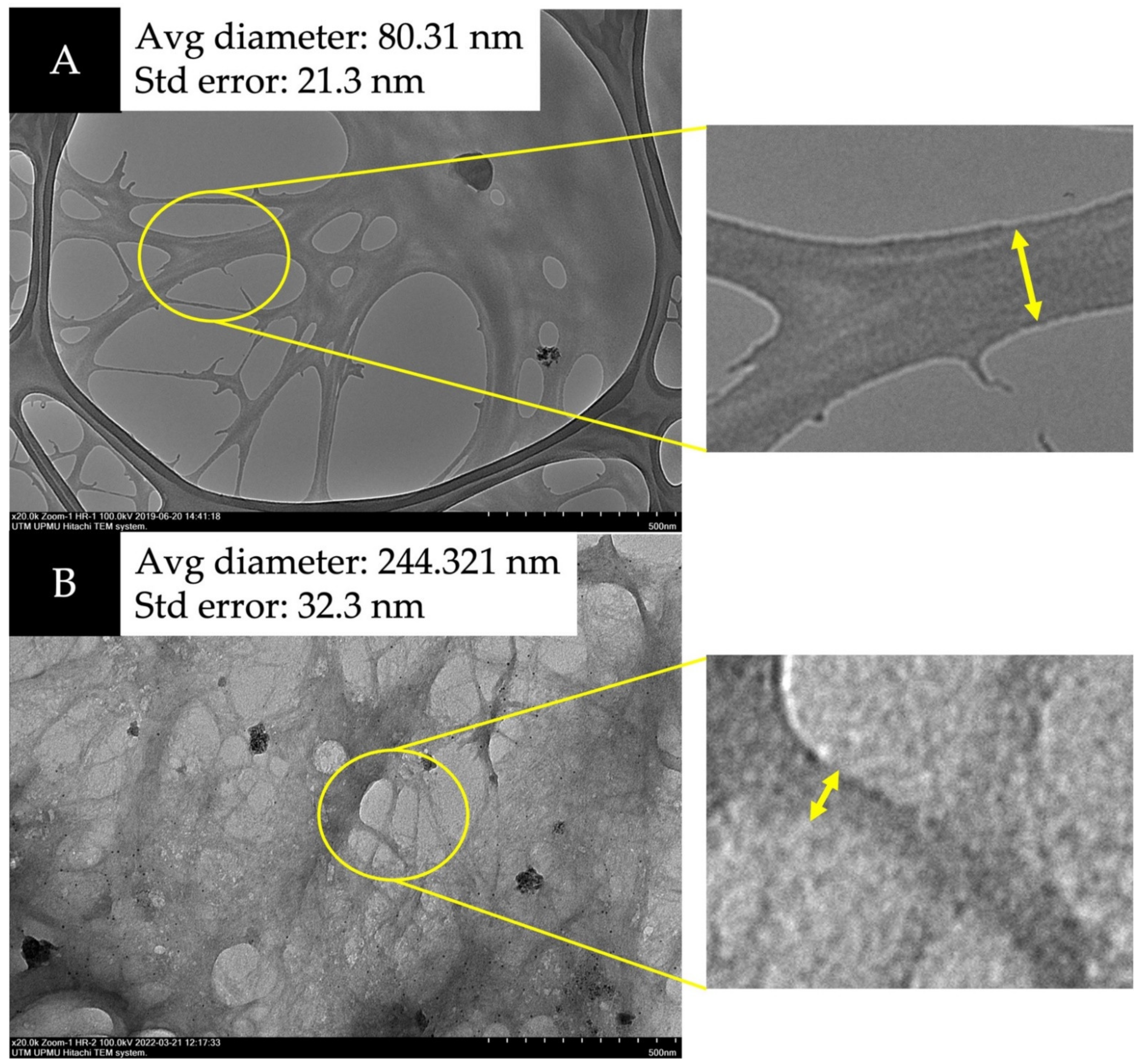
TEM was utilized to verify the nano structure of the fiber. Figure 4 and Figure 5 show that CNFs undergoing acid hydrolysis have thinner fiber strands with a 28–87 nm diameter compared to the raw sample before the process. The amorphous area of microfibril (cleaved during hydrolysis with sulfuric acid), decreased the micrometer-sized fiber to nanometers [28,29,30]. The homogenization turned the fiber into NC with a web-like shape.

Figure 4.
Transmission electron microscopy (TEM) images of (A) M. acuminata and (B) M. balbisiana at 20,000× magnification.
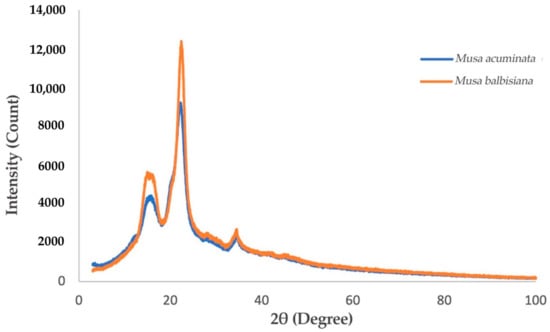
Figure 5.
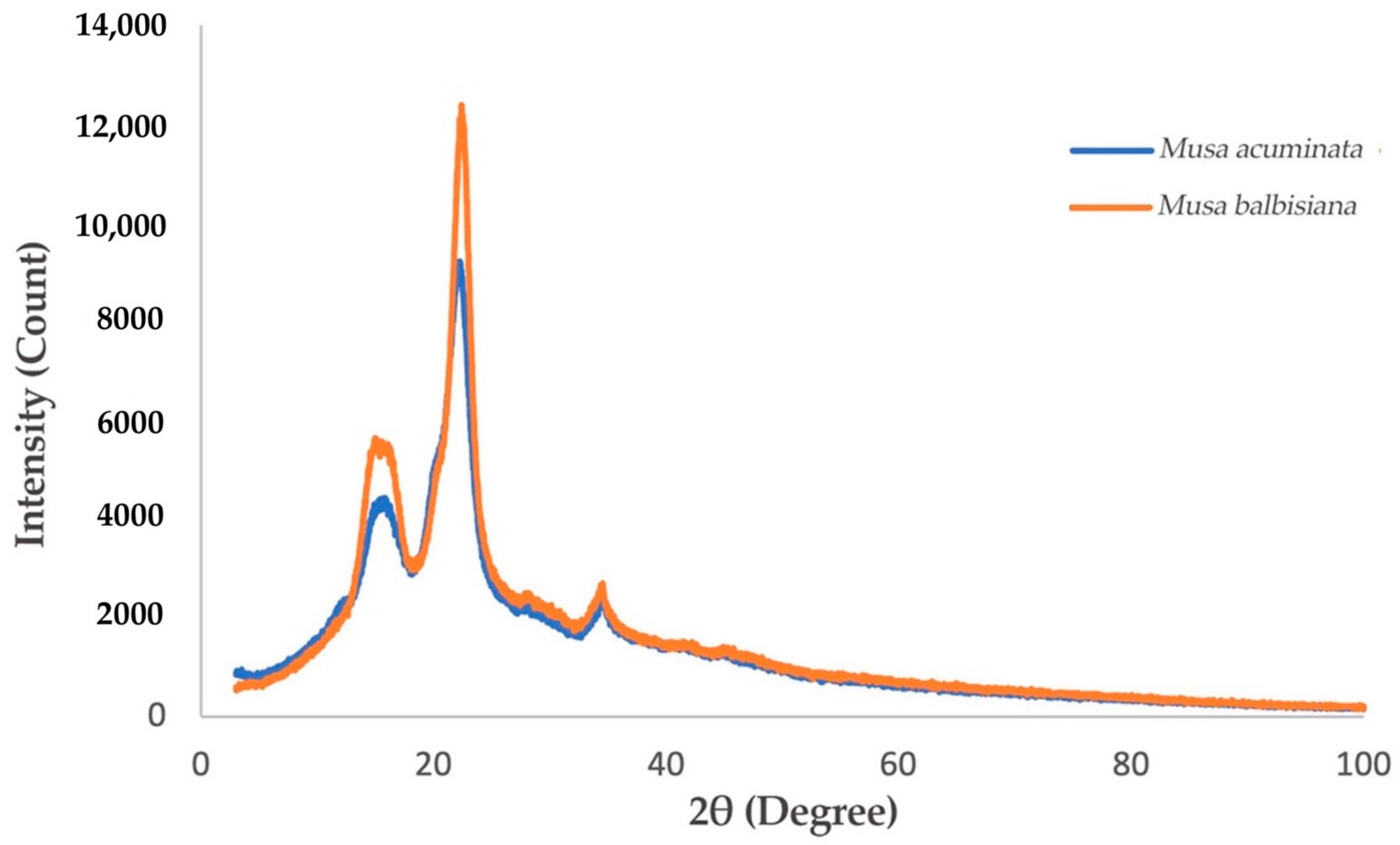
XRD Spectra of M. acuminata and M. balbisiana pseudostem CNF.
3.2.3. Comparison of M. acuminata and M. balbisiana Pseudostem via XRD Analysis
The degree of crystallinity of the CNF fashioned from M. acuminata and M. balbisiana pseudostem was delineated by the intense peaks detected at 16° and 22° in the diffractogram shown in Figure 5. The results display the narrowest/sharpest peaks at 2θ = 22° due to high crystallinity compared to other samples. The expansion and realignment of monocrystals may occur simultaneously throughout the CNC generation, which enhances the polyose crystallinity [31]. Figure 6 shows that the crystallinity index of CNF obtained from M. acuminata is 65.68%, whereas M. balbisiana is higher at 75.37%. It shows that the CNF obtained from M. balbisiana has a higher crystallinity region than M. acuminata. The removal of hemicellulose, lignin, and amorphous non-cellulosic substances during the pre-treatment and bleaching processes increases the crystallinity due to bonding interactions and Van der Waals forces between close molecules and crystalline structure of saccharide [28]. In comparison with other experiments, 64.32% crystallinity was attained in a comparable experiment using banana rachis. Meanwhile, from raw to treated fiber, crystallinity increases by 78% for banana rachis and 450% for banana peel bran. However, the crystallinity of generated nanofibers from poplar wood was found to be 69% [32]. In addition, softwood pulp has a 78.3% crystallinity index while hardwood pulp has an index of 72.7% [33]. As a result, softwood pulp is projected to have greater cellulose purity than M. balbisiana.
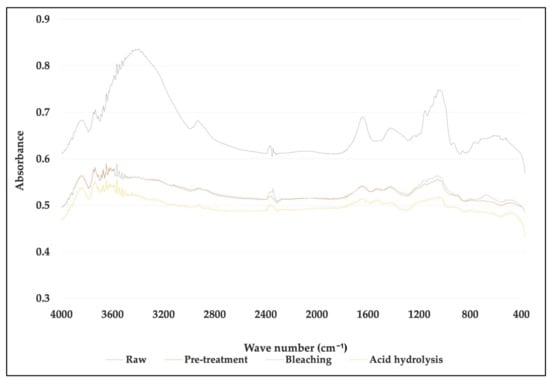
Figure 6.
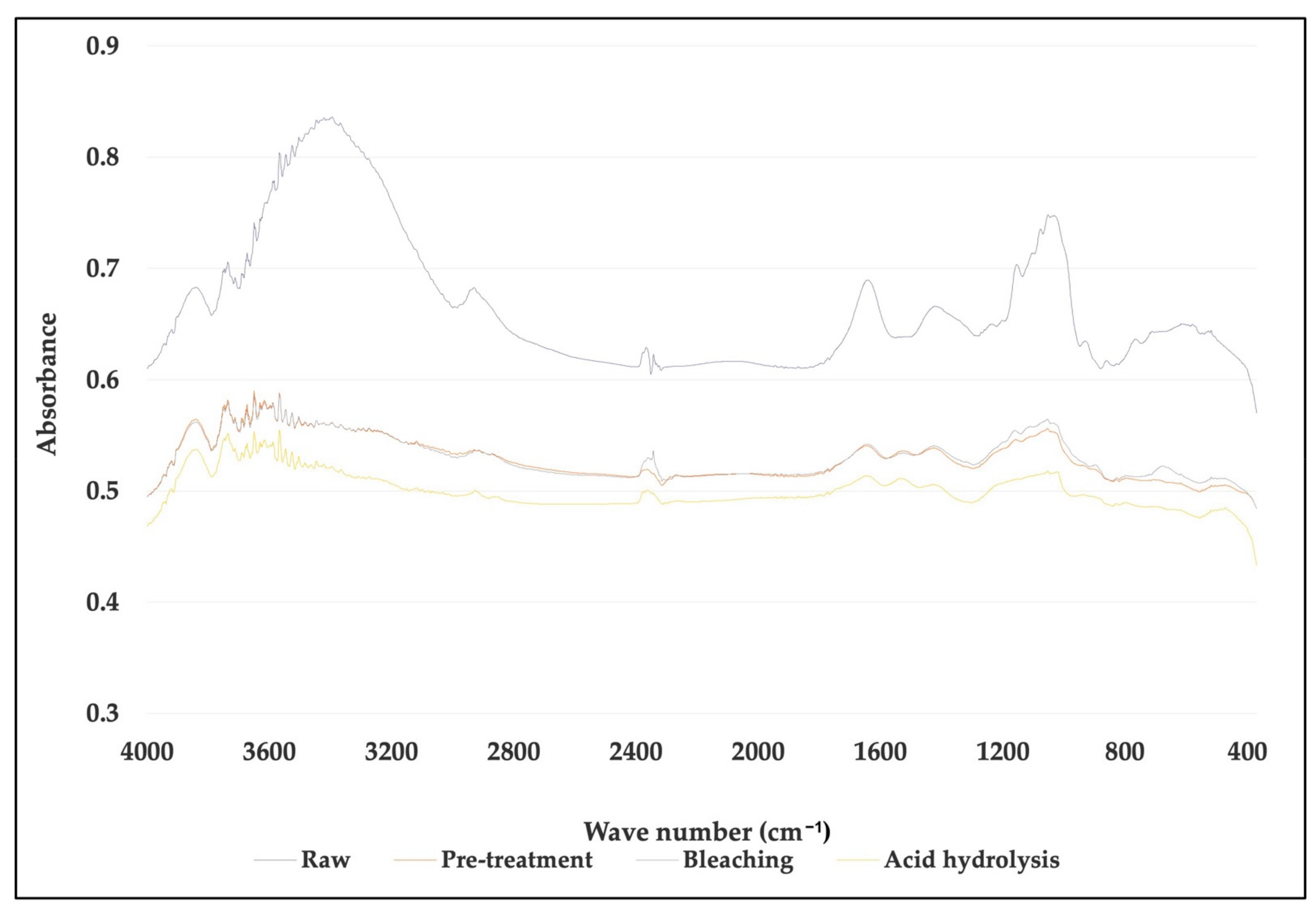
Comparison of FTIR spectra between untreated, treated fiber and cellulose nanofiber (CNF) for Musa acuminata.
The acid hydrolysis approach is the most well-known and commonly utilized of the several methods for generating cellulose nanostructures. This technique separates the cellulose’s disordered and amorphous portions, yielding single, well-defined crystals. The fact that the crystalline areas are insoluble in acids in the conditions under which they are used supports this event [34]. Because the structure of ribbon-shaped crystalline bundles in cellulose is not easily pierced by acid molecules, too brief hydrolysis processes will not result in substantial changes in the crystallinity of the material. On the other side, if the reaction period is too lengthy, the crystalline domains of BC will be digested, resulting in a loss in crystallinity. Previous research has shown that in order to create a material with a crystallinity index >80%, longer hydrolysis periods than those employed for plant cellulose are necessary [35].
3.3. Fourier Transform Infrared Spectroscopy (FTIR) of Banana Pseudostem CNF after Pre-Treatment and Processing
The FTIR spectra showed that structural modifications appeared after the sequential fiber treatments. The comparison of selected bands for each fiber (untreated, after alkali treatment, bleached and CNF) are shown in Figure 7 and Figure 8.

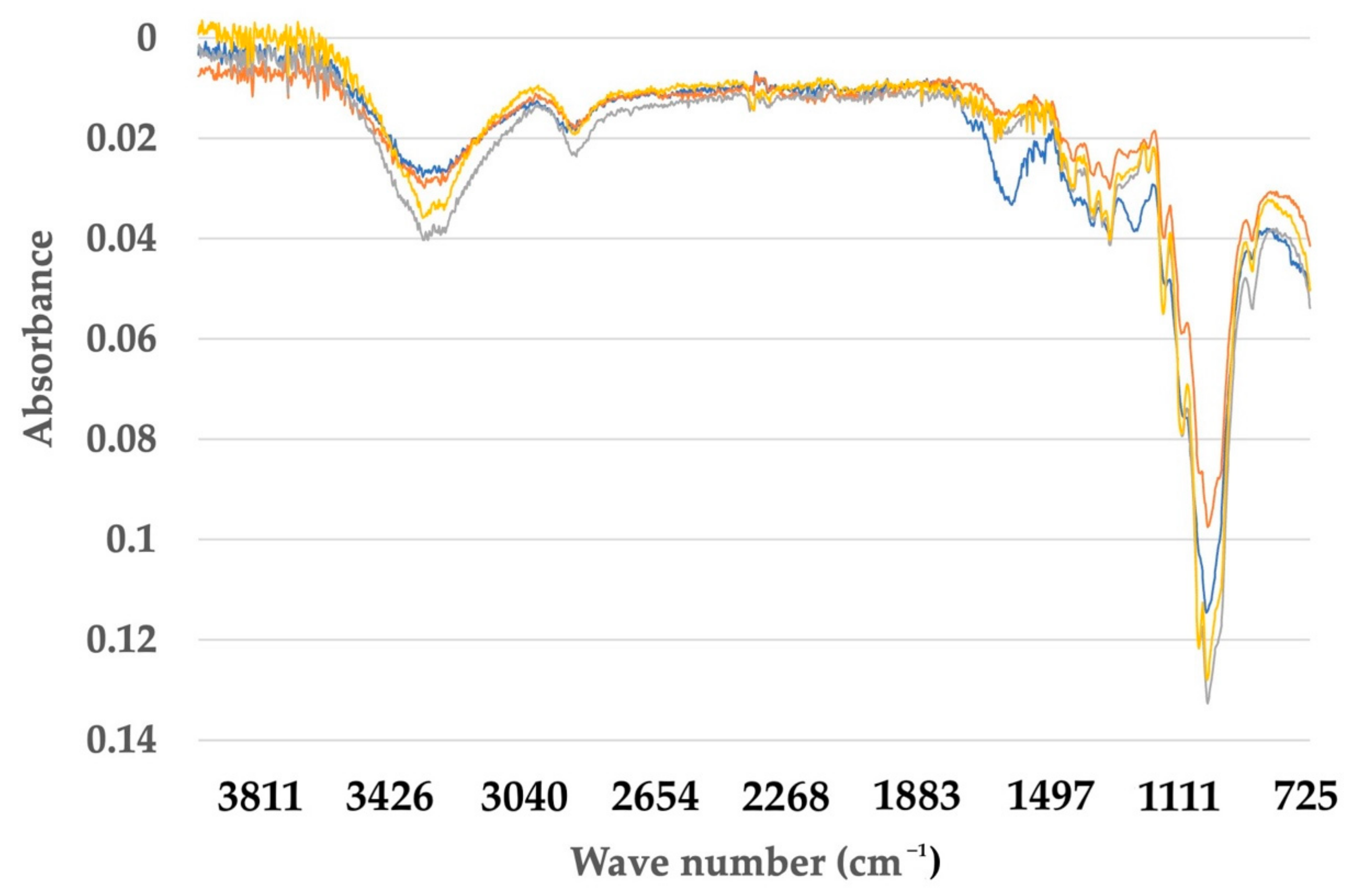
Figure 7.
Comparison of FTIR spectra between untreated, treated fiber and cellulose nanofiber (CNF) for Musa balbisiana.
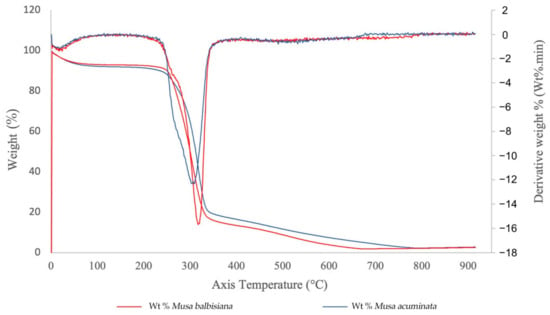
Figure 8.
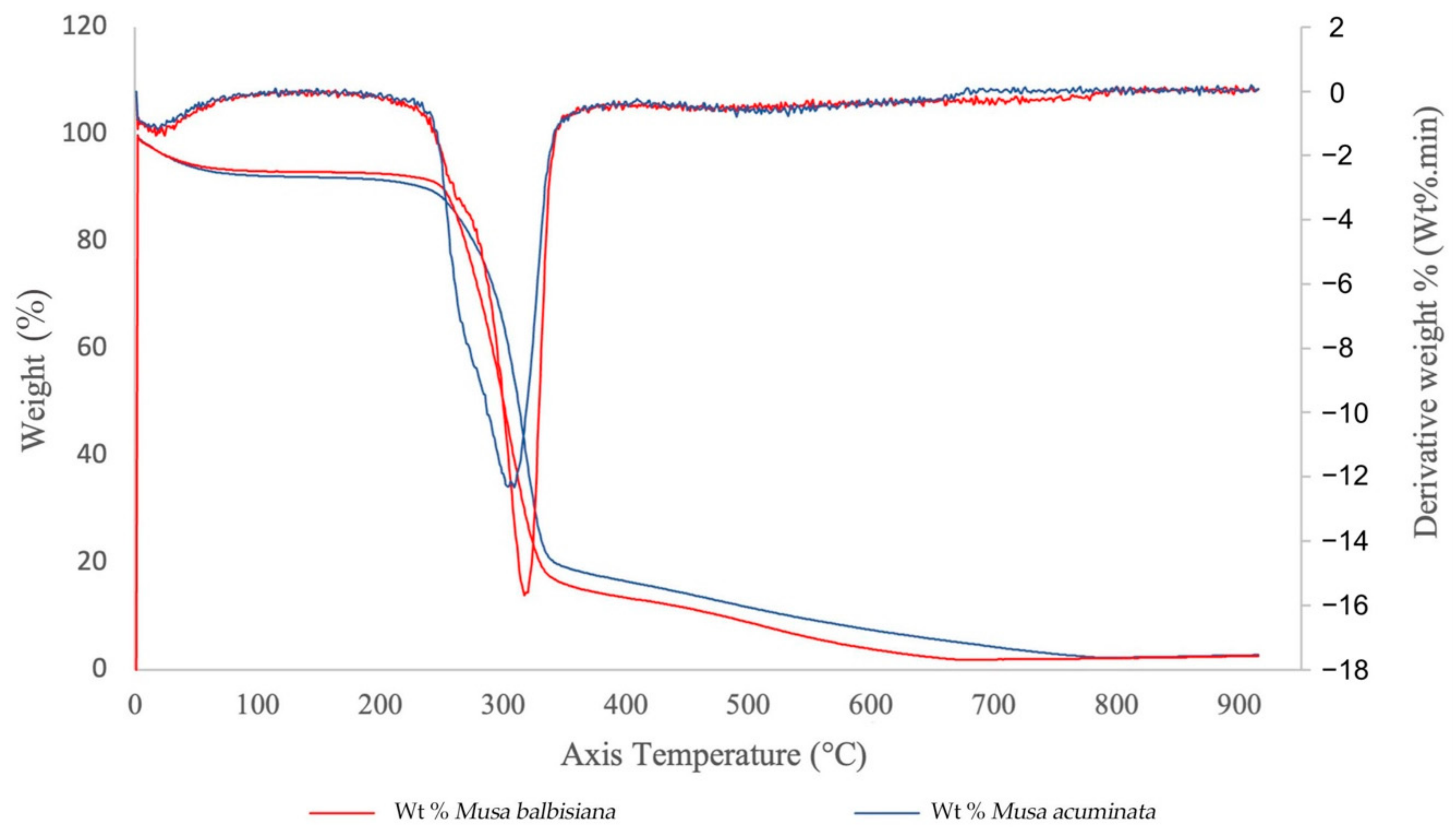
TGA curves of CNFs from M. acuminata and M. balbisiana pseudostem.
For M. acuminata, the most significant contrast is the lack of vibration at 1238 cm−1. The low value at 1242 cm−1 is associated with the C-O-C extension of the lignin aryl-alkyl ether bond [26]. The two peaks disappear from the spectrum of bleached pulp fibers and nanofibers. This was caused by the removal of lignin during chemical treatment. The peak at 1154 cm−1, on the other hand, is because of C-O-C vibration hemicellulose [36]. The peak slowly disappeared as the treatment process proceeded. The disappearance of the peak in the graph indicates that a large portion of hemicelluloses was removed during the pre-treatment and bleaching process. The peak, however, disappeared after the hydrolysis process. This suggests the acid hydrolysis process continuously removed hemicelluloses and lignin from the fiber. The peak 1154 cm−1 also indicates the vibration band for an amorphous substance [37]. Thus, it also suggests that the acid hydrolyzing process produces highly crystalline cellulose and reduces the amorphous content. In addition, the 1640 cm−1 peak in the spectrum of nanofibers and bleached pulp fibers is associated with cellulosic water absorption [38]. As for M. balbisiana, the broad region 3700–3200 cm−1 is related to OH vibrations. The solid and broad band at 3413 cm−1 was attributed to the stretching vibration of hydroxyl groups (-OH).
3.4. Thermogravimetric Analysis (TGA) of Commercial and Native Banana Pseudostem
The TGA curves for the M. acuminata and M. balbisiana pseudostem CNFs are shown in Figure 8. Only one degradation step was observed for both materials. The CNF from both sources generally started its decomposition easily, while the weight loss mainly happened at 263–365 °C. From the observation, the weight loss for both samples are related to evaporation and evacuation of absorbed and bound water [39]. The CNF from M. acuminata pseudostem started to degrade at 263 °C, whereas the M. balbisiana pseudostem started to degrade at higher temperature 280 °C indicating higher thermal stability. The degradation temperature difference is caused by the higher crystallinity of the CNF, which resulted in the further elimination of hemicelluloses and pectin. However, the difference in the solid residue from both samples is not statistically significant (~2.5–2.7% solid residue) for both. The pyrolysis of non-cellulosic materials produces a higher residual weight [40]. Thus, the low residual weight of CNF from both pseudostems indicate that the sample is highly cellulosic.
The initial decomposition temperature of CNF with some modifications, such as hexagonal boron nitride (h-BN), which is an excellent thermally conductive and electrically insulative material, is 205 °C, which demonstrates acceptable thermal stability and mechanical properties for electronics as a thermal interface and packing material [41]. In addition, modified aerogels containing methylene diphenyl dissocyanate (MDI) reached 353.6 °C. Because of the low oxygen concentration of the aromatic moiety in the MDI structure, it is thought that the mass was preserved in modified lignocellulosic nanofibril (LCNF) aerogel at the same temperature. Additionally, since there were more MDI structures, the modified aerogel had greater residual mass after pyrolysis with a higher MDI content. These comparisons clearly demonstrate the benefits of using MDI to maintain CNF’s thermal stability [42].
3.5. Materials Composition of Banana Stem Fiber before Pre-Treatment and Bleaching
The results depicted in Table 2 show that the polyose fraction in the treated fiber was enhanced to 93.16% for M. acuminata and 93.36% for M. balbisiana, whereas the hemicellulose, polymer and extractives contents was reduced. This high cellulose content provides toughness, strength, rigidity, and structural constancy to the banana pseudostem fiber, whereas hemicellulose decreases the fiber strength [43]. In this study, the hemicellulose content in M. acuminata and M. balbisiana is 5.76% and 5.60%, respectively. Lignin protects the plant against microorganism attack and conjointly influences the morphology, structural properties, bonding nature and wetness resistance of the fiber [44]. In this study, M. acuminata and M. balbisiana contain 0.73% and 1.08% lignin, respectively, in comparison to cellulose from hop stem (Humulus lupulus), which contains 44% cellulose, 13% hemicellulose, and 26% lignin [45].

Table 2.
Lignocellulosic and extractives content of M. acuminata and M. balbisiana pseudostem before pre-treatment and after bleaching.
4. Conclusions
Nanocellulose was successfully extracted from the pseudostem of the banana tree. The findings showed that lignin and hemicellulose removal can be achieved through pre-treatment, bleaching, and acid hydrolysis. The nano size can be obtained from these processes and the initial weight was reduced by 62%. The diameter size of the CNF was successfully determined as 80.31 ± 21.3 nm using TEM. Evidence of the degradation of non-cellulosic materials was found in the FTIR spectra. For M. acuminata, the disappearance of peak 1154 cm−1 was due to C-O-C vibration hemicellulose. Meanwhile, the peak 1238 cm−1 was found to be associated with the C-O-C stretching of the aryl-alkyl ether linkage in lignin. For M. balbisiana, a solid and broad band at 3413 cm−1 was attributed to the stretching vibration of hydroxyl groups (-OH). The XRD analysis showed that the crystallinity index of CNF for M. acuminata was 65.68%, whereas M. balbisiana was higher at 75.37%. TGA analysis showed that M. balbisiana pseudostem started degrading at a higher temperature (280 °C) compared to M. acuminata at 263 °C.
Author Contributions
Conceptualization, N.K.; Data curation, M.S.M.; Formal analysis, M.H.S.; Funding acquisition, M.B.M.S.; Methodology, M.S.M. and M.H.S.; Project administration, N.K.; Resources, P.L. and W.S.C.; Supervision, N.K. and M.B.M.S.; Writing—original draft, M.S.M.; Writing—review & editing, N.K. and M.B.M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ministry of Higher Education Malaysia (MoHE) grant number FRGS/1/2020/TK0/SWIN/03/5 and the APC was funded by grant number FRGS/1/2020/TK0/SWIN/03/5.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors gratefully acknowledge the Ministry of Higher Education (MoHE) Malaysia for the FRGS grant (FRGS/1/2020/TK0/SWIN/03/5) and the Research Management Centre (RMC) of Universiti Putra Malaysia for the grant (GP-IPM 9653300) that supported this research study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Soni, R.; Asoh, T.-A.; Uyama, H. Cellulose nanofiber reinforced starch membrane with high mechanical strength and durability in water. Carbohydr. Polym. 2020, 238, 116203. [Google Scholar] [CrossRef] [PubMed]
- Kargarzadeh, H.; Mariano, M.; Huang, J.; Lin, N.; Ahmad, I.; Dufresne, A.; Thomas, S. Recent developments on nanocellulose reinforced polymer nanocomposites: A review. Polymer 2017, 132, 368–393. [Google Scholar] [CrossRef]
- Shen, G.; Yu, G.; Wu, H.; Li, S.; Hou, X.; Li, M.; Li, Q.; Liu, X.; Zhou, M.; Chen, A.; et al. Incorporation of Lipids into Wheat Bran Cellulose/Wheat Gluten Composite Film Improves Its Water Resistance Properties. Membranes 2021, 12, 18. [Google Scholar] [CrossRef] [PubMed]
- Subagyo, A.; Chafidz, A. Banana Pseudo-Stem Fiber: Preparation, Characteristics, and Applications. In Banana Nutrition—Function and Processing Kinetics; IntechOpen: London, UK, 2020; pp. 1–19. [Google Scholar]
- Abdullah, N.; Sulaiman, F.; Taib, R.M. Characterization of banana (Musa spp.) plantation wastes as a potential renewable energy source. AIP Conf. Proc. 2013, 1528, 325–330. [Google Scholar] [CrossRef]
- Ingale, S.; Joshi, S.J.; Gupte, A. Production of bioethanol using agricultural waste: Banana pseudo stem. Braz. J. Microbiol. 2014, 45, 885–892. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, T.; Danish, M. Prospects of banana waste utilization in wastewater treatment: A review. J. Environ. Manag. 2018, 206, 330–348. [Google Scholar] [CrossRef]
- Cordeiro, N.; Belgacem, M.; Torres, I.; Moura, J. Chemical composition and pulping of banana pseudo-stems. Ind. Crop. Prod. 2004, 19, 147–154. [Google Scholar] [CrossRef]
- Li, K.; Fu, S.; Zhan, H.; Zhan, Y.; Lucia, L.A. Analysis of the chemical composition and morphological structure of banana pseudo-stem. BioResources 2010, 5, 576–585. [Google Scholar] [CrossRef]
- Shaghaleh, H.; Xu, X.; Wang, S. Current progress in production of biopolymeric materials based on cellulose, cellulose nanofibers, and cellulose derivatives. RSC Adv. 2018, 8, 825–842. [Google Scholar] [CrossRef] [Green Version]
- Boufi, S.; González, I.; Delgado-Aguilar, M.; Tarrés, Q.; Pèlach, M.; Mutjé, P. Nanofibrillated cellulose as an additive in papermaking process: A review. Carbohydr. Polym. 2016, 154, 151–166. [Google Scholar] [CrossRef]
- Espinosa, E.; Tarrés, Q.; Domínguez-Robles, J.; Delgado-Aguilar, M.; Mutjé, P.; Rodríguez, A. Recycled fibers for fluting production: The role of lignocellulosic micro/nanofibers of banana leaves. J. Clean. Prod. 2018, 172, 233–238. [Google Scholar] [CrossRef]
- Pelissari, F.M.; Andrade-Mahecha, M.M.; Sobral, P.J.D.A.; Menegalli, F.C. Nanocomposites based on banana starch reinforced with cellulose nanofibers isolated from banana peels. J. Colloid Interface Sci. 2017, 505, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Tibolla, H.; Pelissari, F.M.; Menegalli, F.C. Cellulose nanofibers produced from banana peel by chemical and enzymatic treatment. LWT-Food Sci. Technol. 2014, 59, 1311–1318. [Google Scholar] [CrossRef]
- Razab, M.K.A.A.; Ghani, R.S.M.; Zin, F.A.M.; Yusoff, N.A.A.N.; Noor, A.M. Isolation and Characterization of Cellulose Nanofibrils from Banana Psof Cellulose Nanofibrils from Banana Pseudostem, Oil Palm Trunk, and Kenaf Bast Fibers Using Chemicals and High-intensity Ultrasonication. J. Nat. Fibers 2021, 1–14. [Google Scholar] [CrossRef]
- Hidayati, S.; Zuidar, A.S.; Satyajaya, W.; Murhadi; Retnowati, D. Isolation and characterization of formacell Lignins from oil empty fruits bunches. IOP Conf. Ser. Mater. Sci. Eng. 2018, 344, 012006. [Google Scholar] [CrossRef] [Green Version]
- Aziz, N.A.A.; Ho, L.H.; Bin Azahari, B.; Bhat, R.; Cheng, L.-H.; Ibrahim, M.N.M. Chemical and functional properties of the native banana (Musaacuminata × balbisiana Colla cv. Awak) pseudo-stem and pseudo-stem tender core flours. Food Chem. 2011, 128, 748–753. [Google Scholar] [CrossRef]
- Salehudin, M.H.; Salleh, E.; Mamat, S.N.H.; Muhamad, I.I. Starch based Active Packaging Film Reinforced with Empty Fruit Bunch (EFB) Cellulose Nanofiber. Procedia Chem. 2014, 9, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.M.; Kadirgama, K. Influence Pre-Treatment on the Properties of Lignocellulose Based Biocomposite. In National Conference on Postgraduate Research; University Malaysia Pahang: Pekan, Malaysia, 2009; pp. 67–78. [Google Scholar]
- Liping, T.; Yongcheng, Y.; Xuezhi, L.; Jian, Z.; Yinbo, Q.; Yuen, M.C.; Soh, K.L. Pretreatment of empty fruit bunch from oil palm for fuel ethanol production and proposed biorefinery process. Bioresour. Technol. 2013, 135, 275–282. [Google Scholar] [CrossRef]
- Griffith, O.M. Practical Techniques for Centrifugal Separations Application Guide; Thermo Fisher Scientific Inc.: Waltham, MA, USA, 2010; pp. 1–28. Available online: https://thermofisher.co.nz/Uploads/file/Scientific/Applications/Equipment-Furniture/Practical-Techniques-for-Centrifugal-Separations.pdf (accessed on 20 December 2021).
- Lani, N.S.; Ngadi, N.; Johari, A.; Jusoh, M. Isolation, Characterization, and Application of Nanocellulose from Oil Palm Empty Fruit Bunch Fiber as Nanocomposites. J. Nanomater. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Fazeli, M.; Keley, M.; Biazar, E. Preparation and characterization of starch-based composite films reinforced by cellulose nanofibers. Int. J. Biol. Macromol. 2018, 116, 272–280. [Google Scholar] [CrossRef]
- Mansor, A.M.; Lim, J.S.; Ani, F.N.; Hashim, H.; Ho, W.S. Characteristics of cellulose, hemicellulose and lignin of MD2 pineapple biomass. Chem. Eng. Trans. 2019, 72, 79–84. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E., Jr.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Hamzah, F.; Idris, A.; Shuan, T.K. Preliminary study on enzymatic hydrolysis of treated oil palm (Elaeis) empty fruit bunches fibre (EFB) by using combination of cellulase and β 1-4 glucosidase. Biomass Bioenergy 2011, 35, 1055–1059. [Google Scholar] [CrossRef]
- Meng, F.; Wang, G.; Du, X.; Wang, Z.; Xu, S.; Zhang, Y. Extraction and characterization of cellulose nanofibers and nanocrystals from liquefied banana pseudo-stem residue. Compos. Part B Eng. 2018, 160, 341–347. [Google Scholar] [CrossRef]
- Phanthong, P.; Reubroycharoen, P.; Hao, X.; Xu, G.; Abudula, A.; Guan, G. Nanocellulose: Extraction and application. Carbon Resour. Convers. 2018, 1, 32–43. [Google Scholar] [CrossRef]
- Rajinipriya, M.; Nagalakshmaiah, M.; Robert, M.; Elkoun, S. Importance of Agricultural and Industrial Waste in the Field of Nanocellulose and Recent Industrial Developments of Wood Based Nanocellulose: A Review. ACS Sustain. Chem. Eng. 2018, 6, 2807–2828. [Google Scholar] [CrossRef]
- Li, R.; Fei, J.; Cai, Y.; Li, Y.; Feng, J.; Yao, J. Cellulose whiskers extracted from mulberry: A novel biomass production. Carbohydr. Polym. 2009, 76, 94–99. [Google Scholar] [CrossRef]
- Zope, G.; Goswami, A.; Kulkarni, S. Isolation and Characterization of Cellulose Nanocrystals Produced by Acid Hydrolysis from Banana Pseudostem. BioNanoScience 2022, 1–9. [Google Scholar] [CrossRef]
- Sanchez-Salvador, J.L.; Campano, C.; Balea, A.; Tarrés, Q.; Delgado-Aguilar, M.; Mutjé, P.; Blanco, A.; Negro, C. Critical comparison of the properties of cellulose nanofibers produced from softwood and hardwood through enzymatic, chemical and mechanical processes. Int. J. Biol. Macromol. 2022, 205, 220–230. [Google Scholar] [CrossRef]
- Teixeira, E.D.M.; Bondancia, T.J.; Teodoro, K.B.R.; Corrêa, A.C.; Marconcini, J.M.; Mattoso, L.H.C. Sugarcane bagasse whiskers: Extraction and characterizations. Ind. Crop. Prod. 2011, 33, 63–66. [Google Scholar] [CrossRef]
- Martínez-Sanz, M.; Olsson, R.T.; Lopez-Rubio, A.; Lagaron, J.M. Development of electrospun EVOH fibres reinforced with bacterial cellulose nanowhiskers. Part I: Characterization and method optimization. Cellulose 2010, 18, 335–347. [Google Scholar] [CrossRef]
- Feldman, D. Wood—Chemistry, ultrastructure, reactions, by D. Fengel and G. Wegener, Walter de Gruyter, Berlin and New York, 1984, 613 pp. Price: 245 DM. J. Polym. Sci. Part C Polym. Lett. 1985, 23, 601–602. [Google Scholar] [CrossRef]
- Yano, S.; Hatakeyama, H.; Hatakeyama, T. Effect of hydrogen bond formation on dynamic mechanical properties of amorphous cellulose. J. Appl. Polym. Sci. 1976, 20, 3221–3231. [Google Scholar] [CrossRef]
- Jonoobi, M.; Khazaeian, A.; Tahir, P.M.; Azry, S.S.; Oksman, K. Characteristics of cellulose nanofibers isolated from rubberwood and empty fruit bunches of oil palm using chemo-mechanical process. Cellulose 2011, 18, 1085–1095. [Google Scholar] [CrossRef]
- Khawas, P.; Das, A.J.; Deka, S.C. Production of renewable cellulose nanopaper from culinary banana (Musa ABB) peel and its characterization. Ind. Crop. Prod. 2016, 86, 102–112. [Google Scholar] [CrossRef]
- Fahma, F.; Iwamoto, S.; Hori, N.; Iwata, T.; Takemura, A. Effect of pre-acid-hydrolysis treatment on morphology and properties of cellulose nanowhiskers from coconut husk. Cellulose 2010, 18, 443–450. [Google Scholar] [CrossRef]
- Wang, X.; Sun, M.; Wang, R.; Jiao, L.; Bian, H.; Dai, H. Promoting h-BN dispersion in cellulose-based composite by lignosulfonate for regulatable effectual thermal management. Mater. Des. 2022, 214, 110379. [Google Scholar] [CrossRef]
- Bian, H.; Duan, S.; Wu, J.; Fu, Y.; Yang, W.; Yao, S.; Zhang, Z.; Xiao, H.; Dai, H.; Hu, C. Lignocellulosic nanofibril aerogel via gas phase coagulation and diisocyanate modification for solvent absorption. Carbohydr. Polym. 2022, 278, 119011. [Google Scholar] [CrossRef]
- Sarala, R. Characterization of a new natural cellulosic fiber extracted from Derris scandens stem. Int. J. Biol. Macromol. 2020, 165, 2303–2313. [Google Scholar] [CrossRef]
- Prasanna, N.S.; Mitra, J. Isolation and characterization of cellulose nanocrystals from Cucumis sativus peels. Carbohydr. Polym. 2020, 247, 116706. [Google Scholar] [CrossRef] [PubMed]
- Kanai, N.; Nishimura, K.; Umetani, S.; Saito, Y.; Saito, H.; Oyama, T.; Kawamura, I. Upcycling of Waste Hop Stems into Cellulose Nanofibers: Isolation and Structural Characterization. ACS Agric. Sci. Technol. 2021, 1, 347–354. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).