Comparison of the Electrodialysis Performance in Tartrate Stabilization of a Red Wine Using Aliphatic and Aromatic Commercial and Modified Ion-Exchange Membranes
Abstract
1. Introduction
2. Materials and Methods
2.1. Membranes
2.2. Solutions
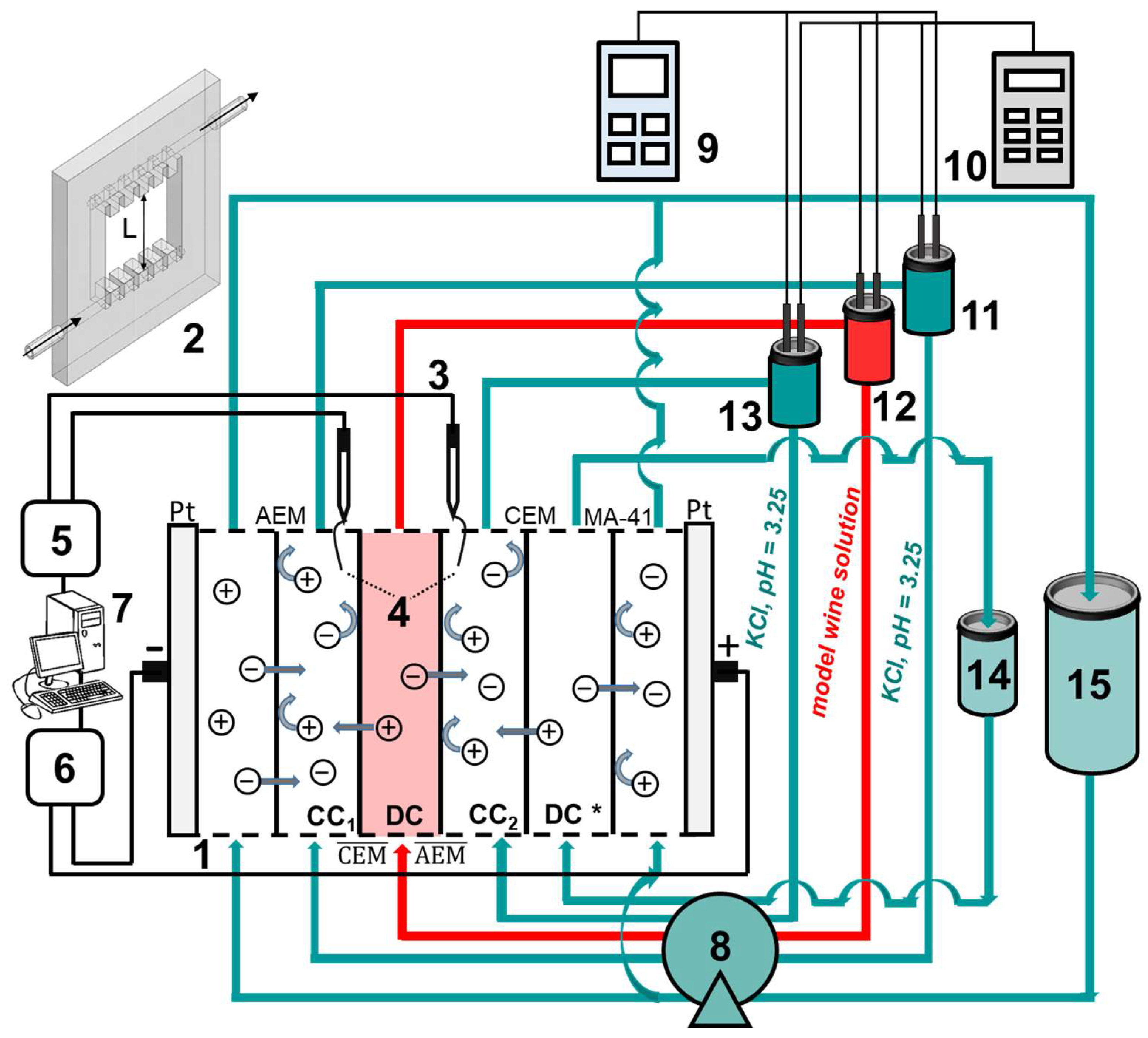
2.3. Electrodialysis Removal of Tartrates
2.4. Membrane Fouling Study
2.5. Membrane Surface Modification
3. Results and Discussion
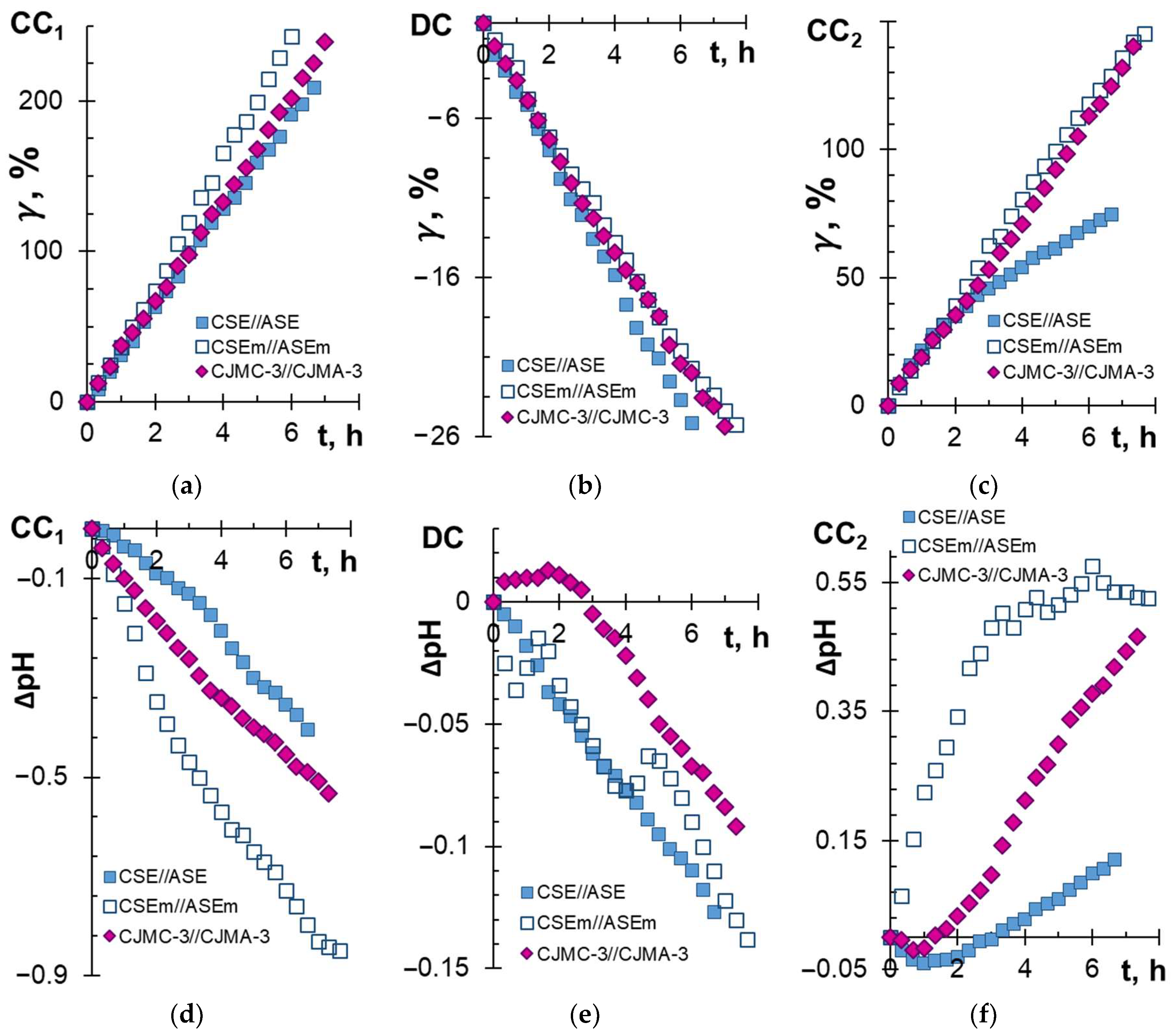
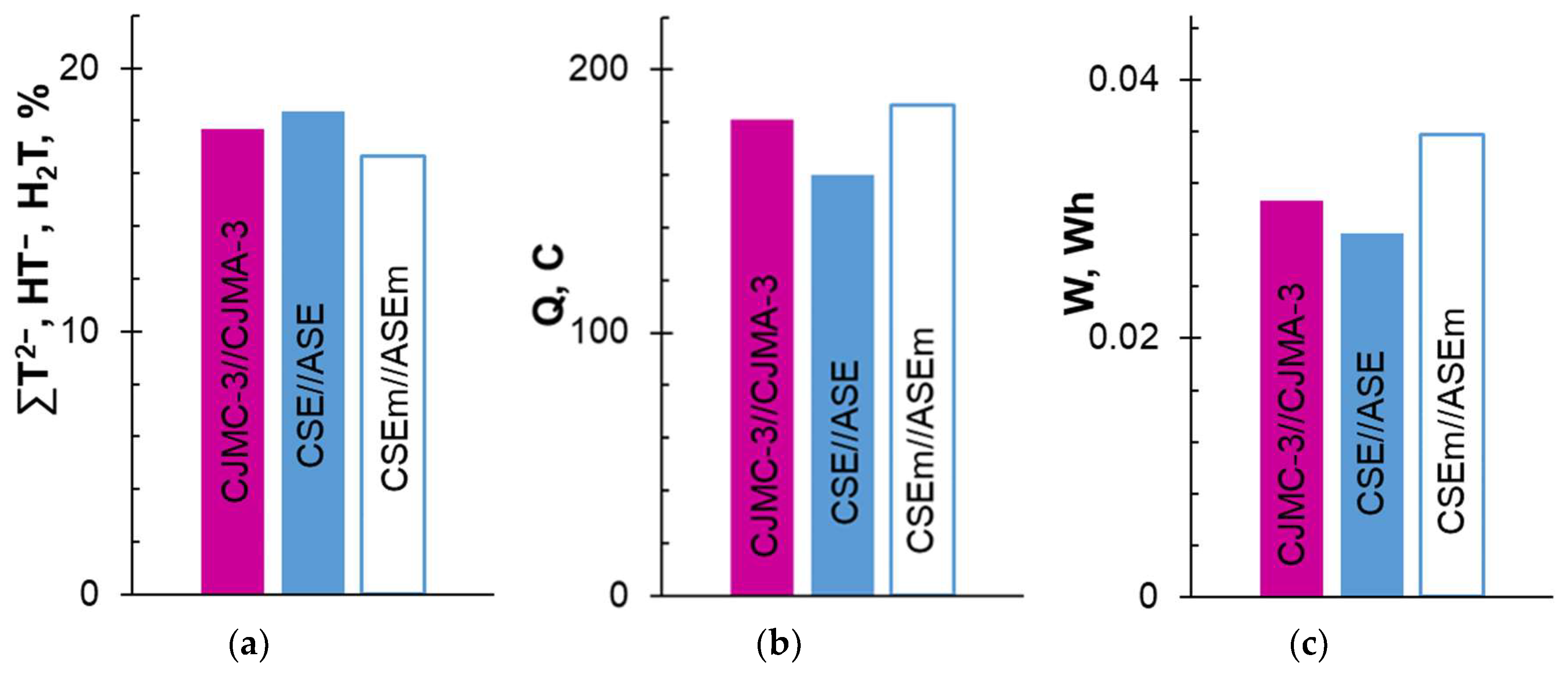
3.1. ED Processing of the Model Solution
3.2. Fouling of Membranes in Electrodialysis
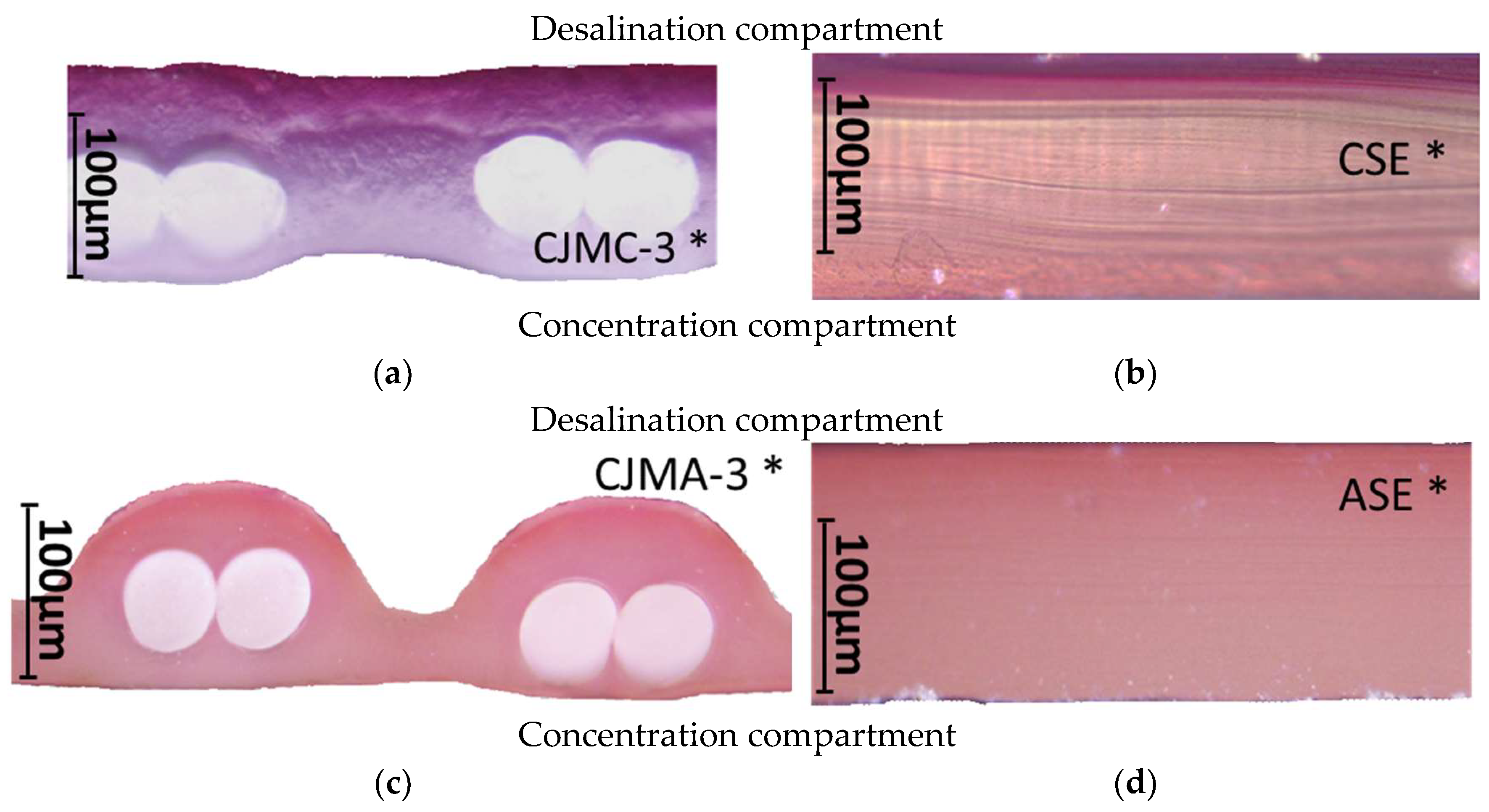
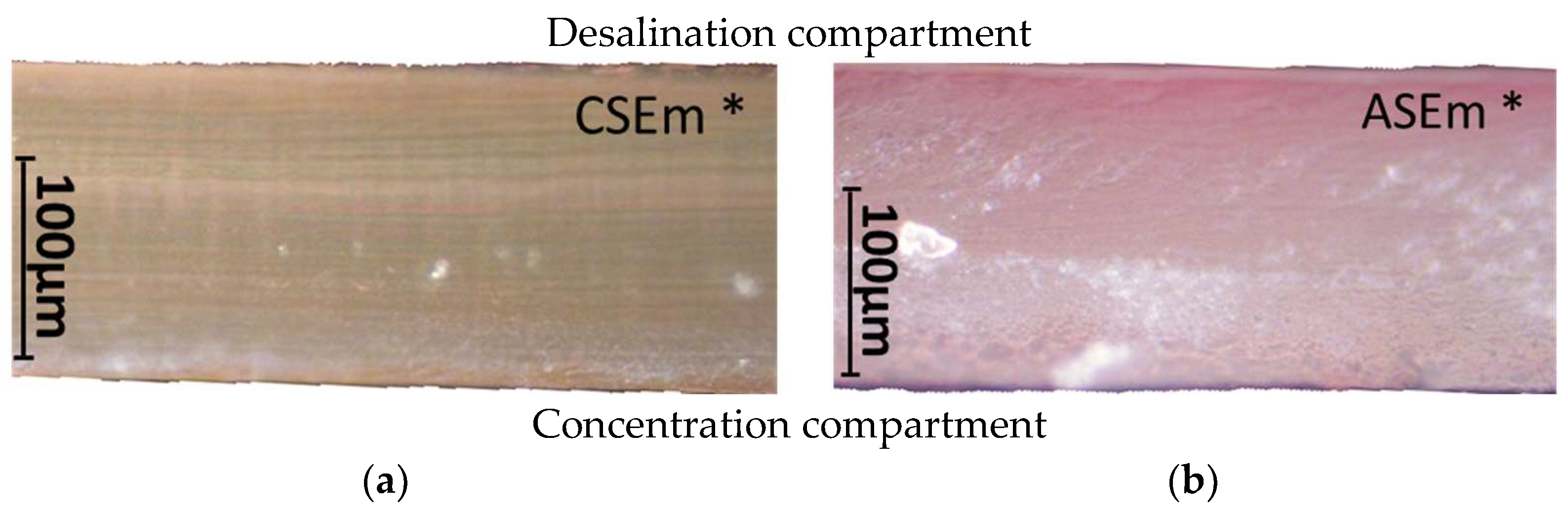
3.2.1. Fouling Color Indication
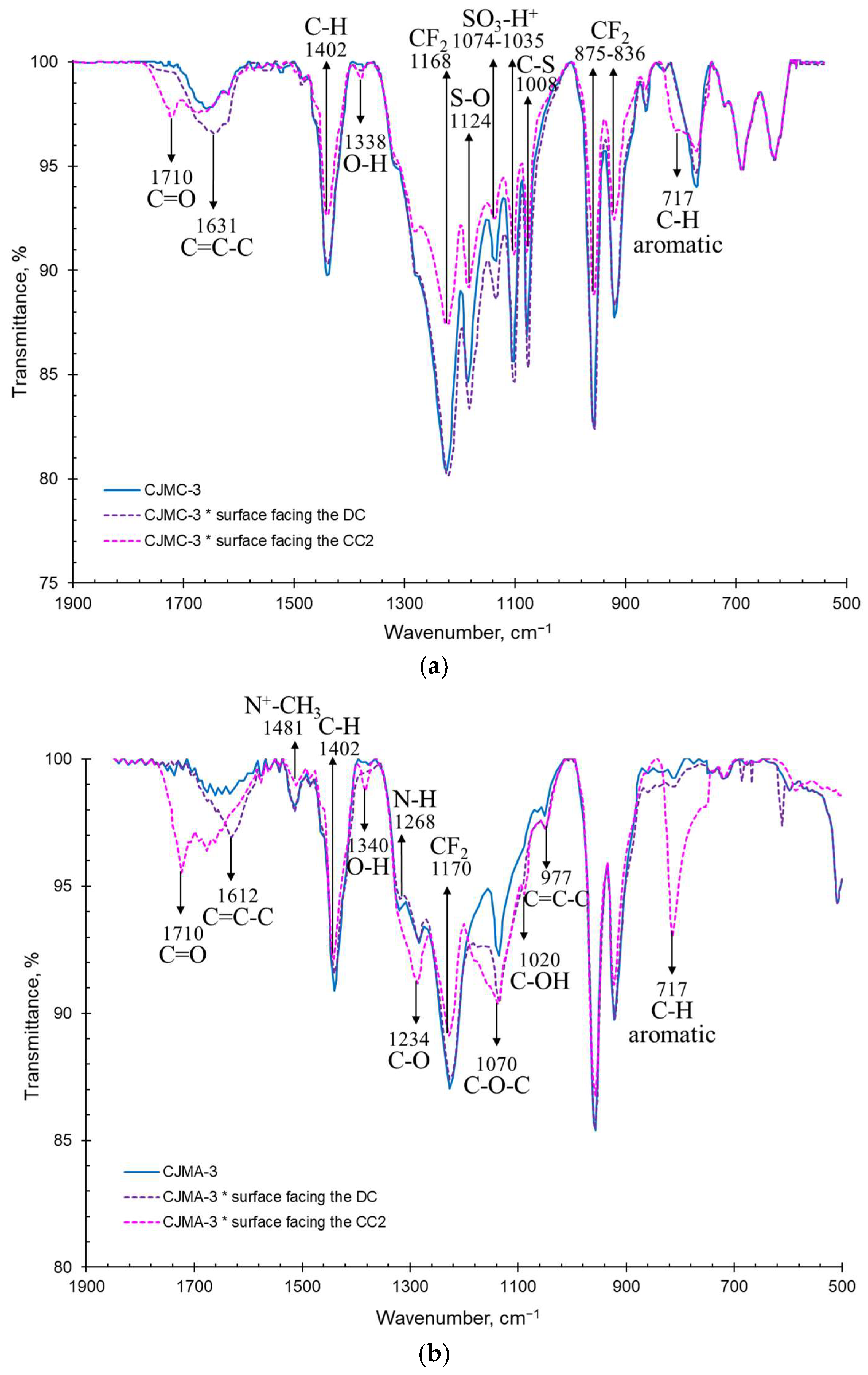
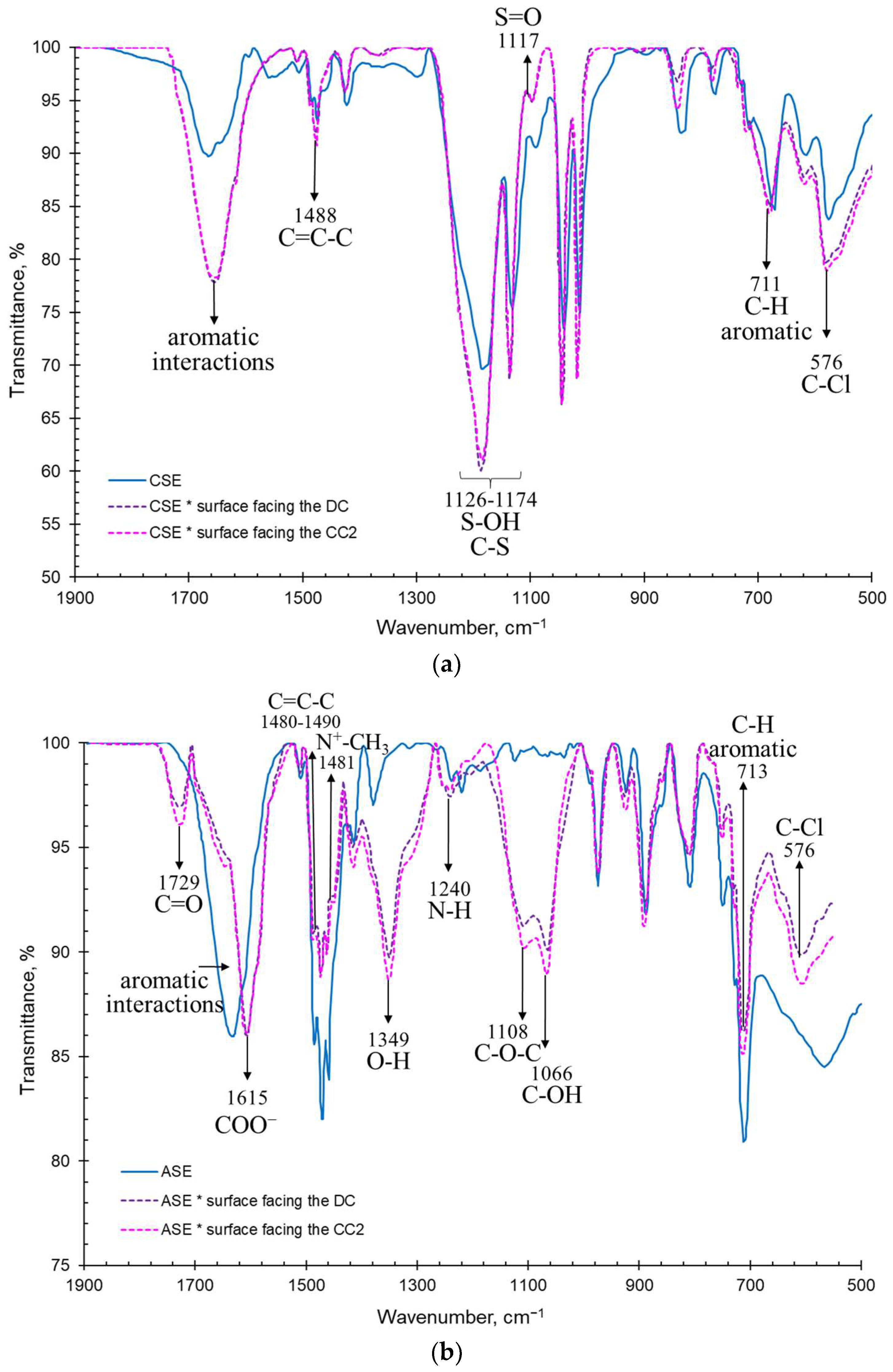
3.2.2. IR Spectra and Surface Contact Angles of the Membranes Surfaces
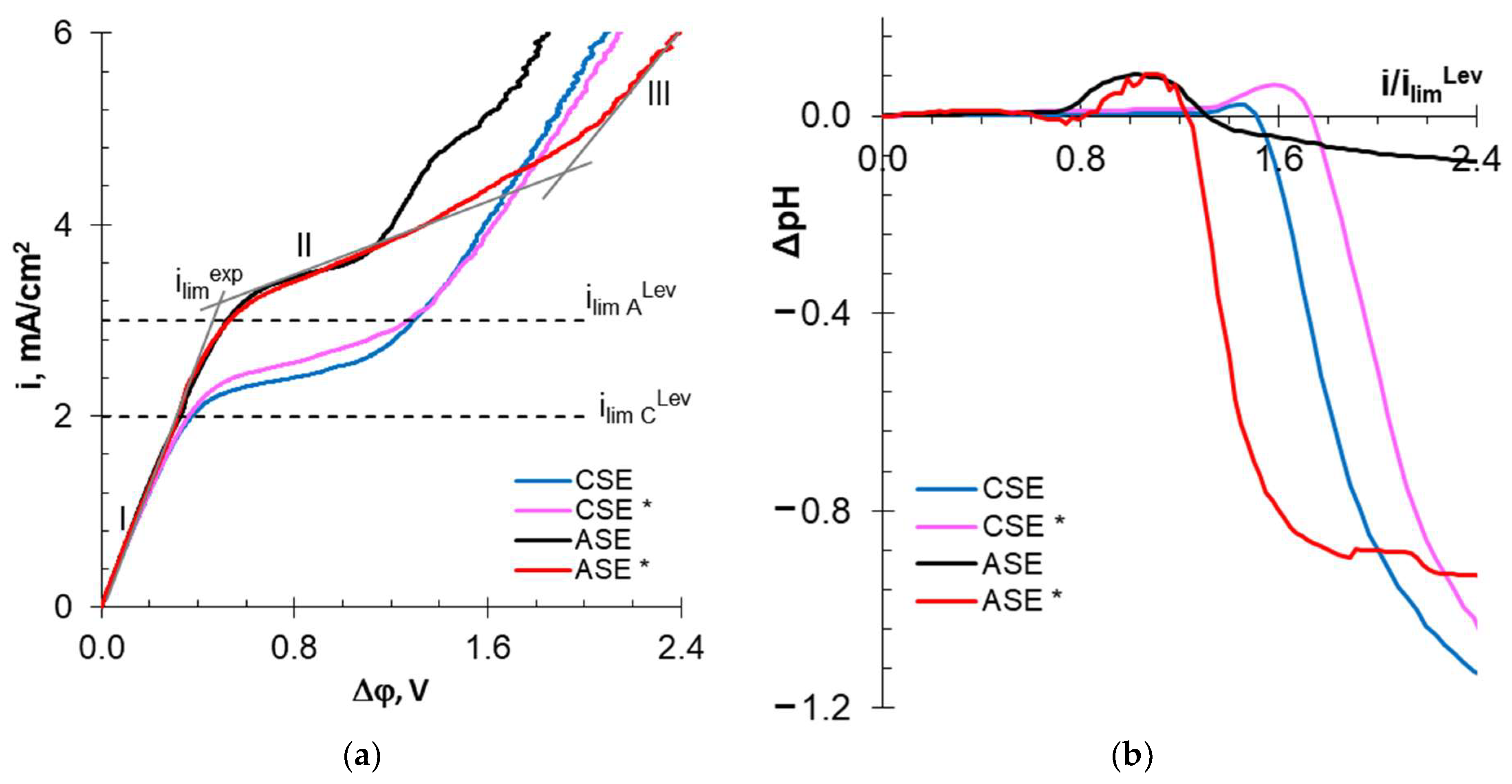
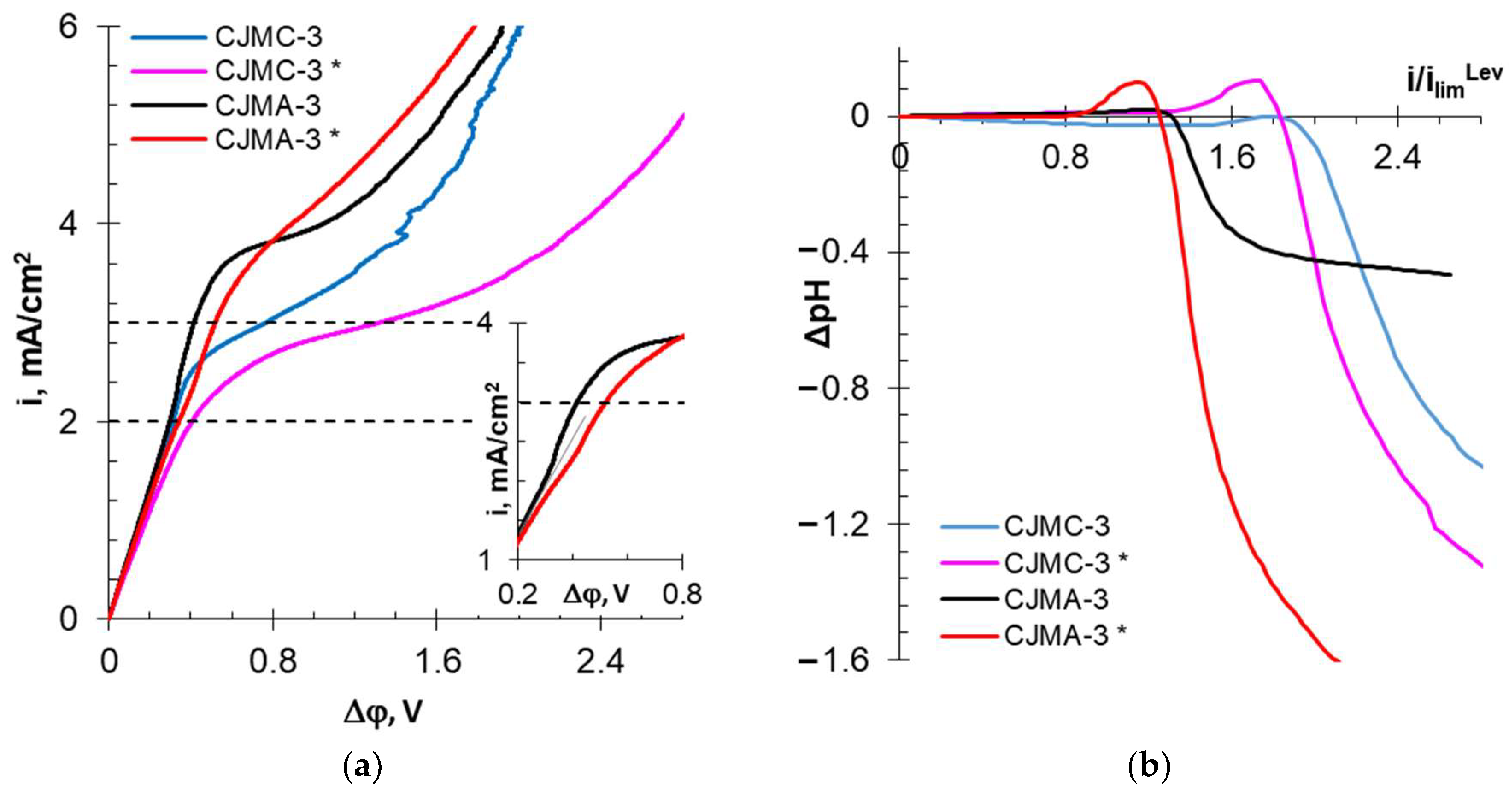
3.2.3. Current–Voltage Curves and Electrical Conductivity of Membranes
3.3. Impact of Membrane Modification on the Fouling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yakovlev, V.A.; Stepanova, A.N. Analysis and Prospects for the Development of Agribusiness: Regional Aspect. IOP Conf. Ser. Earth Environ. Sci. 2020, 548, 022034. [Google Scholar] [CrossRef]
- Wollan, D. Membrane and Other Techniques for the Management of Wine Composition. In Managing Wine Quality; Elsevier: Amsterdam, The Netherlands, 2010; pp. 133–163. [Google Scholar]
- Poirier, D.; Bennasar, M.; Tarodo de La Fuente, B.; Gillot, J.; Garcera, D. Clarification et Stabilisation Des Vins Par Ultrafiltration Tangentielle Sur Membranes Minérales. Lait 1984, 64, 141–142. [Google Scholar] [CrossRef]
- El Rayess, Y.; Mietton-Peuchot, M. Membrane Technologies in Wine Industry: An Overview. Crit. Rev. Food Sci. Nutr. 2016, 56, 2005–2020. [Google Scholar] [CrossRef]
- Tamime, A.Y. (Ed.) Membrane Processing: Dairy and Beverage Applications; John Wiley & Sons Ltd.: Chichester, UK, 2013; ISBN 978-0-4706-5584-9. [Google Scholar]
- Anderson, K.; Nelgen, S. Global Wine Markets, 1961 to 2009: A Statistical Compendium; University of Adelaide Press: Adelaide, Australia, 2011. [Google Scholar]
- Thoukis, G. Chemistry of Wine Stabilization: A Review. In Chemistry of Winemaking; Advances in Chemistry; American Chemical Society: Washington, DC, USA, 1974; Volume 137, pp. 116–133. ISBN 9780841202085. [Google Scholar]
- Gnilomedova, N.; Anikina, N.; Vesyutova, A.; Oleinikova, V.; Gavrish, V.; Chayka, T. Identifying Tartrate Salt Crystals in Wine Sediment. Food Process. Tech. Technol. 2022, 52, 490–499. [Google Scholar] [CrossRef]
- Low, L.L.; O’Neill, B.; Ford, C.; Godden, J.; Gishen, M.; Colby, C. Economic Evaluation of Alternative Technologies for Tartrate Stabilisation of Wines. Int. J. Food Sci. Technol. 2008, 43, 1202–1216. [Google Scholar] [CrossRef]
- Jackson, R.S. Wine Science: Principles and Applications, 3rd ed.; Academic Press: Cambridge, MA, USA, 2008; ISBN 978-0-12-373646-8. [Google Scholar]
- Cabrita, M.J.; Garcia, R.; Catarino, S. Recent Developments in Wine Tartaric Stabilization; Jordão, A.M., Cosme, F., Eds.; Nova Science Publishers: New York, NY, USA, 2016; pp. 49–63. ISBN 978-1-63484-883-1. [Google Scholar]
- Filipe-Ribeiro, L.; Milheiro, J.; Guise, R.; Vilamarim, R.; Fraga, J.B.; Martins-Gomes, C.; Nunes, F.M.; Cosme, F. Efficiency of Carboxymethylcellulose in Red Wine Tartaric Stability: Effect on Wine Phenolic Composition, Chromatic Characteristics and Colouring Matter Stability. Food Chem. 2021, 360, 129996. [Google Scholar] [CrossRef]
- Bosso, A.; Motta, S.; Panero, L.; Lucini, S.; Guaita, M. Use of Potassium Polyaspartate for Stabilization of Potassium Bitartrate in Wines: Influence on Colloidal Stability and Interactions with Other Additives and Enological Practices. J. Food Sci. 2020, 85, 2406–2415. [Google Scholar] [CrossRef]
- Ortega-Heras, M.; González-SanJosé, M.L. Mannoproteins and Enology: Tartrate and Protein Stabilization. In Recent Advances in Wine Stabilization and Conservation Technologies; Nova Science Publishers: Hauppauge, NY, USA, 2016; pp. 95–109. [Google Scholar]
- Lankhorst, P.P.; Voogt, B.; Tuinier, R.; Lefol, B.; Pellerin, P.; Virone, C. Prevention of Tartrate Crystallization in Wine by Hydrocolloids: The Mechanism Studied by Dynamic Light Scattering. J. Agric. Food Chem. 2017, 65, 8923–8929. [Google Scholar] [CrossRef]
- Lasanta, C.; Gomez Benitez, J. Tartrate Stabilization of Wines. Trends Food Sci. Technol. 2012, 28, 52–59. [Google Scholar] [CrossRef]
- Ponce, F.; Mirabal-Gallardo, Y.; Versari, A.; Laurie, V. The Use of Cation Exchange Resins in Wines: Effects on PH, Tartrate Stability, and Metal Content. Cienc. Investig. Agrar. 2018, 45, 82–92. [Google Scholar] [CrossRef]
- Houldsworth, D.W. Demineralization of Whey by Means of Ion Exchange and Electrodialysis. Int. J. Dairy Technol. 1980, 33, 45–51. [Google Scholar] [CrossRef]
- Purolite FDA Conditioning of Ion Exchange Resin before Food Use. Available online: https://www.purolite.com/index/core-technologies/industry/food-and-beverage/sweetener-applications/corn-sweetener-refining-with-ion-exchange-resins/fda-conditioning-before-use (accessed on 29 December 2022).
- Fernandes, C.; Dos Santos, P.C.; De Pinho, M.N. Wine Tartaric Stabilization by Electrodialysis. In Proceedings of the CHISA 2006—17th International Congress of Chemical and Process Engineering, Prague, Czech Republic, 27–31 August 2006. [Google Scholar]
- Claus, H.; Tenzer, S.; Sobe, M.; Schlander, M.; König, H.; Fröhlich, J. Effect of Carboxymethyl Cellulose on Tartrate Salt, Protein and Colour Stability of Red Wine. Aust. J. Grape Wine Res. 2014, 20, 186–193. [Google Scholar] [CrossRef]
- Moutounet, M.; Bouissou, D.; Escudier, J.L. Effets de Traitement de Stabilisation Tartrique de Vins Rouges Par Une Gomme de Cellulose (Carboxymethylcellulose). Available online: https://www.infowine.com/intranet/libretti/libretto8096-01-1.pdf (accessed on 29 December 2022).
- Fischler, F. Commission Regulation (EC) No 2053/97 of 20 October 1997 Amending Regulation (EEC) No 3220/90 Laying down Conditions for the Use of Certain Oenological Practices Provided for in Council Regulaiton (EEC) No 822/87. Available online: https://www.efta.int/eea-lex/31997R2053 (accessed on 29 December 2022).
- Oenodia. The 2022 Innovation. Available online: https://eurodia.com/en/linnovation-2022-smart-by-oenodia-une-nouvelle-etoile-est-nee/ (accessed on 29 December 2022).
- Straits Research. Electrodialysis Equipment Market. Available online: https://straitsresearch.com/report/electrodialysis-equipment-market (accessed on 29 December 2022).
- Transfert, I. Eurodia/Oenodia: Eco-Friendly Membrane Processes. Available online: https://www.inrae-transfert.fr/en/107-bioprocedes-biotechnologies-blanches/327-eurodia-oenodia-eco-friendly-membrane-processes (accessed on 21 December 2021).
- Audinos, R.; Roson, J.P.; Jouret, C. Application of Electrodialysis to the Elimination of Certain Grape Juice and Wine Components. Connaiss. Vigne Vin 1979, 13, 229–239. [Google Scholar]
- Paronetto, L.; Paronetto, L.; Braido, A. Some Tests on Tartrate Stabilization of Musts and Wines by Electrodialysis. Vignevini 1977, 4, 9–15. [Google Scholar]
- Wucherpfennig, K.; Krueger, R. Stabilization of Grape Juice and Wine against Tartar by Means of Electrodialysis. In Membrane Ion-Exchange Freeze-Cone. Food Industry, Proceedings of the International Symposium on Separation Processes, Paris, France, 5–9 January 1975; A.P.R.I.A.: Paris, France, 1975. [Google Scholar]
- Escudier, J.L.; Moutounet, M.; Saint-Pierre, B.; Battle, J.L. Stabilisation Tartrique Des Vins Par Membranes: Resultats et Developments Technologiques. In Proceedings of the 11 ème Colloque Viticole et Oenologique, Montpellier, France, 1997. [Google Scholar]
- Gonçalves, F.; Fernandes, C.; Cameira dos Santos, P.; de Pinho, M.N. Wine Tartaric Stabilization by Electrodialysis and Its Assessment by the Saturation Temperature. J. Food Eng. 2003, 59, 229–235. [Google Scholar] [CrossRef]
- El Rayess, Y.; Achcouty, S.; Ghanem, C.; Rizk, Z.; Nehme, N. Clarification and Stabilization of Wines Using Membrane Processes. In Recent Advances in Wine Stabilization and Conservation Technologies; Nova Science Publishers: Hauppauge, NY, USA, 2016; pp. 111–135. ISBN 9781634848831. [Google Scholar]
- Vecino, X.; Reig, M.; Gibert, O.; Valderrama, C.; Cortina, J.L. Integration of Monopolar and Bipolar Electrodialysis Processes for Tartaric Acid Recovery from Residues of the Winery Industry. ACS Sustain. Chem. Eng. 2020, 8, 13387–13399. [Google Scholar] [CrossRef]
- Soares, P.A.M.H.; Geraldes, V.; Fernandes, C.; dos Santos, P.C.; de Pinho, M.N. Wine Tartaric Stabilization by Electrodialysis: Prediction of Required Deionization Degree. Am. J. Enol. Vitic. 2009, 60, 183–188. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology, Volume 2: The Chemistry of Wine—Stabilization and Treatments, 2nd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2006; ISBN 978-0-470-01038-9. [Google Scholar]
- Gómez Benítez, J.; Palacios Macías, V.; Szekely Gorostiaga, P.; Veas López, R.; Pérez Rodríguez, L. Comparison of Electrodialysis and Cold Treatment on an Industrial Scale for Tartrate Stabilization of Sherry Wines. J. Food Eng. 2003, 58, 373–378. [Google Scholar] [CrossRef]
- Mourgues, J. Utilisation Des Résines Échangeuses d’ions. Rev. Oenol. 1993, 19, 51–54. [Google Scholar]
- Cosme, F.; Vilela, A.; Jordão, A. The Role of Tartaric Acid in Grapes and Wines. In Advances in Chemistry Research; Nova Science Publishers: Hauppauge, NY, USA, 2017; ISBN 978-1-53612-791-1. [Google Scholar]
- Brilenok, N.S.; Vershinin, V.I.; Bakhareva, M.V. Evaluation of Polyphenols Antioxidant Capacity in the Presence of Complexants by FRAP Assay. Anal. Kontrol 2016, 20, 209–217. [Google Scholar] [CrossRef]
- Pellerin, G.; Bazinet, L.; Grenier, D. Effect of Cranberry Juice Deacidification on Its Antibacterial Activity against Periodontal Pathogens and Its Anti-Inflammatory Properties in an Oral Epithelial Cell Model. Food Funct. 2021, 12, 10470–10483. [Google Scholar] [CrossRef] [PubMed]
- Riponi, C.; Nauleau, F.; Amati, A.; Arfelli, G.; Castellari, M. Essais de Stabilisation Tartrique Des Vins Au Moyen de l’électrodialyse. Rev. Fr. D’oenol. 1992, 137, 59–63. [Google Scholar]
- Dammak, L.; Fouilloux, J.; Bdiri, M.; Larchet, C.; Renard, E.; Baklouti, L.; Sarapulova, V.; Kozmai, A.; Pismenskaya, N. A Review on Ion-Exchange Membrane Fouling during the Electrodialysis Process in the Food Industry, Part 1: Types, Effects, Characterization Methods, Fouling Mechanisms and Interactions. Membranes 2021, 11, 789. [Google Scholar] [CrossRef] [PubMed]
- Bdiri, M.; Perreault, V.; Mikhaylin, S.; Larchet, C.; Hellal, F.; Bazinet, L.; Dammak, L. Identification of Phenolic Compounds and Their Fouling Mechanisms in Ion-Exchange Membranes Used at an Industrial Scale for Wine Tartaric Stabilization by Electrodialysis. Sep. Purif. Technol. 2020, 233, 115995. [Google Scholar] [CrossRef]
- Zhao, Q.; Du, G.; Wang, S.; Zhao, P.; Cao, X.; Cheng, C.; Liu, H.; Xue, Y.; Wang, X. Investigating the Role of Tartaric Acid in Wine Astringency. Food Chem. 2023, 403, 134385. [Google Scholar] [CrossRef]
- Perreault, V.; Sarapulova, V.; Tsygurina, K.; Pismenskaya, N.; Bazinet, L. Understanding of Adsorption and Desorption Mechanisms of Anthocyanins and Proanthocyanidins on Heterogeneous and Homogeneous Cation-Exchange Membranes. Membranes 2021, 11, 136. [Google Scholar] [CrossRef]
- Mikhaylin, S.; Bazinet, L. Fouling on Ion-Exchange Membranes: Classification, Characterization and Strategies of Prevention and Control. Adv. Colloid Interface Sci. 2016, 229, 34–56. [Google Scholar] [CrossRef]
- Pismenskaya, N.; Bdiri, M.; Sarapulova, V.; Kozmai, A.; Fouilloux, J.; Baklouti, L.; Larchet, C.; Renard, E.; Dammak, L. A Review on Ion-Exchange Membranes Fouling during Electrodialysis Process in Food Industry, Part 2: Influence on Transport Properties and Electrochemical Characteristics, Cleaning and Its Consequences. Membranes 2021, 11, 811. [Google Scholar] [CrossRef]
- Ioannidou, S.M.; Filippi, K.; Kookos, I.K.; Koutinas, A.; Ladakis, D. Techno-Economic Evaluation and Life Cycle Assessment of a Biorefinery Using Winery Waste Streams for the Production of Succinic Acid and Value-Added Co-Products. Bioresour. Technol. 2022, 348, 126295. [Google Scholar] [CrossRef]
- Ncube, A.; Fiorentino, G.; Colella, M.; Ulgiati, S. Upgrading Wineries to Biorefineries within a Circular Economy Perspective: An Italian Case Study. Sci. Total Environ. 2021, 775, 145809. [Google Scholar] [CrossRef]
- Han, L. Current Strategies for the Design of Anti-Fouling Ion-Exchange Membranes; Springer: Berlin/Heidelberg, Germany, 2021; pp. 13–25. ISBN 978-3-030-41294-4. [Google Scholar]
- Astom Detailed Specification of IEMs Produced Astom Corporation. Available online: http://www.astom-corp.jp/en/product/10.html (accessed on 29 December 2022).
- Wang, B.; Liu, F.; Zhang, F.; Tan, M.; Jiang, H.; Liu, Y.; Zhang, Y. Efficient Separation and Recovery of Cobalt(II) and Lithium(I) from Spent Lithium Ion Batteries (LIBs) by Polymer Inclusion Membrane Electrodialysis (PIMED). Chem. Eng. J. 2022, 430, 132924. [Google Scholar] [CrossRef]
- Zhou, Y.; Yan, H.; Wang, X.; Wu, L.; Wang, Y.; Xu, T. Electrodialytic Concentrating Lithium Salt from Primary Resource. Desalination 2018, 425, 30–36. [Google Scholar] [CrossRef]
- Yan, H.; Xu, C.; Li, W.; Wang, Y.; Xu, T. Electrodialysis To Concentrate Waste Ionic Liquids: Optimization of Operating Parameters. Ind. Eng. Chem. Res. 2016, 55, 2144–2152. [Google Scholar] [CrossRef]
- Wang, H.; Yan, J.; Fu, R.; Yan, H.; Jiang, C.; Wang, Y.; Xu, T. Bipolar Membrane Electrodialysis for Cleaner Production of Gluconic Acid: Valorization of the Regenerated Base for the Upstream Enzyme Catalysis. Ind. Eng. Chem. Res. 2022, 61, 7634–7644. [Google Scholar] [CrossRef]
- Porozhnyy, M.V.; Kozmai, A.E.; Mareev, A.A.; Gil, V.V. Theoretical and Experimental Study of Neutralization Dialysis of Phenylalanine–Mineral Salt Equimolar Mixture of Different Concentrations. Membr. Membr. Technol. 2022, 4, 306–318. [Google Scholar] [CrossRef]
- Cao, R.; Shi, S.; Cao, H.; Li, Y.; Duan, F.; Li, Y. Surface Composite Modification of Anion Exchange Membrane by Electrodeposition and Self-Polymerization for Improved Antifouling Performance. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 648, 129402. [Google Scholar] [CrossRef]
- Yao, Y.; Mu, J.; Liao, J.; Dong, J.; Luo, B.; Ruan, H.; Shen, Z.; Shen, J. Imparting Antibacterial Adhesion Property to Anion Exchange Membrane by Constructing Negatively Charged Functional Layer. Sep. Purif. Technol. 2022, 288, 120628. [Google Scholar] [CrossRef]
- Wang, J.; Liu, M.; Feng, Z.; Liu, J.; Liao, S.; Li, X.; Yu, Y. Highly Conductive Anion Exchange Membrane with a Stable Double-Sided Anti-Fouling Structure for Electrodialysis Desalination of Protein Systems. Desalination 2023, 545, 116167. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Jiang, C.; Xu, T. Recovery of Gamma-Aminobutyric Acid (GABA) from Reaction Mixtures Containing Salt by Electrodialysis. Sep. Purif. Technol. 2016, 170, 353–359. [Google Scholar] [CrossRef]
- Yan, H.; Wang, Y.; Xu, T. K6-5: Developing Ion Exchange Membrane for Treating High Salinity Water Using Electrodialysis. In Proceedings of the 5th International Conference on Sustainable Chemical Production Process Engineering (SCPPE), Tianjin, China, 30 July 2019; p. 65. [Google Scholar]
- Sarapulova, V.; Pismenskaya, N.; Butylskii, D.; Titorova, V.; Wang, Y.; Xu, T.; Zhang, Y.; Nikonenko, V. Transport and Electrochemical Characteristics of CJMCED Homogeneous Cation Exchange Membranes in Sodium Chloride, Calcium Chloride, and Sodium Sulfate Solutions. Membranes 2020, 10, 165. [Google Scholar] [CrossRef]
- Sarapulova, V.; Pismenskaya, N.; Titorova, V.; Sharafan, M.; Wang, Y.; Xu, T.; Zhang, Y.; Nikonenko, V. Transport Characteristics of CJMAEDTM Homogeneous Anion Exchange Membranes in Sodium Chloride and Sodium Sulfate Solutions. Int. J. Mol. Sci. 2021, 22, 1415. [Google Scholar] [CrossRef] [PubMed]
- Giusti, M.; Wrolstad, R.E. Anthocyanins. In Handbook of Food Analytical Chemistry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; pp. 5–69. [Google Scholar]
- Newman, J.S. Electrochemical Systems; Prentice Hall: Englewood Cliffs, NJ, USA, 1973. [Google Scholar]
- Lteif, R.; Dammak, L.; Larchet, C.; Auclair, B. Conductivité Électrique Membranaire: Étude de l’effet de La Concentration, de La Nature de l’électrolyte et de La Structure Membranaire. Eur. Polym. J. 1999, 35, 1187–1195. [Google Scholar] [CrossRef]
- Karpenko-Jereb, L.; Demina, O.; Dvorkina, G.; Parshikov, S.; Larchet, C.; Auclair, B.; Berezina, N. Comparative Study of Methods Used for the Determination of Electroconductivity of Ion-Exchange Membranes. Russ. J. Electrochem. 2001, 37, 287–293. [Google Scholar] [CrossRef]
- Ponomar, M.; Krasnyuk, E.; Butylskii, D.; Nikonenko, V.; Wang, Y.; Jiang, C.; Xu, T.; Pismenskaya, N. Sessile Drop Method: Critical Analysis and Optimization for Measuring the Contact Angle of an Ion-Exchange Membrane Surface. Membranes 2022, 12, 765. [Google Scholar] [CrossRef] [PubMed]
- Gil, V.; Porozhnyy, M.; Rybalkina, O.; Butylskii, D.; Pismenskaya, N. The Development of Electroconvection at the Surface of a Heterogeneous Cation-Exchange Membrane Modified with Perfluorosulfonic Acid Polymer Film Containing Titanium Oxide. Membranes 2020, 10, 125. [Google Scholar] [CrossRef]
- Safronova, E.Y.; Yaroslavtsev, A.B. Synthesis of MF-4SC Composite Membranes Exhibiting an Anisotropic Distribution of Zirconia and Ion Transport Asymmetry. Pet. Chem. 2015, 55, 862–865. [Google Scholar] [CrossRef]
- Hayashi, K.; Abe, Y.; Mitsui, S. Blue Anthocyanin from the Flowers of Commelina, the Crystallisation and Some Properties Thereof. Proc. Jpn. Acad. 1958, 34, 373–378. [Google Scholar] [CrossRef]
- Mata, R. Flavonoids: Chemistry, Biochemistry and Applications, 1st ed.; Andersen, O.M., Markham, K.R., Eds.; CRC Press: Boca Raton, FL, USA, 2005; Volume 70, ISBN 9780429121586. [Google Scholar]
- Pismenskaya, N.; Sarapulova, V.; Klevtsova, A.; Mikhaylin, S.; Bazinet, L. Adsorption of Anthocyanins by Cation and Anion Exchange Resins with Aromatic and Aliphatic Polymer Matrices. Int. J. Mol. Sci. 2020, 21, 7874. [Google Scholar] [CrossRef] [PubMed]
- Danilczuk, M.; Lin, L.; Schlick, S.; Hamrock, S.J.; Schaberg, M.S. Understanding the Fingerprint Region in the Infra-Red Spectra of Perfluorinated Ionomer Membranes and Corresponding Model Compounds: Experiments and Theoretical Calculations. J. Power Sources 2011, 196, 8216–8224. [Google Scholar] [CrossRef]
- Bellamy, L.J. The Infra-Red Spectra of Complex Molecules, 3rd ed.; Springer: Dordrecht, The Netherlands, 1975; ISBN 978-94-011-6019-3. [Google Scholar]
- Wang, Y.; Peng, J.; Li, J.; Zhai, M. PVDF Based Ion Exchange Membrane Prepared by Radiation Grafting of Ethyl Styrenesulfonate and Sequent Hydrolysis. Radiat. Phys. Chem. 2017, 130, 252–258. [Google Scholar] [CrossRef]
- Garcia-Vasquez, W.; Ghalloussi, R.; Dammak, L.; Larchet, C.; Nikonenko, V.; Grande, D. Structure and Properties of Heterogeneous and Homogeneous Ion-Exchange Membranes Subjected to Ageing in Sodium Hypochlorite. J. Membr. Sci. 2014, 452, 104–116. [Google Scholar] [CrossRef]
- Hansima, M.A.C.K.; Jayaweera, A.T.; Ketharani, J.; Ritigala, T.; Zheng, L.; Samarajeewa, D.R.; Nanayakkara, K.G.N.; Herath, A.C.; Makehelwala, M.; Jinadasa, K.B.S.N.; et al. Characterization of Humic Substances Isolated from a Tropical Zone and Their Role in Membrane Fouling. J. Environ. Chem. Eng. 2022, 10, 107456. [Google Scholar] [CrossRef]
- Scano, P. Characterization of the Medium Infrared Spectra of Polyphenols of Red and White Wines by Integrating FT IR and UV–Vis Spectral Data. LWT 2021, 147, 111604. [Google Scholar] [CrossRef]
- Cui, Z.; Drioli, E.; Lee, Y.M. Recent Progress in Fluoropolymers for Membranes. Prog. Polym. Sci. 2014, 39, 164–198. [Google Scholar] [CrossRef]
- OIV. International Oenological CODEX. Available online: https://www.oiv.int/index.php/standards/international-oenological-codex (accessed on 29 December 2022).
- Chesnokova, N.; Prikhodko, Y.; Kuznetsova, A.; Kushnarenko, L.; Gerasimova, V. Anthocyanin Films in Freshness Assessment of Minced Fish. Food Process. Tech. Technol. 2021, 51, 349–362. [Google Scholar] [CrossRef]
- Burattini, E.; Cavagna, M.; Dell’Anna, R.; Malvezzi Campeggi, F.; Monti, F.; Rossi, F.; Torriani, S. A FTIR Microspectroscopy Study of Autolysis in Cells of the Wine Yeast Saccharomyces Cerevisiae. Vib. Spectrosc. 2008, 47, 139–147. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S. The Use of Fourier Transform Infrared (FTIR) Spectroscopy and Artificial Neural Networks (ANNs) to Assess Wine Quality. Mod. Chem. Appl. 2013, 1, 4. [Google Scholar] [CrossRef]
- Mukhamediev, M.G.; Bekchanov, D.Z. New Anion Exchanger Based on Polyvinyl Chloride and Its Application in Industrial Water Treatment. Russ. J. Appl. Chem. 2019, 92, 1499–1505. [Google Scholar] [CrossRef]
- Krol, J. Concentration Polarization with Monopolar Ion Exchange Membranes: Current-Voltage Curves and Water Dissociation. J. Membr. Sci. 1999, 162, 145–154. [Google Scholar] [CrossRef]
- Park, J.-S.; Choi, J.-H.; Woo, J.-J.; Moon, S.-H. An Electrical Impedance Spectroscopic (EIS) Study on Transport Characteristics of Ion-Exchange Membrane Systems. J. Colloid Interface Sci. 2006, 300, 655–662. [Google Scholar] [CrossRef]
- Korzhova, E.; Pismenskaya, N.; Lopatin, D.; Baranov, O.; Dammak, L.; Nikonenko, V. Effect of Surface Hydrophobization on Chronopotentiometric Behavior of an AMX Anion-Exchange Membrane at Overlimiting Currents. J. Membr. Sci. 2016, 500, 161–170. [Google Scholar] [CrossRef]
- Abu-Rjal, R.; Prigozhin, L.; Rubinstein, I.; Zaltzman, B. Equilibrium Electro-Convective Instability in Concentration Polarization: The Effect of Non-Equal Ionic Diffusivities and Longitudinal Flow. Russ. J. Electrochem. 2017, 53, 903–918. [Google Scholar] [CrossRef]
- Zabolotskii, V.I.; Shel’deshov, N.V.; Gnusin, N.P. Dissociation of Water Molecules in Systems with Ion-Exchange Membranes. Russ. Chem. Rev. 1988, 57, 801–808. [Google Scholar] [CrossRef]
- Rubinstein, I.; Zaltzman, B. Electro-Convective versus Electroosmotic Instability in Concentration Polarization. Adv. Colloid Interface Sci. 2007, 134–135, 190–200. [Google Scholar] [CrossRef]
- Zhang, Y.; Pinoy, L.; Meesschaert, B.; Van der Bruggen, B. Separation of Small Organic Ions from Salts by Ion-Exchange Membrane in Electrodialysis. AIChE J. 2011, 57, 2070–2078. [Google Scholar] [CrossRef]
- Titorova, V.D.; Moroz, I.A.; Mareev, S.A.; Pismenskaya, N.D.; Sabbatovskii, K.G.; Wang, Y.; Xu, T.; Nikonenko, V.V. How Bulk and Surface Properties of Sulfonated Cation-Exchange Membranes Response to Their Exposure to Electric Current during Electrodialysis of a Ca2+ Containing Solution. J. Membr. Sci. 2022, 644, 120149. [Google Scholar] [CrossRef]
- de Freitas, V.; Carvalho, E.; Mateus, N. Study of Carbohydrate Influence on Protein–Tannin Aggregation by Nephelometry. Food Chem. 2003, 81, 503–509. [Google Scholar] [CrossRef]
- Alberti, G.; Narducci, R.; Sganappa, M. Effects of Hydrothermal/Thermal Treatments on the Water-Uptake of Nafion Membranes and Relations with Changes of Conformation, Counter-Elastic Force and Tensile Modulus of the Matrix. J. Power Sources 2008, 178, 575–583. [Google Scholar] [CrossRef]
- Nebavskaya, X.; Sarapulova, V.; Butylskii, D.; Larchet, C.; Pismenskaya, N. Electrochemical Properties of Homogeneous and Heterogeneous Anion Exchange Membranes Coated with Cation Exchange Polyelectrolyte. Membranes 2019, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Titorova, V.; Sabbatovskiy, K.; Sarapulova, V.; Kirichenko, E.; Sobolev, V.; Kirichenko, K. Characterization of MK-40 Membrane Modified by Layers of Cation Exchange and Anion Exchange Polyelectrolytes. Membranes 2020, 10, 20. [Google Scholar] [CrossRef]
- Tsygurina, K.; Rybalkina, O.; Sabbatovskiy, K.; Kirichenko, E.; Sobolev, V.; Kirichenko, K. Layer-by-Layer Coating of MK-40 Heterogeneous Membrane with Polyelectrolytes Creates Samples with Low Electrical Resistance and Weak Generation of H+ and OH−Ions. Membranes 2021, 11, 145. [Google Scholar] [CrossRef] [PubMed]
- Fidaleo, M.; Moresi, M. Electrodialysis Applications in the Food Industry; Academic Press: Cambridge, MA, USA, 2006; Volume 51, pp. 265–360. ISSN 1043-4526. [Google Scholar]
- Rybalkina, O.A.; Sharafan, M.V.; Nikonenko, V.V.; Pismenskaya, N.D. Two Mechanisms of H+/OH− Ion Generation in Anion-Exchange Membrane Systems with Polybasic Acid Salt Solutions. J. Membr. Sci. 2022, 651, 120449. [Google Scholar] [CrossRef]
- Chandra, A.; Tadimeti, J.G.D.; Bhuvanesh, E.; Pathiwada, D.; Chattopadhyay, S. Switching Selectivity of Carboxylic Acids and Associated Physico-Chemical Changes with PH during Electrodialysis of Ternary Mixtures. Sep. Purif. Technol. 2018, 193, 327–344. [Google Scholar] [CrossRef]
- Helfferich, F. Ion Exchange; McGraw-Hill: New York, NY, USA, 1962; ISBN 0-486-68784-8. [Google Scholar]


| Membrane | Thickness in 0.02 M NaCl Solution (µm) | Water Content (gH2O/gdry, %) | Ion-Exchange Capacity of Swollen Membrane (mmol/gwet) |
|---|---|---|---|
| CJMA-3 | 151 ± 5 | 17 ± 1 [62] | 0.57 ± 0.05 [62] |
| CJMC-3 | 185 ± 5 | 44 ± 3 [63] | 0.63 ± 0.05 [63] |
| ASE | 150 ± 5 | 24 ± 1 [56] | 1.93 ± 0.05 [56] |
| CSE | 140 ± 5 | 42 ± 1 [56] | 1.57 ± 0.05 [56] |
| Inorganic Matter | Concentration | Organic Matter | Concentration | ||
|---|---|---|---|---|---|
| mg/L | %mass | mg/L | %vol | ||
| Cl− | 193 ± 5 | – | acetic aldehyde | 32 ± 5 | – |
| SO42− | 969 ± 5 | – | diacetyl | 4 ± 5 | – |
| K+ | 1281 ± 5 | – | acetoin | 48 ± 5 | – |
| Na+ | 136 ± 5 | – | furfurol | 14 ± 5 | – |
| Mg2+ | 110 ± 5 | – | 2,3-butyleneglycol racemate | 560 ± 5 | – |
| Ca2+ | 124 ± 5 | – | 2,3-butyleneglycol meso | 259 ± 5 | – |
| Organic acids | methyl acetat | 45 ± 5 | – | ||
| ascorbic | 31 ± 5 | – | ethyl acetat | 82 ± 5 | – |
| tartaric | 1872 ± 5 | – | ethyl butyrate | 1 ± 5 | – |
| citric | 405 ± 5 | – | ethyl caproate | 7 ± 5 | – |
| lactic | 5020 ± 5 | – | ethyl lactate | 83 ± 5 | – |
| malic | 156 ± 5 | – | ethyl caprylate | 2 ± 5 | – |
| succinic | 1622 ± 5 | – | ethyl caprate | 4 ± 5 | – |
| chlorogenic | 11 ± 5 | – | ethanol | – | 11 ± 1 |
| niacin | 8 ± 5 | – | methanol | 142 ± 5 | – |
| orotic | 973 ± 5 | – | 1-propanol | 33 ± 5 | – |
| caffeic | 37 ± 5 | – | isobutanol | 50 ± 5 | – |
| gallic | 10 ± 5 | – | 1-butanol | 2 ± 5 | – |
| acetic | 1955 ± 5 | – | amyl alcohol | 253 ± 5 | – |
| propionic | 9 ± 5 | – | octanol | 16 ± 5 | – |
| isobutyric | 1 ± 5 | – | benzyl alcohol | 15 ± 5 | – |
| 3-methylbutanoic | 7 ± 5 | – | phenethyl alcohol | 128 ± 5 | – |
| Saccharides | 1,2-propylene glycol | 24 ± 5 | – | ||
| ** total | – | 10 ± 1 | * polyphenols, total | 670 ± 10 | – |
| Membrane | Cation-Exchange Membrane | Anion-Exchange Membrane | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| H+ | K+ | Cl− | ∑T | |||||||
| ji | 1,2 mmol | γi, % | 3ji | mmol | γi, % | ji | mmol | γi, % | ji | |
| CJMC-3//CJMA-3 | 0.010 ± 0.002 | 1.09 | 19 ± 1 | 0.07 | 0.29 | 18 ± 1 | 0.02 | 0.70 | 18 ± 1 | 0.05 |
| CSE//ASE | 0.012 ± 0.002 | 1.19 | 21 ± 1 | 0.09 | 0.30 | 18 ± 1 | 0.02 | 0.77 | 20 ± 1 | 0.06 |
| CSEm//ASEm | 0.018 ± 0.002 | 1.20 | 21 ± 1 | 0.08 | 0.58 | 35 ± 1 | 0.04 | 0.65 | 17 ± 1 | 0.04 |
| Membranes | Pristine | * After ED |
|---|---|---|
| CJMC-3 |  |  |
| 50 ± 3 | 36 ± 3 | |
| CJMA-3 |  |  |
| 48 ± 3 | 19 ± 5 | |
| CSE |  |  |
| 53 ± 2 | 36 ± 3 | |
| ASE |  |  |
| 57 ± 3 | 30 ± 3 | |
| CSEm |  |  |
| 67 ± 4 | 48 ± 3 | |
| ASEm |  |  |
| 51 ± 3 | 52 ± 3 |
| Parameter | CJMC-3 | CJMA-3 | CSE | ASE | |
|---|---|---|---|---|---|
| ilimexp, mA/cm2 | Pristine | 2.62 | 3.64 | 2.18 | 3.22 |
| After ED | 2.52 | 3.48 | 2.34 | 3.15 | |
| “plateau length”, V | Pristine | 1.11 | 0.70 | 0.95 | 0.61 |
| After ED | 1.71 | 0.83 | 0.98 | 1.36 | |
| i/ilimLev value corresponding to the beginning of water splitting | Pristine | 1.50 | 1.25 | 1.35 | 1.06 |
| After ED | 1.39 | 1.18 | 1.35 | 1.11 | |
| ΔpH value at i/ilimLev = 2.0 | Pristine | –0.09 | –0.42 | –0.88 | –0.08 |
| After ED | –0.42 | –1.54 | –0.53 | –0.88 |
| Membrane | CJMC-3 | CJMA-3 | CSE | ASE | CSEm | ASEm |
|---|---|---|---|---|---|---|
| Pristine | 5.7 ± 0.3 | 3.1 ± 0.3 | 8.0 ± 0.3 | 4.9 ± 0.3 | 2.0 ± 0.3 | 4.6 ± 0.3 |
| After ED | 5.9 ± 0.3 | 2.3 ± 0.5 | 5.8 ± 0.3 | 4.1 ± 0.3 | 1.1 ± 0.5 | 1.9 ± 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasechnaya, E.; Tsygurina, K.; Ponomar, M.; Chuprynina, D.; Nikonenko, V.; Pismenskaya, N. Comparison of the Electrodialysis Performance in Tartrate Stabilization of a Red Wine Using Aliphatic and Aromatic Commercial and Modified Ion-Exchange Membranes. Membranes 2023, 13, 84. https://doi.org/10.3390/membranes13010084
Pasechnaya E, Tsygurina K, Ponomar M, Chuprynina D, Nikonenko V, Pismenskaya N. Comparison of the Electrodialysis Performance in Tartrate Stabilization of a Red Wine Using Aliphatic and Aromatic Commercial and Modified Ion-Exchange Membranes. Membranes. 2023; 13(1):84. https://doi.org/10.3390/membranes13010084
Chicago/Turabian StylePasechnaya, Evgeniia, Kseniia Tsygurina, Maria Ponomar, Daria Chuprynina, Victor Nikonenko, and Natalia Pismenskaya. 2023. "Comparison of the Electrodialysis Performance in Tartrate Stabilization of a Red Wine Using Aliphatic and Aromatic Commercial and Modified Ion-Exchange Membranes" Membranes 13, no. 1: 84. https://doi.org/10.3390/membranes13010084
APA StylePasechnaya, E., Tsygurina, K., Ponomar, M., Chuprynina, D., Nikonenko, V., & Pismenskaya, N. (2023). Comparison of the Electrodialysis Performance in Tartrate Stabilization of a Red Wine Using Aliphatic and Aromatic Commercial and Modified Ion-Exchange Membranes. Membranes, 13(1), 84. https://doi.org/10.3390/membranes13010084









