Influence of Silane Treatment on CNM/PAC/PVDF Properties and Performance for Water Desalination by VMD
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals Materials
2.2. Membrane Preparation
3. Membrane Characterization
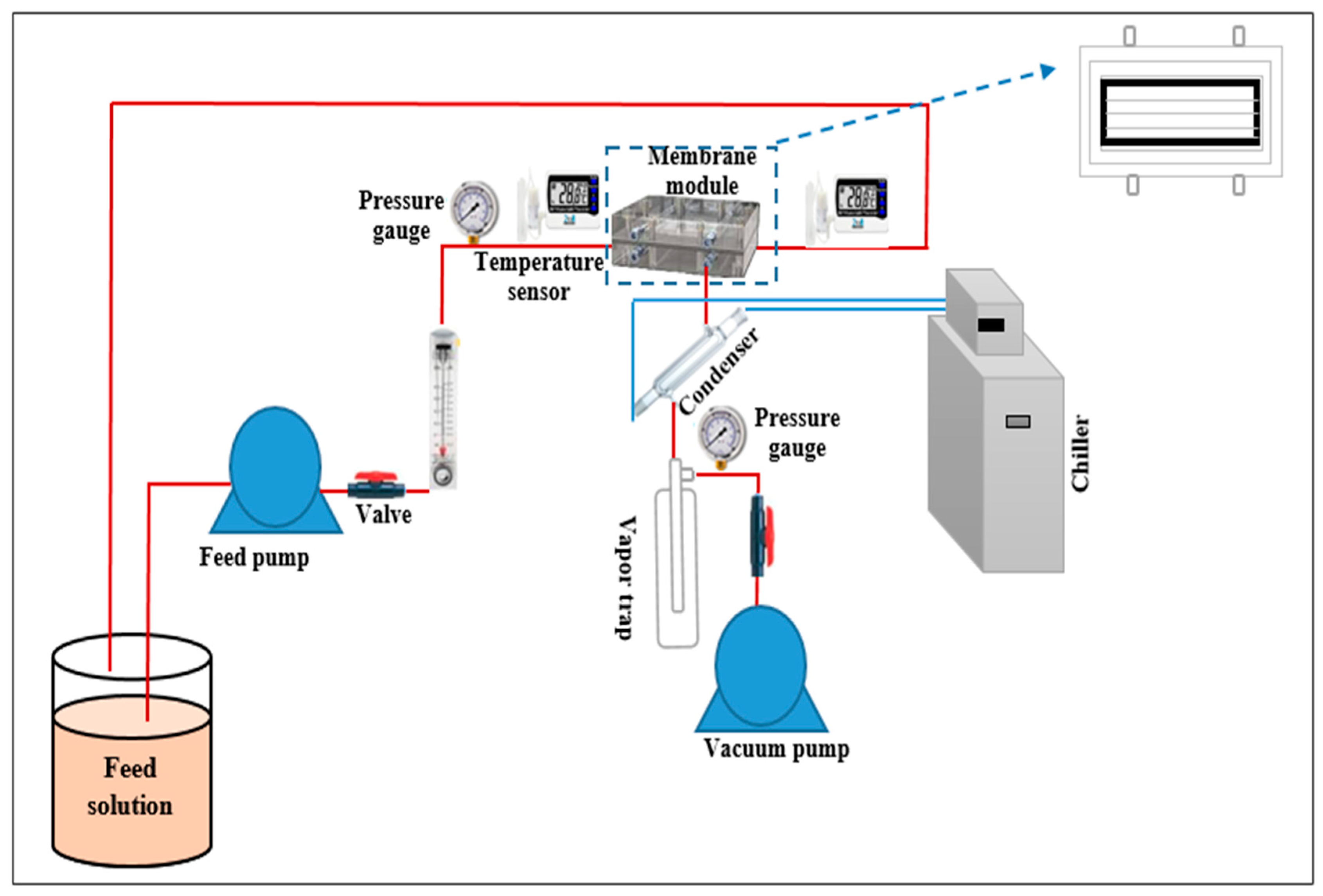
4. Membrane Performance
5. Results and Discussion
5.1. FTIR Analysis
5.2. X-Ray Diffraction (XRD)
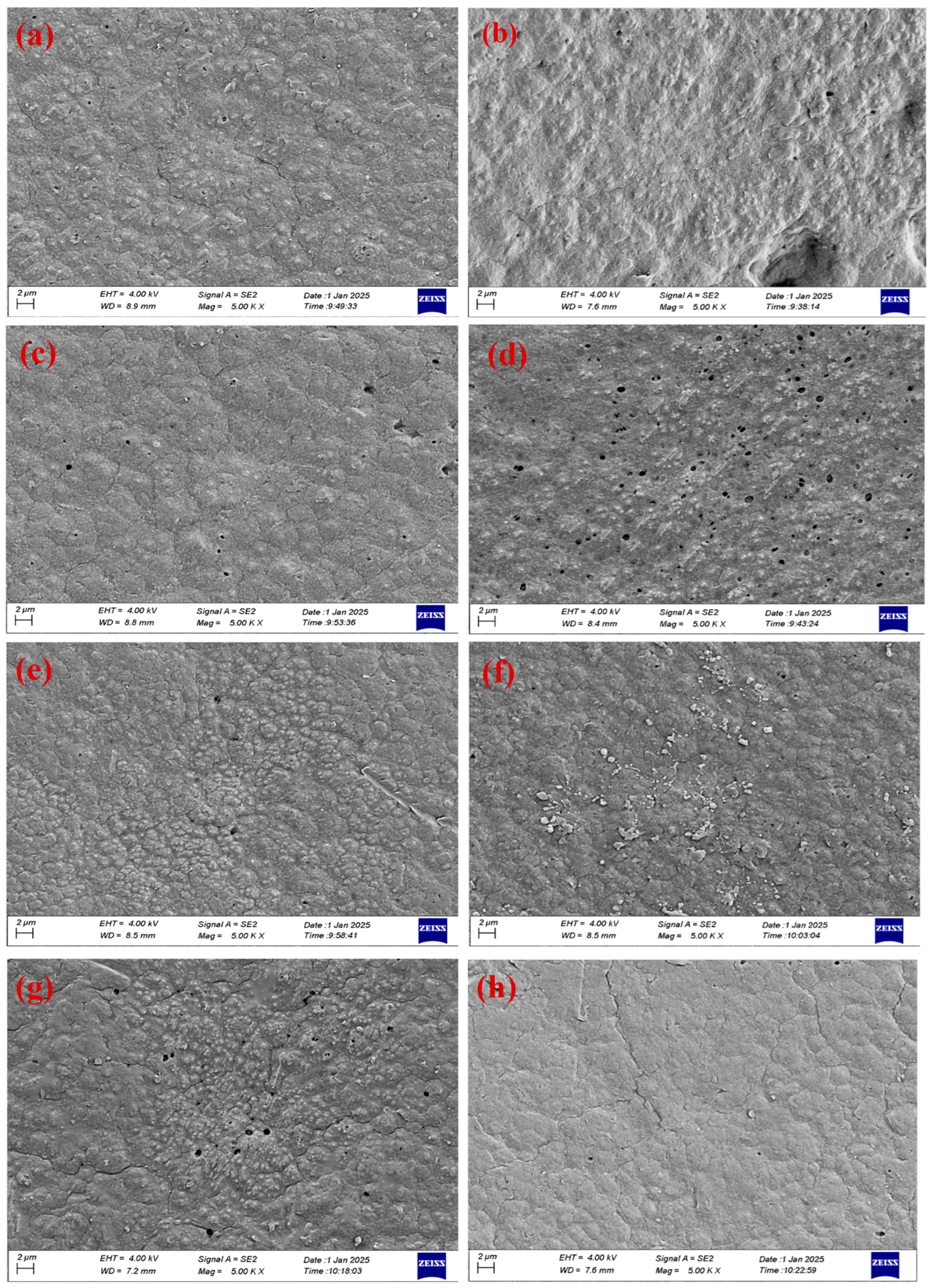
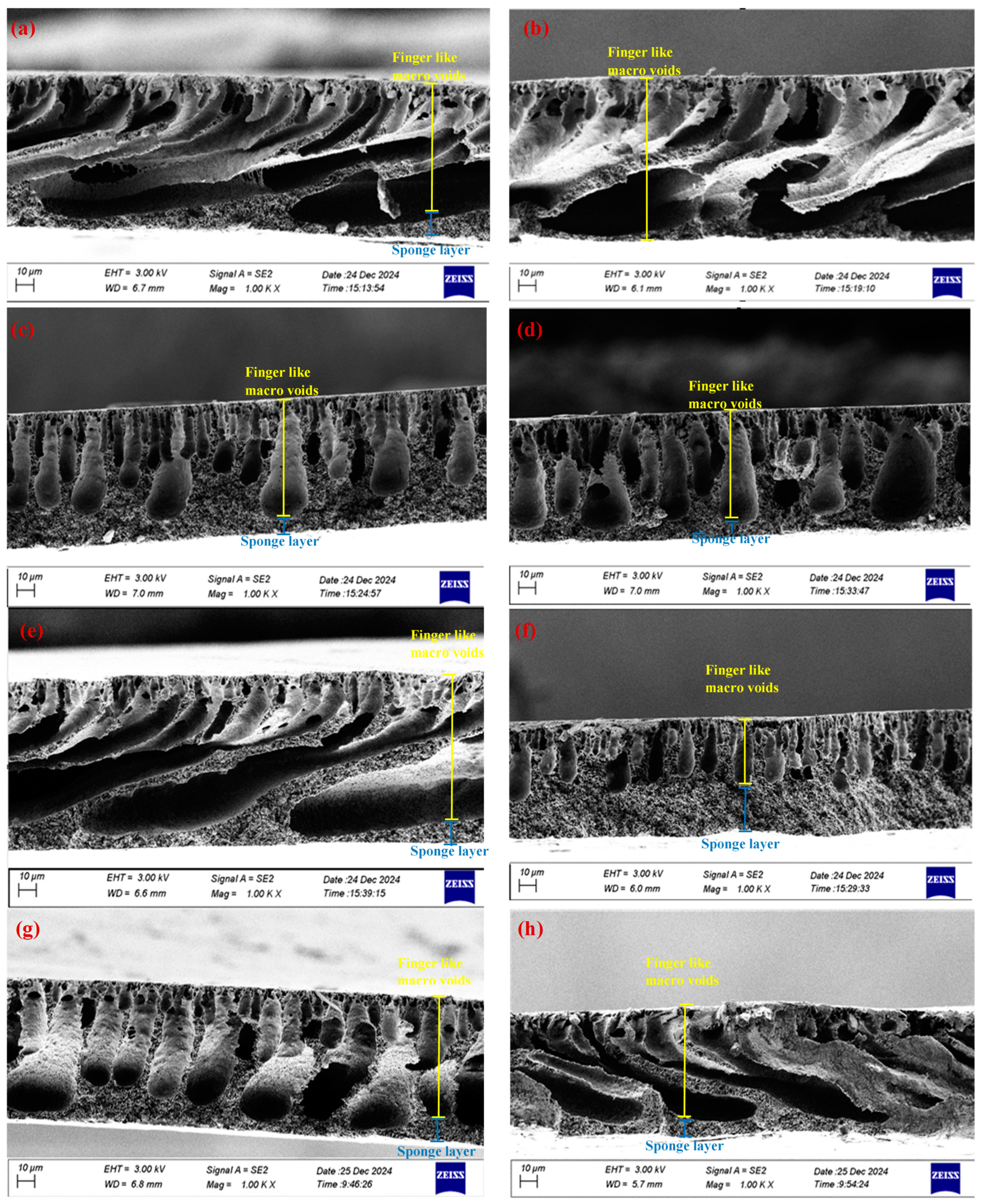
5.3. Field Emission Scanning Electron Microscopy and Energy Dispersive X-Ray Spectrometer (FE-SEM and EDX)
5.4. Membrane Thickness, Porosity, Pore Size, and LEP
5.5. Water Contact Angle
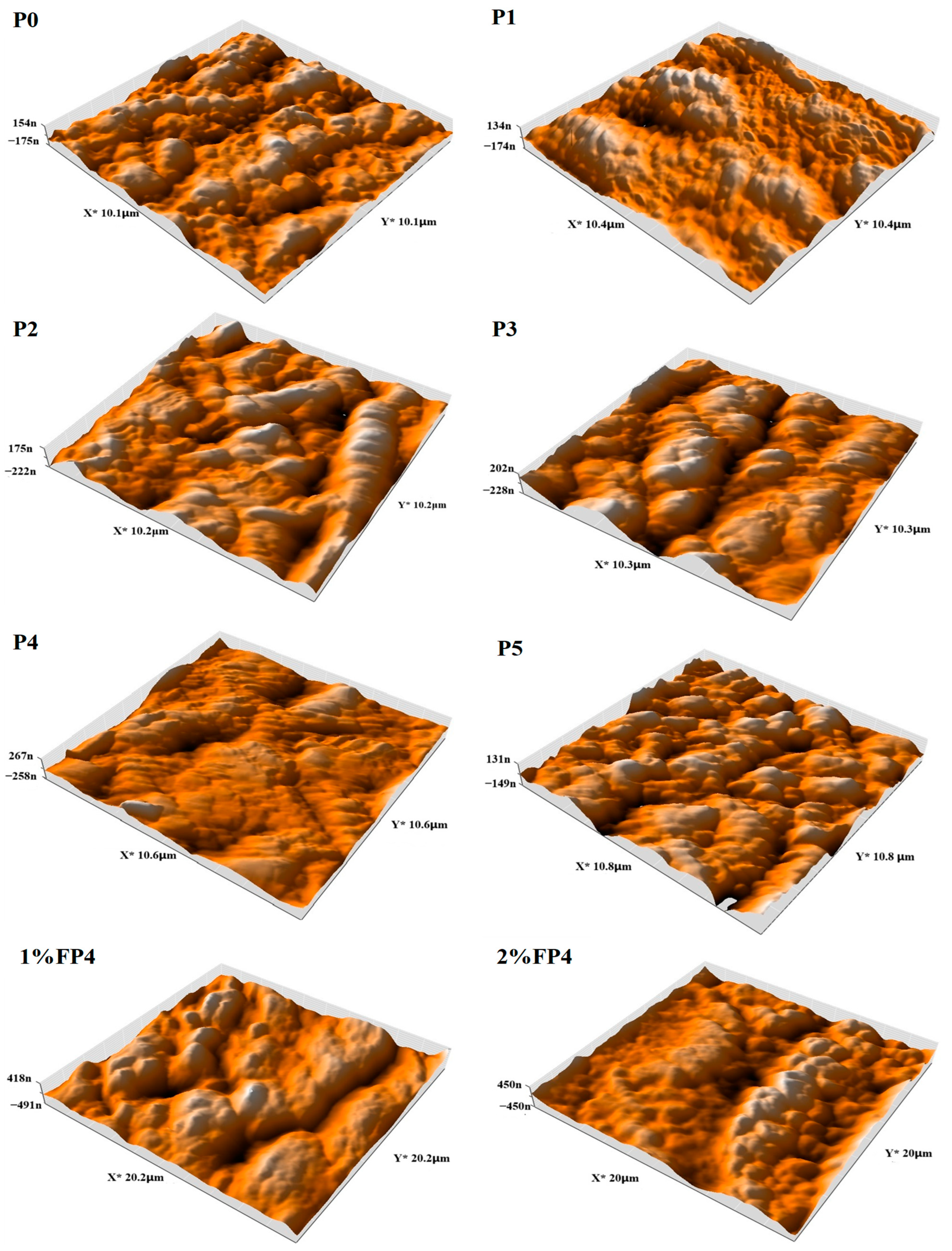
5.6. Atomic Force Microscopy (AFM)
5.7. Mechanical Characteristic of Membrane
6. Membrane Performance
6.1. Pure Water and Salt Solution
6.2. Effect of Operating Conditions
6.3. Leaching Nanomaterials and Longtime of Operation
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Patel, R.V.; Chaubey, S.; Yadav, A.; Shahi, V.K. Chemical grafting of hydrophobic functional groups on polyvinylidene fluoride side chain for vacuum membrane distillation applications. J. Environ. Chem. Eng. 2024, 12, 112746. [Google Scholar] [CrossRef]
- Asadolahi, M.; Fashandi, H.; Zamani, H. Disclosing the pivotal role of Poly(vinylidene fluoride) omniphobic membrane surface morphology in performance of photothermal vacuum membrane distillation. Chem. Eng. J. 2024, 496, 153970. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, J.; Gao, C.; Yuan, S.; Wang, Z. Emerging advanced membranes for removal of volatile organic compounds during membrane distillation. Desalination 2025, 597, 118372. [Google Scholar] [CrossRef]
- Sun, B.; Wu, M.; Zhen, H.; Jia, Y.; Li, P.; Yuan, Z.; Li, X.; He, G.; Jiang, X. Tailored PVDF membrane with coordinated interfacial nano/micro-structure for enhanced membrane distillation. Desalination 2024, 573, 117177. [Google Scholar] [CrossRef]
- Julian, H.; Nurgirisia, N.; Qiu, G.; Ting, Y.-P.; Wenten, I.G. Membrane distillation for wastewater treatment: Current trends, challenges and prospects of dense membrane distillation. J. Water Process Eng. 2022, 46, 102615. [Google Scholar] [CrossRef]
- Chin, J.Y.; Ahmad, A.L.; Low, S.C. Anti-Wetting Membrane Distillation to Treat High Salinity Wastewater: Review. J. Membr. Sci. Res. 2020, 6, 401–415. [Google Scholar] [CrossRef]
- Asadolahi, M.; Fashandi, H.; Ghodsi, A. One-step development of photothermal omniphobic membrane for vacuum membrane distillation towards sustainable water desalination using solar energy. Chem. Eng. J. 2024, 500, 157428. [Google Scholar] [CrossRef]
- Samadi, A.; Ni, T.; Fontananova, E.; Tang, G.; Shon, H.; Zhao, S. Engineering antiwetting hydrophobic surfaces for membrane distillation: A review. Desalination 2023, 563, 116722. [Google Scholar] [CrossRef]
- Pangarkar, B.L.; Deshmukh, S.K.; Sapkal, V.S.; Sapkal, R.S. Review of membrane distillation process for water purification. Desalin. Water Treat. 2016, 57, 2959–2981. [Google Scholar] [CrossRef]
- Ahmadi, H.; Ziapour, B.M.; Ghaebi, H.; Nematollahzadeh, A. Optimization of vacuum membrane distillation and advanced design of compact solar water heaters with heat recovery. J. Water Process Eng. 2024, 67, 106212. [Google Scholar] [CrossRef]
- Rashid, K.T.; Rahman, S.B.A.; Alsalhy, Q.F. Optimum Operating Parameters for Hollow Fiber Membranes in Direct Contact Membrane Distillation. Arab. J. Sci. Eng. 2016, 41, 2647–2658. [Google Scholar] [CrossRef]
- Abid, M.B.; Wahab, R.A.; Salam, M.A.; Gzara, L.; Moujdin, I.A. Desalination technologies, membrane distillation, and electrospinning, an overview. Heliyon 2023, 9, e12810. [Google Scholar] [CrossRef]
- Francis, L.; Ahmed, F.E.; Hilal, N. Advances in Membrane Distillation Module Configurations. Membranes 2022, 12, 81. [Google Scholar] [CrossRef]
- Alsebaeai, M.K.; Ahmad, A.L. Membrane distillation: Progress in the improvement of dedicated membranes for enhanced hydrophobicity and desalination performance. J. Ind. Eng. Chem. 2020, 86, 13–34. [Google Scholar] [CrossRef]
- Suga, Y.; Takagi, R.; Matsuyama, H. Effect of the characteristic properties of membrane on long-term stability in the vacuum membrane distillation process. Membranes 2021, 11, 252. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Li, F.; Wang, H.; Zhang, X.; Di, Y.; Wang, L.; Yan, Z.; Wang, W.; Lu, J.; Liu, M.; et al. Preparation and properties of cellulose triacetate (CTA)-PVDF composite membrane with hydrophobic/hydrophilic properties for membrane distillation. Appl. Surf. Sci. 2025, 684, 161891. [Google Scholar] [CrossRef]
- Zheng, R.; Chen, Y.; Wang, J.; Song, J.; Li, X.-M.; He, T. Preparation of omniphobic PVDF membrane with hierarchical structure for treating saline oily wastewater using direct contact membrane distillation. J. Memb. Sci. 2018, 555, 197–205. [Google Scholar] [CrossRef]
- Schnittger, J.; McCutcheon, J.; Hoyer, T.; Weyd, M.; Fischer, G.; Puhlfürß, P.; Halisch, M.; Voigt, I.; Lerch, A. Hydrophobic ceramic membranes in MD processes—Impact of material selection and layer characteristics. J. Membr. Sci. 2021, 618, 118678. [Google Scholar] [CrossRef]
- Jin, Q.; Zhang, X.; Li, F.; Zhao, X. Hydrophobic modification of a PVDF hollow fiber membrane by plasma activation and silane grafting for membrane distillation. Water Sci. Technol. 2023, 87, 2806–2819. [Google Scholar] [CrossRef]
- Swaminathan, M.; Swaminathan, G. An Insight into Enhanced Membrane Hydrophobicity Using Silane Functionalization for Effective Membrane Distillation. Sep. Purif. Rev. 2024, 31, 1–21. [Google Scholar] [CrossRef]
- Van Tran, T.T.; Nguyen, C.H.; Lin, W.-C.; Juang, R.-S. Improved stability of a supported liquid membrane process via hydrophobic modification of PVDF support by plasma activation and chemical vapor deposition. Sep. Purif. Technol. 2021, 277, 119615. [Google Scholar] [CrossRef]
- Arkles, B. Hydrophobicity, hydrophilicity and silanes. Paint. Coat. Ind. 2006, 22, 114–135. [Google Scholar]
- Arkles, B. Tailoring Surfaces with Silanes. Chemtech 1977, 7, 766–778. [Google Scholar]
- Aljumaily, M.M.; Alsaadi, M.A.; Das, R.; Abd Hamid, S.B.; Hashim, N.A.; AlOmar, M.K.; Alayan, H.M.; Novikov, M.; Alsalhy, Q.F.; Hashim, M.A. Optimization of the synthesis of superhydrophobic carbon nanomaterials by chemical vapor deposition. Sci. Rep. 2018, 8, 2778. [Google Scholar] [CrossRef]
- Almajras, Q.A.; Hassan, A.K.; Al-Juboori, R.A.; Alsalhy, Q.F. Green and sustainable biosynthesis of hybrid iron/palladium nanoparticles functionalized PES membranes for dye removal. Desalin. Water Treat. 2025, 321, 100973. [Google Scholar] [CrossRef]
- Tai, B.; Ma, X. Superhydrophobic Surface Modification of Carbon Nanotubes for Polymer Membrane in Direct Contact Membrane Distillation. Master’s Thesis, Texas A&M University, College Station, TX, USA, 2019. Available online: https://oaktrust.library.tamu.edu/server/api/core/bitstreams/beb4b8af-dc27-4f48-8838-dbfc91de714c/content (accessed on 19 March 2025).
- Mpala, T.J.; Richards, H.; Etale, A.; Mahlangu, O.T.; Nthunya, L.N. Carbon nanotubes and silver nanoparticles modification of PVDF membranes for improved seawater desalination in direct contact membrane distillation. Front. Membr. Sci. Technol. 2023, 2, 1165678. [Google Scholar] [CrossRef]
- Hussein Al-Timimi, D.A.; Alsalhy, Q.F.; AbdulRazak, A.A. Polyethersulfone/amine grafted silica nanoparticles mixed matrix membrane: A comparative study for mebeverine hydrochloride wastewater treatment. Alex. Eng. J. 2023, 66, 167–190. [Google Scholar] [CrossRef]
- Ibraheem, B.M.; AlAani, S.; Alsalhy, Q.F.; Nemeth, Z.; Hernadi, K. Improving surface characteristics and operational parameters of polyvinyl chloride ultrafiltration membranes: How to manipulate the performance of thin film composite forward osmosis membranes? Desalination 2023, 565, 116896. [Google Scholar] [CrossRef]
- Francis, L.; Hilal, N. Electrosprayed CNTs on Electrospun PVDF-Co-HFP Membrane for Robust Membrane Distillation. Nanomaterials 2022, 12, 4331. [Google Scholar] [CrossRef]
- Saffarini, R.; Arafat, H.; Thomas, R. Influence of Pore Structure on Membrane Wettability in Membrane. In Proceedings of the Sixth Jordan International Chemical Engineering Conference, Amman, Jordan, 12–14 March 2012; pp. 1–5. [Google Scholar]
- Racz, G.; Kerker, S.; Schmitz, O.; Schnabel, B.; Kovacs, Z.; Vatai, G.; Ebrahimi, M.; Czermak, P. Experimental determination of liquid entry pressure (LEP) in vacuum membrane distillation for oily wastewaters. Membr. Water Treat. 2015, 6, 237–249. [Google Scholar] [CrossRef]
- Yang, C.; Peng, X.; Zhao, Y.; Wang, X.; Cheng, L.; Wang, F.; Li, Y.; Li, P. Experimental study on VMD and its performance comparison with AGMD for treating copper-containing solution. Chem. Eng. Sci. 2019, 207, 876–891. [Google Scholar] [CrossRef]
- Rao, L.; You, X.; Chen, B.; Shen, L.; Xu, Y.; Zhang, M.; Hong, H.; Li, R.; Lin, H. A novel composite membrane for simultaneous separation and catalytic degradation of oil/water emulsion with high performance. Chemosphere 2022, 288, 132490. [Google Scholar] [CrossRef]
- Aljumaily, M.M.; Alsaadi, M.A.; Hashim, N.A.; Alsalhy, Q.F.; Das, R.; Mjalli, F.S.; Awanis Hashim, N.; Alsalhy, Q.F.; Das, R.; Mjalli, F.S. Embedded high-hydrophobic CNMs prepared by CVD technique with PVDF-co-HFP membrane for application in water desalination by DCMD. Desalin. Water Treat. 2019, 142, 37–48. [Google Scholar] [CrossRef]
- Tian, X.; Jiang, X. Poly(vinylidene fluoride-co-hexafluoropropene) (PVDF-HFP) membranes for ethyl acetate removal from water. J. Hazard. Mater. 2008, 153, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.L.S.; Morales-Torres, S.; Figueiredo, J.L.; Silva, A.M.T. Multi-walled carbon nanotubes/PVDF blended membranes with sponge-and finger-like pores for direct contact membrane distillation. Desalination 2015, 357, 233–245. [Google Scholar]
- Avilés, F.; Cauich-Rodríguez, J.V.; Moo-Tah, L.; May-Pat, A.; Vargas-Coronado, R. Evaluation of mild acid oxidation treatments for MWCNT functionalization. Carbon 2009, 47, 2970–2975. [Google Scholar] [CrossRef]
- Wu, T.; Zhou, B.; Zhu, T.; Shi, J.; Xu, Z.; Hu, C.; Wang, J. Facile and low-cost approach towards a PVDF ultrafiltration membrane with enhanced hydrophilicity and antifouling performance via graphene oxide/water-bath coagulation. RSC Adv. 2014, 5, 7880–7889. [Google Scholar] [CrossRef]
- Khayet, M.; Cojocaru, C.; García-Payo, M.C. Experimental design and optimization of asymmetric flat-sheet membranes prepared for direct contact membrane distillation. J. Memb. Sci. 2010, 351, 234–245. [Google Scholar] [CrossRef]
- Baig, U.; Azeem, M.A.; Lawal, D.U.; Al Abdulgader, H.; Baroud, T.N. Facile and scalable fabrication of superhydrophobic PVDF membrane for the desalination of highly saline water using air-gap membrane distillation system. J. Environ. Chem. Eng. 2022, 10, 109025. [Google Scholar] [CrossRef]
- Zhou, R.; Rana, D.; Matsuura, T.; Lan, C.Q. Effects of multi-walled carbon nanotubes (MWCNTs) and integrated MWCNTs/SiO2 nano-additives on PVDF polymeric membranes for vacuum membrane distillation. Sep. Purif. Technol. 2019, 217, 154–163. [Google Scholar] [CrossRef]
- El-Zanati, E.; Taha, E.; Ettouney, R.; El-Rifai, M. Functionalized CNT/PVDF Blended Flat Sheet Membrane for Water Desalination via Vacuum Membrane Distillation, Fabrication and Characterization. Water Energy Food Environ. J. Int. J. 2022, 3, 53. [Google Scholar]
- Jiménez-Robles, R.; Moreno-Torralbo, B.M.; Badia, J.D.; Martínez-Soria, V.; Izquierdo, M. Flat PVDF Membrane with Enhanced Hydrophobicity through Alkali Activation and Organofluorosilanisation for Dissolved Methane Recovery. Membranes 2022, 12, 426. [Google Scholar] [CrossRef]
- Jiménez-Robles, R.; Izquierdo, M.; Martínez-Soria, V.; Martí, L.; Monleón, A.; Badia, J.D. Stability of Superhydrophobicity and Structure of PVDF Membranes Treated by Vacuum Oxygen Plasma and Organofluorosilanisation. Membranes 2023, 13, 314. [Google Scholar] [CrossRef]
- Chimanlal, I.; Nthunya, L.N.; Mahlangu, O.T.; Kirkebæk, B.; Ali, A.; Quist-Jensen, C.A.; Richards, H. Nanoparticle-Enhanced PVDF Flat-Sheet Membranes for Seawater Desalination in Direct Contact Membrane Distillation. Membranes 2023, 13, 317. [Google Scholar] [CrossRef] [PubMed]
- Abdelrasoul, A. (Ed.) Advances in Membrane Technologies; IntechOpen: London, UK, 2020. [Google Scholar]
- Madaeni, S.S.; Zinadini, S.; Vatanpour, V. Preparation of superhydrophobic nanofiltration membrane by embedding multiwalled carbon nanotube and polydimethylsiloxane in pores of microfiltration membrane. Sep. Purif. Technol. 2013, 111, 98–107. [Google Scholar] [CrossRef]
- Aljumaily, M.M.; Alayan, H.M.; Mohammed, A.A.; Alsaadi, M.A.; Alsalhy, Q.F.; Figoli, A.; Criscuoli, A. The influence of coating super-hydrophobic carbon nanomaterials on the performance of membrane distillation. Appl. Water Sci. 2022, 12, 28. [Google Scholar] [CrossRef]
- Aljumaily, M.M.; Alsaadi, M.A.; Hashim, N.A.; Alsalhy, Q.F.; Mjalli, F.S.; Atieh, M.A.; Al-Harrasi, A. PVDF-co-HFP/superhydrophobic acetylene-based nanocarbon hybrid membrane for seawater desalination via DCMD. Chem. Eng. Res. Des. 2018, 138, 248–259. [Google Scholar] [CrossRef]
- Devi, S.; Ray, P.; Singh, K.; Singh, P.S. Preparation and characterization of highly micro-porous PVDF membranes for desalination of saline water through vacuum membrane distillation. Desalination 2014, 346, 9–18. [Google Scholar] [CrossRef]
- Pagliero, M.; Comite, A.; Soda, O.; Costa, C. Effect of support on PVDF membranes for distillation process. J. Memb. Sci. 2021, 635, 119528. [Google Scholar] [CrossRef]
- Liu, J.; Albdoor, A.K.; Lin, W.; Hai, F.I.; Ma, Z. Membrane fouling in direct contact membrane distillation for liquid desiccant regeneration: Effects of feed temperature and flow velocity. J. Memb. Sci. 2022, 642, 119936. [Google Scholar] [CrossRef]
- Chen, L.; Xu, P.; Wang, H. Interplay of the Factors Affecting Water Flux and Salt Rejection in Membrane Distillation: A State-of-the-Art Critical Review. Water 2020, 12, 2841. [Google Scholar] [CrossRef]
- Xu, J.; Singh, Y.B.; Amy, G.L.; Ghaffour, N. Effect of operating parameters and membrane characteristics on air gap membrane distillation performance for the treatment of highly saline water. J. Memb. Sci. 2016, 512, 73–82. [Google Scholar] [CrossRef]
- Iqbal, F.; Asif, M.; Jafry, A.T.; Bibi, W. Experimental Investigation of Vacuum Membrane Distillation (VMD) Performance Based on Operational Parameters for Clean Water Production. MATEC Web Conf. 2024, 398, 01028. [Google Scholar] [CrossRef]
- Pangarkar, B.L.; Sane, M.G.; Parjane, S.B.; Abhang, R.M.; Guddad, M. The heat and mass transfer phenomena in vacuum membrane distillation for desalination. World Acad. Sci. Eng. Technol. 2010, 37, 977–982. [Google Scholar]
- Anvari, A.; Azimi Yancheshme, A.; Kekre, K.M.; Ronen, A. State-of-the-art methods for overcoming temperature polarization in membrane distillation process: A review. J. Memb. Sci. 2020, 616, 118413. [Google Scholar] [CrossRef]
- Abu-Zeid, M.A.E.R.; Zhang, Y.; Dong, H.; Zhang, L.; Chen, H.L.; Hou, L. A comprehensive review of vacuum membrane distillation technique. Desalination 2015, 356, 1–14. [Google Scholar] [CrossRef]
- Hussain Mana, T.; Alam, J.; Shukla, A.K.; Alkhudhiri, A.; Mohammed, A.N.; Alhoshan, M. Performance investigation of poly(vinylidene fluoride-cohexafluoropropylene) membranes containing SiO2 nanoparticles in a newly designed single vacuum membrane distillation system. Water Environ. Res. 2024, 96, e10980. [Google Scholar] [CrossRef]
- Madupathi, M.M.; Srishti, S.; Fatima, S.; Sridhar, S. Sea and brackish water desalination through a novel PVDF-PTFE composite hydrophobic membrane by vacuum membrane distillation. Discov. Chem. Eng. 2024, 4, 7. [Google Scholar] [CrossRef]
- Kim, H.; Yun, T.; Hong, S.; Lee, S. Experimental and theoretical investigation of a high performance PTFE membrane for vacuum-membrane distillation. J. Memb. Sci. 2021, 617, 118524. [Google Scholar] [CrossRef]
- Giraldo-Mejía, H.; Quintero, Y.M.; Mery, F.; Rodriguez, F.; Curcio, E.; Estay, H.; Garcia, A. Plasma-grafting surface modifications to enhance membrane hydrophobicity for brine membrane distillation. Desalination 2023, 567, 116942. [Google Scholar] [CrossRef]
- Ji, J.; Li, H.; Wang, W.; Li, J.; Zhang, W.; Li, K.; Yang, T.; Jin, W.; Tang, Y.; Li, W.; et al. Silane-crosslinked polybenzimidazole with different hydroxyl content for high-temperature proton exchange membrane. J. Memb. Sci. 2024, 694, 122423. [Google Scholar] [CrossRef]
- An, X.; Liu, Z.; Hu, Y. Amphiphobic surface modification of electrospun nanofibrous membranes for anti-wetting performance in membrane distillation. Desalination 2018, 432, 23–31. [Google Scholar] [CrossRef]
- Li, B.; Yun, Y.; Wang, M.; Li, C.; Yang, W.; Li, J.; Liu, G. Superhydrophobic polymer membrane coated by mineralized β-FeOOH nanorods for direct contact membrane distillation. Desalination 2021, 500, 114889. [Google Scholar] [CrossRef]





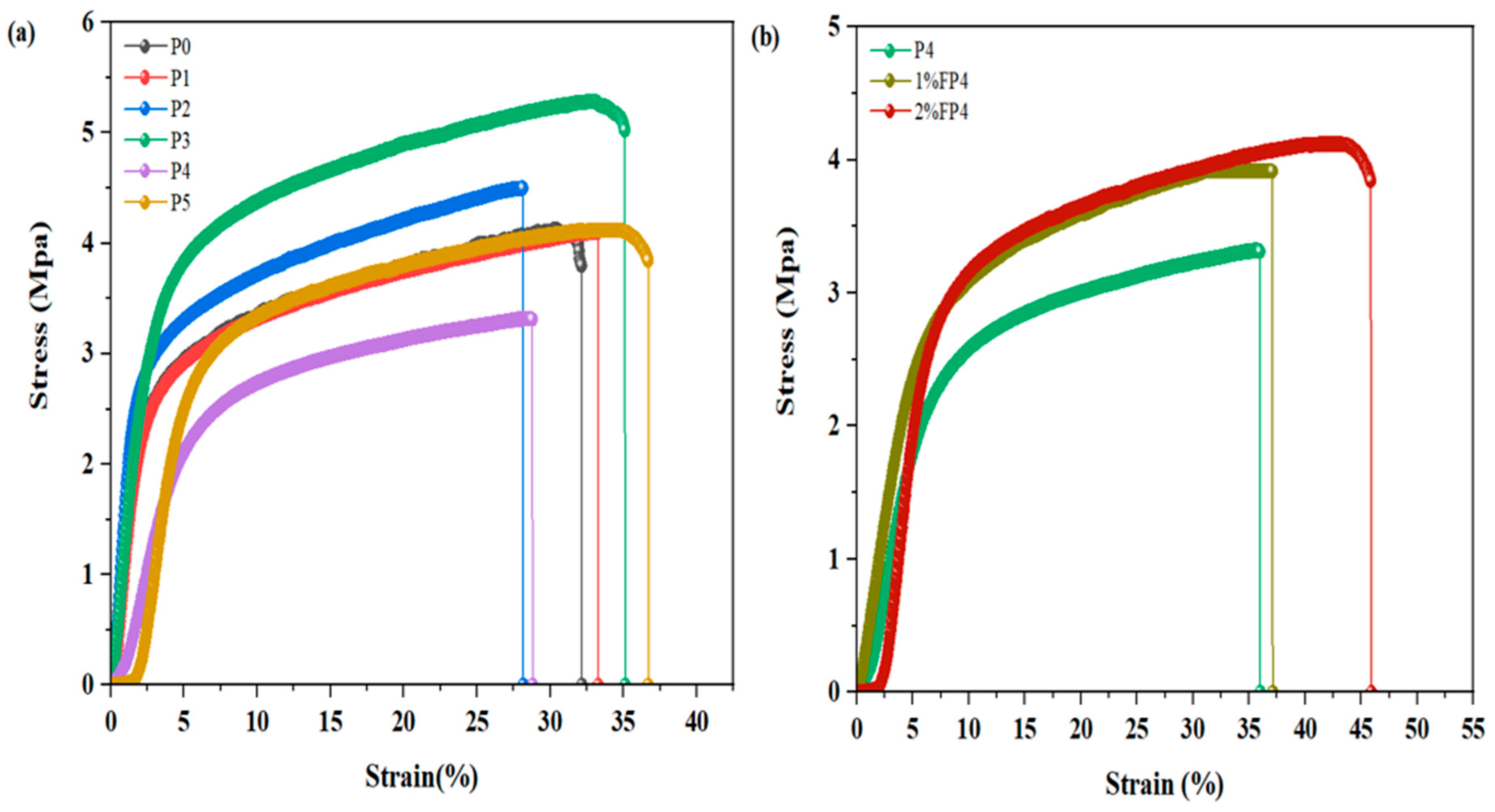
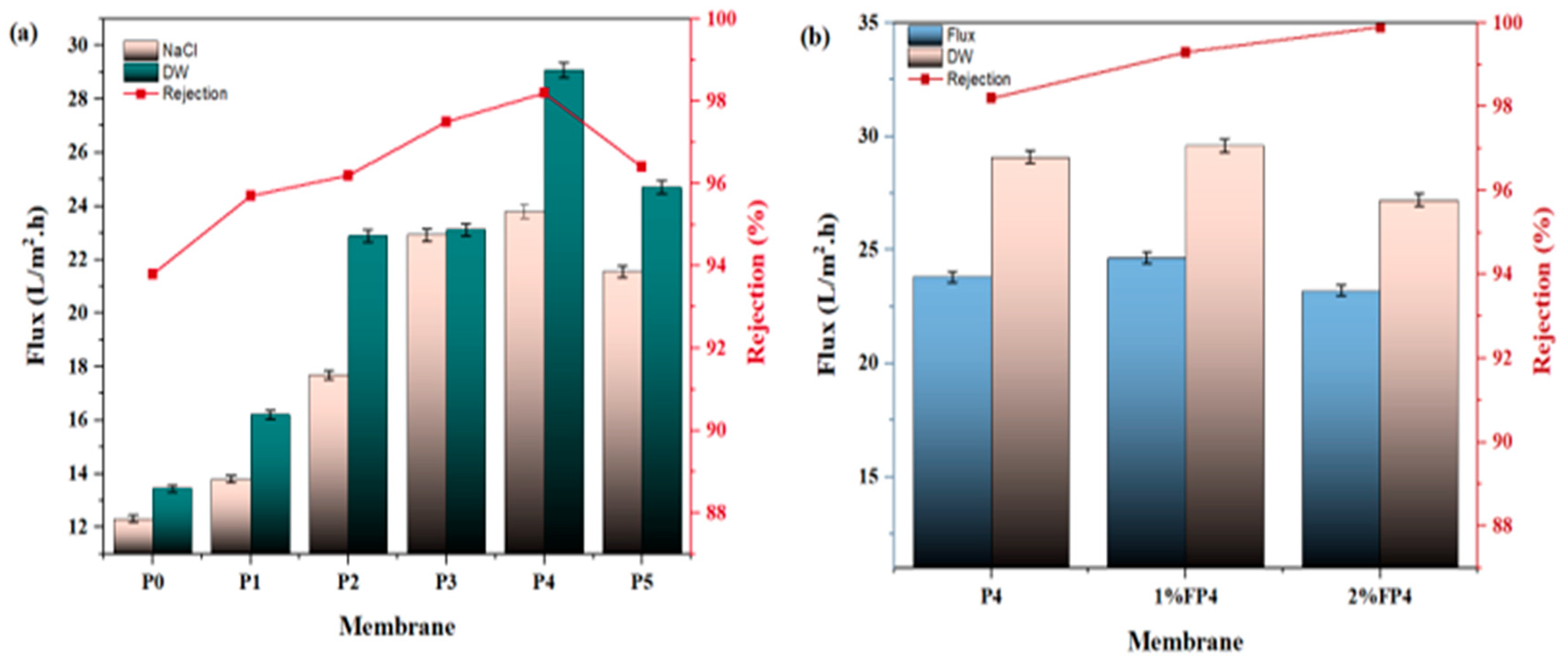
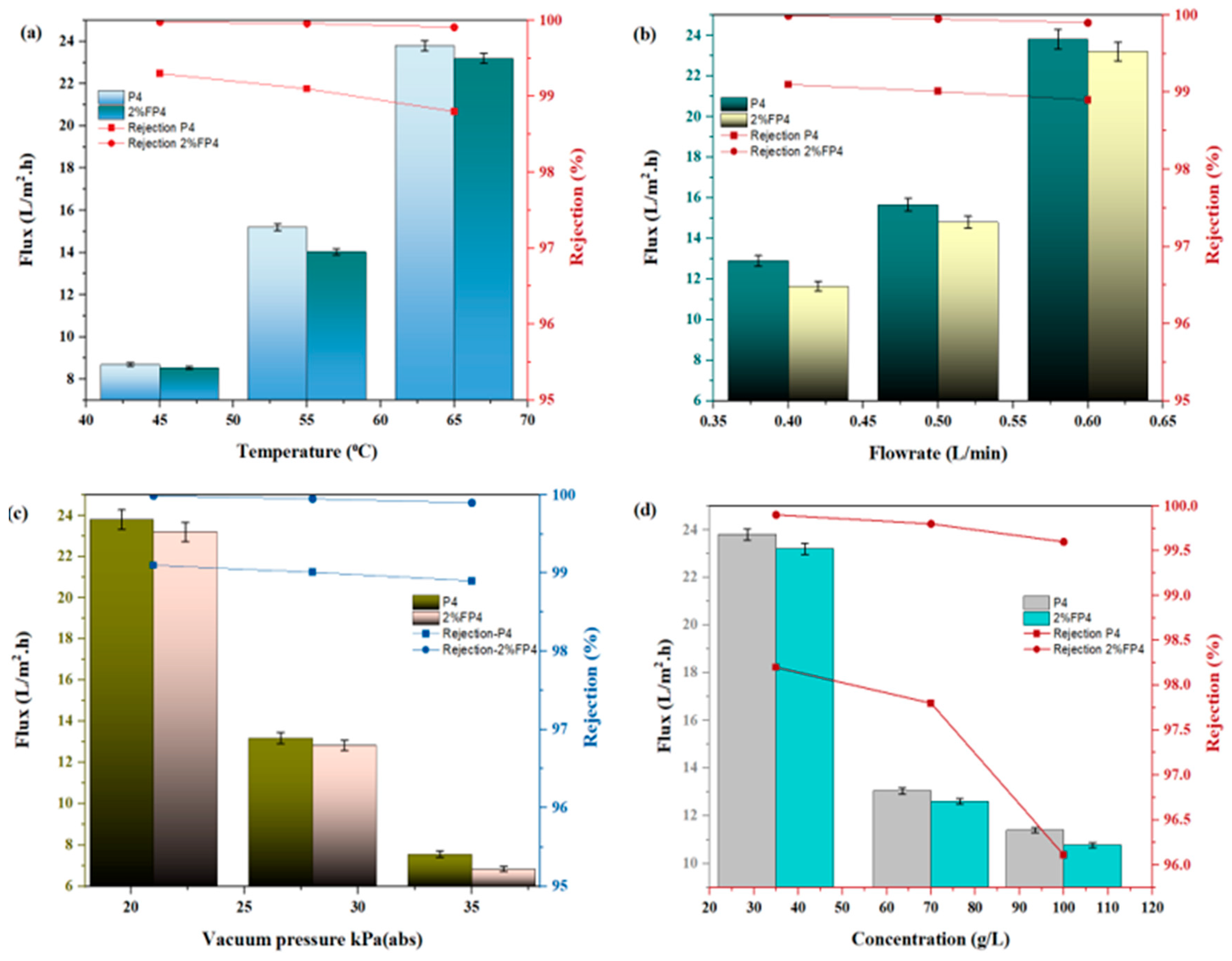
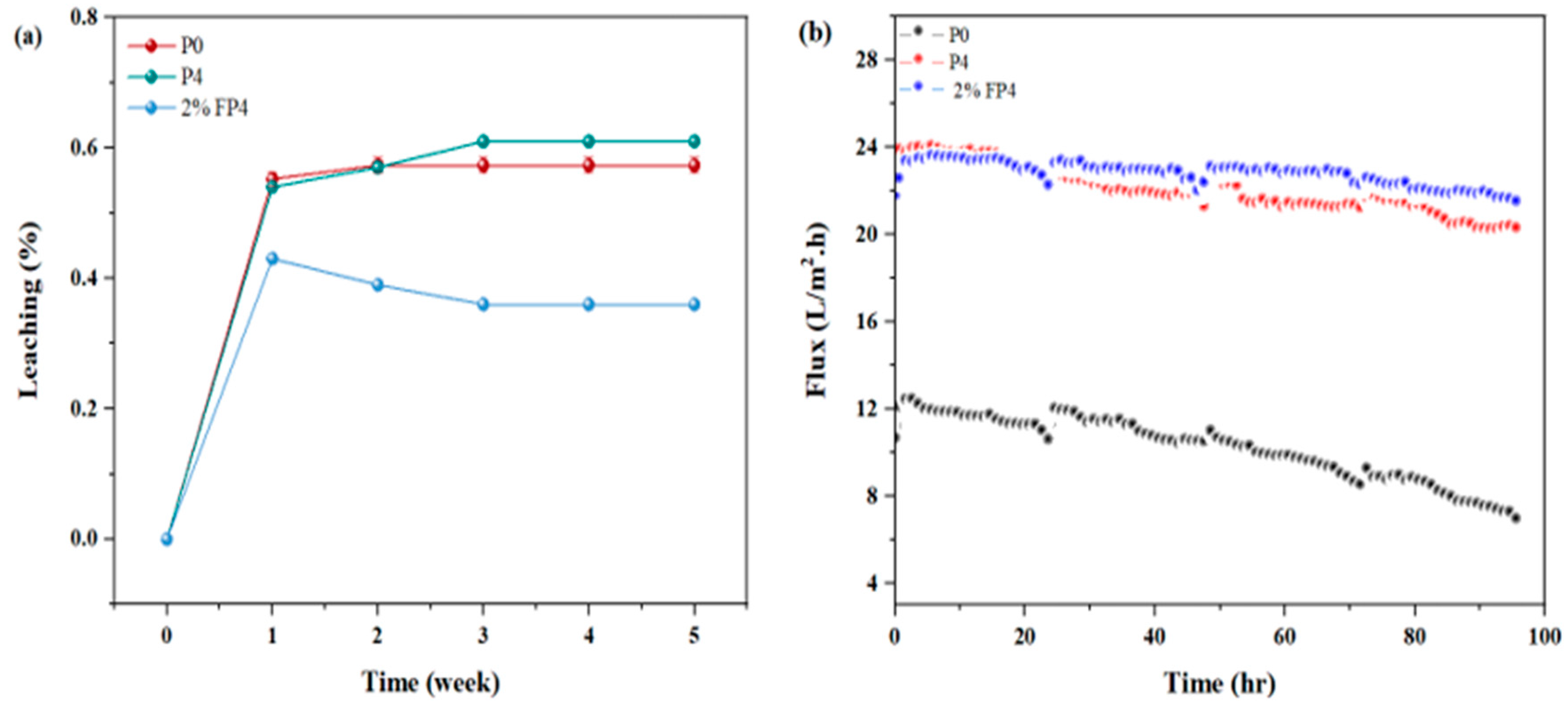
| Sample | PVDF (wt.%) | NMP (wt.%) | CNM/PAC (wt.%) |
|---|---|---|---|
| P0 | 14 | 86 | -- |
| P1 | 14 | 86 | 0.1 |
| P2 | 14 | 86 | 0.2 |
| P3 | 14 | 86 | 0.3 |
| P4 | 14 | 86 | 0.4 |
| P5 | 14 | 86 | 0.5 |
| Membrane Code | Average Surface Roughness (Ra) nm | Root Mean Square (Rq) nm | Maximum Height (Rz) nm |
|---|---|---|---|
| P0 | 64.76 | 78.68 | 455.6 |
| P1 | 66.96 | 83.28 | 499.9 |
| P2 | 81.53 | 100.2 | 641.6 |
| p3 | 93.94 | 115.2 | 706.3 |
| p4 | 95.93 | 115.1 | 600 |
| P5 | 56.17 | 69.56 | 466.2 |
| 1% FP4 | 156.3 | 196.3 | 1294 |
| 2% FP4 | 175 | 212.7 | 1122 |
| MD Conf. | Polymer | Fluorosilane Type | Contact Angle (°) | Operating Time (h) | Flux (kg/m2·h) | Rejection (%) | Ref. |
|---|---|---|---|---|---|---|---|
| DCMD | PVDF-HFP | perfluorodecyldimethylchlorosilane (FAS-17) | 138 | / | 19 | 99.9 | [65] |
| DCMD | PVDF | Perfluorodecyltriethoxysilane (PFDES) | 162 | / | / | 99.9 | [66] |
| AGMD | PVDF | Heneicosafluorododecyltrichlorosilane (FAS-21) | >150 | / | 19.09 | 99.9 | [41] |
| DCMD | PVDF | methyl trichloroalkyl silane (MTCS) perfluorooctane trichlorosilane silanes (PTCS) | 112 116 | / | / | / | [19] |
| VMD | PVDF | Perfluorodecyltriethoxysilane (PFTES) | 117 | 96 | 23.2 | 99.9 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khaleel, S.R.; Ibrahim, S.S.; Criscuoli, A.; Figoli, A.; Lawal, D.U.; Alsalhy, Q.F. Influence of Silane Treatment on CNM/PAC/PVDF Properties and Performance for Water Desalination by VMD. Membranes 2025, 15, 104. https://doi.org/10.3390/membranes15040104
Khaleel SR, Ibrahim SS, Criscuoli A, Figoli A, Lawal DU, Alsalhy QF. Influence of Silane Treatment on CNM/PAC/PVDF Properties and Performance for Water Desalination by VMD. Membranes. 2025; 15(4):104. https://doi.org/10.3390/membranes15040104
Chicago/Turabian StyleKhaleel, Samraa R., Salah S. Ibrahim, Alessandra Criscuoli, Alberto Figoli, Dahiru U. Lawal, and Qusay F. Alsalhy. 2025. "Influence of Silane Treatment on CNM/PAC/PVDF Properties and Performance for Water Desalination by VMD" Membranes 15, no. 4: 104. https://doi.org/10.3390/membranes15040104
APA StyleKhaleel, S. R., Ibrahim, S. S., Criscuoli, A., Figoli, A., Lawal, D. U., & Alsalhy, Q. F. (2025). Influence of Silane Treatment on CNM/PAC/PVDF Properties and Performance for Water Desalination by VMD. Membranes, 15(4), 104. https://doi.org/10.3390/membranes15040104









