Exploring Risk and Resilient Profiles for Functional Impairment and Baseline Predictors in a 2-Year Follow-Up First-Episode Psychosis Cohort Using Latent Class Growth Analysis
Abstract
1. Introduction
2. Experimental Section
2.1. Participants
2.2. Assessment
2.2.1. Baseline Sociodemographic Data
2.2.2. Baseline Clinical and Functional Assessment
2.2.3. 2-Month Follow-Up Neuropsychological Assessment
2.3. Statistical Analysis
2.3.1. Identification of Functional Trajectories: Latent Class Growth Analysis
2.3.2. Identification of Baseline Predictors of Functional Trajectory Membership
2.3.3. Diagnosis Distribution within the Identified Functional Trajectories
3. Results
3.1. Sample Characteristics and Attrition Analysis
3.2. Latent Classes of Functional Trajectories
3.3. Baseline Predictors of Trajectory Membership
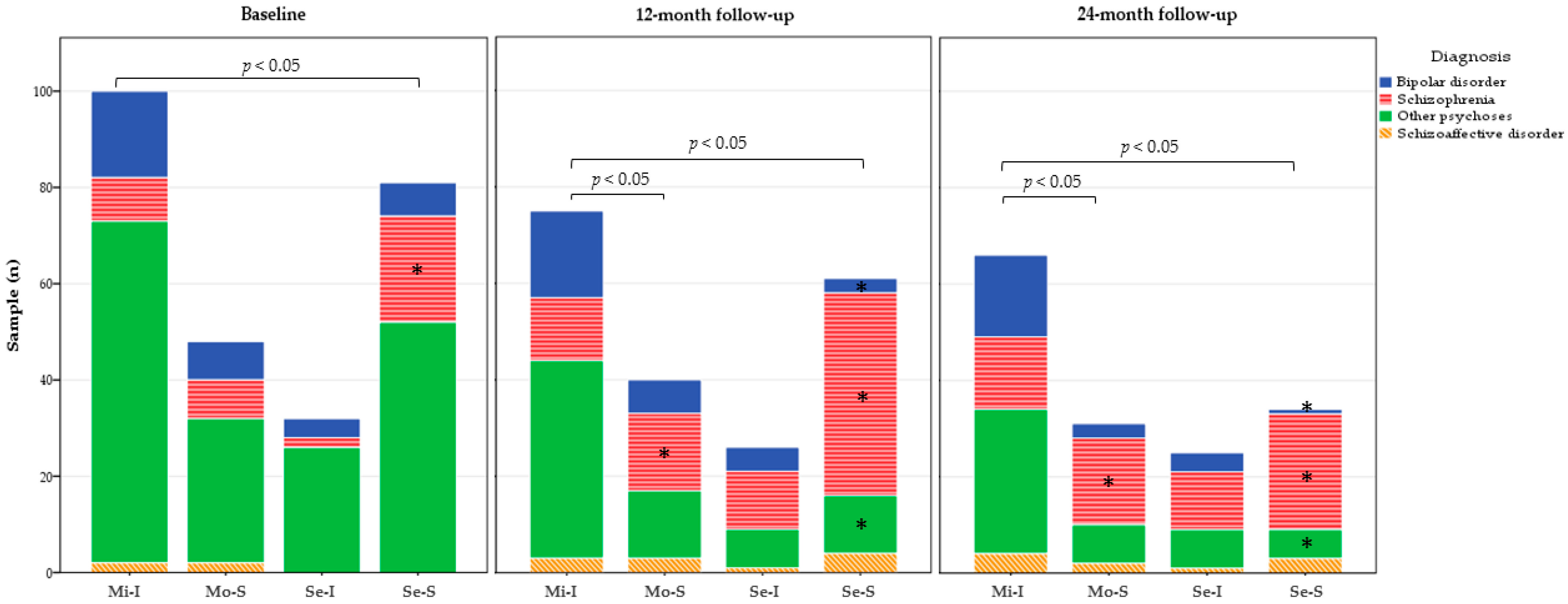
3.4. Exploring Diagnoses Distribution among Functional Trajectories throughout the Follow-Up
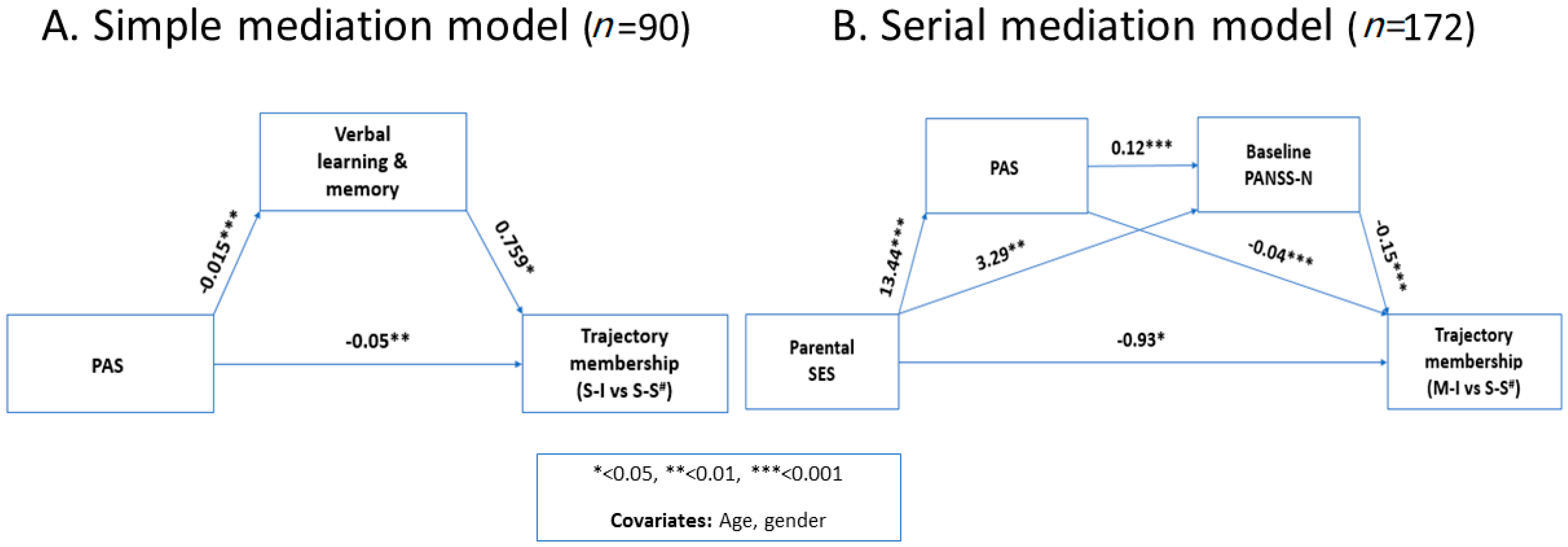
3.5. Post-Hoc Mediation Analysis
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Rosa, A.; Sanchez-Moreno, J.; Martinez-Aran, A.; Salamero, M.; Torrent, C.; Reinares, M.; Comes, M.; Colom, F.; Van Riel, W.; Ayuso-Mateos, J.; et al. Validity and reliability of the Functioning Assessment Short Test (FAST) in bipolar disorder. Clin. Pr. Epidemiol. Ment. Health CP EMH 2007, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Kahn, R.S.; Sommer, I.E.; Murray, R.M.; Meyer-Lindenberg, A.; Weinberger, D.R.; Cannon, T.D.; O’Donovan, M.; Correll, C.U.; Kane, J.M.; van Os, J.; et al. Schizophrenia. Nat. Rev. Dis. Primers 2015, 1, 15067. [Google Scholar] [CrossRef] [PubMed]
- Weissman, M.M.; Sholomskas, D.; John, K. The assessment of social adjustment. An update. Arch. Gen. Psychiatry 1981, 38, 1250–1258. [Google Scholar] [CrossRef] [PubMed]
- Keck, P.E., Jr. Defining and improving response to treatment in patients with bipolar disorder. J. Clin. Psychiatry 2004, 65 (Suppl. S15), 25–29. [Google Scholar] [PubMed]
- Michalak, E.E.; Murray, G. A clinician’s guide to psychosocial functioning and quality of life in bipolar disorder. In Practical Management of Bipolar Disorder; Young, A.H., Michalak, E.E., Ferrier, I.N., Eds.; Cambridge University Press: Cambridge, UK, 2010; pp. 163–174. [Google Scholar] [CrossRef]
- Álvarez-Jiménez, M.; Gleeson, J.F.; Henry, L.P.; Harrigan, S.M.; Harris, M.G.; Killackey, E.; Bendall, S.; Amminger, G.P.; Yung, A.R.; Herrman, H.; et al. Road to full recovery: Longitudinal relationship between symptomatic remission and psychosocial recovery in first-episode psychosis over 7.5 years. Psychol. Med. 2012, 42, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Birchwood, M.; Todd, P.; Jackson, C. Early intervention in psychosis. The critical period hypothesis. Br. J. Psychiatry. Suppl. 1998, 172, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Santesteban-Echarri, O.; Paino, M.; Rice, S.; González-Blanch, C.; McGorry, P.; Gleeson, J.; Alvarez-Jimenez, M. Predictors of functional recovery in first-episode psychosis: A systematic review and meta-analysis of longitudinal studies. Clin. Psychol. Rev. 2017, 58, 59–75. [Google Scholar] [CrossRef]
- Vieta, E.; Salagre, E.; Grande, I.; Carvalho, A.F.; Fernandes, B.S.; Berk, M.; Birmaher, B.; Tohen, M.; Suppes, T. Early Intervention in Bipolar Disorder. Am. J. Psychiatry 2018, 175, 411–426. [Google Scholar] [CrossRef]
- Silva Ribeiro, J.; Pereira, D.; Salagre, E.; Coroa, M.; Santos Oliveira, P.; Santos, V.; Madeira, N.; Grande, I.; Vieta, E. Risk Calculators in Bipolar Disorder: A Systematic Review. Brain Sci. 2020, 10, 525. [Google Scholar] [CrossRef]
- Bernardo, M.; Cabrera, B.; Arango, C.; Bioque, M.; Castro-Fornieles, J.; Cuesta, M.J.; Lafuente, A.; Parellada, M.; Saiz-Ruiz, J.; Vieta, E. One decade of the first episodes project (PEPs): Advancing towards a precision psychiatry. Rev. De Psiquiatr. Y Salud Ment. 2019, 12, 135–140. [Google Scholar] [CrossRef]
- Miettunen, J.; Nordström, T.; Kaakinen, M.; Ahmed, A.O. Latent variable mixture modeling in psychiatric research—A review and application. Psychol. Med. 2016, 46, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Van der Nest, G.; Lima Passos, V.; Candel, M.J.J.M.; Van Breukelen, G.J.P. An overview of mixture modelling for latent evolutions in longitudinal data: Modelling approaches, fit statistics and software. Adv. Life Course Res. 2020, 43. [Google Scholar] [CrossRef]
- Hodgekins, J.; Birchwood, M.; Christopher, R.; Marshall, M.; Coker, S.; Everard, L.; Lester, H.; Jones, P.; Amos, T.; Singh, S.; et al. Investigating trajectories of social recovery in individuals with first-episode psychosis: A latent class growth analysis. Br. J. Psychiatry J. Ment. Sci. 2015, 207, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.H.; Holton, K.M.; Öngür, D.; Montrose, D.; Keshavan, M.S. Longitudinal trajectory of early functional recovery in patients with first episode psychosis. Schizophr. Res. 2019, 209, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.C.; Chu, A.O.K.; Kwong, V.W.Y.; Wong, C.S.M.; Hui, C.L.M.; Chan, S.K.W.; Lee, E.H.M.; Chen, E.Y.H. Patterns and predictors of trajectories for social and occupational functioning in patients presenting with first-episode non-affective psychosis: A three-year follow-up study. Schizophr. Res. 2018, 197, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Suvisaari, J.; Mantere, O.; Keinänen, J.; Mäntylä, T.; Rikandi, E.; Lindgren, M.; Kieseppä, T.; Raij, T.T. Is It Possible to Predict the Future in First-Episode Psychosis? Front. Psychiatry 2018, 9, 580. [Google Scholar] [CrossRef]
- Bernardo, M.; Bioque, M.; Parellada, M.; Saiz Ruiz, J.; Cuesta, M.J.; Llerena, A.; Sanjuan, J.; Castro-Fornieles, J.; Arango, C.; Cabrera, B. Assessing clinical and functional outcomes in a gene-environment interaction study in first episode of psychosis (PEPs). Rev. De Psiquiatr. Y Salud Ment. 2013, 6, 4–16. [Google Scholar] [CrossRef]
- Salagre, E.; Arango, C.; Artigas, F.; Ayuso-Mateos, J.L.; Bernardo, M.; Castro-Fornieles, J.; Bobes, J.; Desco, M.; Fananas, L.; Gonzalez-Pinto, A.; et al. CIBERSAM: Ten years of collaborative translational research in mental disorders. Rev. De Psiquiatr. Y Salud Ment. 2019, 12, 1–8. [Google Scholar] [CrossRef]
- Salagre, E.; Grande, I.; Vieta, E.; Mezquida, G.; Cuesta, M.J.; Moreno, C.; Bioque, M.; Lobo, A.; González-Pinto, A.; Moreno, D.M.; et al. Predictors of Bipolar Disorder Versus Schizophrenia Diagnosis in a Multicenter First Psychotic Episode Cohort: Baseline Characterization and a 12-Month Follow-Up Analysis. J. Clin. Psychiatry 2020, 81, 19m12996. [Google Scholar] [CrossRef]
- APA. DSM-IV: Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; American Psychiatric Association: Washington, DC, USA, 1994. [Google Scholar]
- Hollingshead, A.B.; Redlich, F.C. Social class and mental illness: A community study. 1958. Am. J. Public Health 2007, 97, 1756–1757. [Google Scholar]
- Kokkevi, A.; Hartgers, C. EuropASI: European Adaptation of a Multidimensional Assessment Instrument for Drug and Alcohol Dependence. Eur. Addict. Res. 1995, 1, 208–210. [Google Scholar] [CrossRef]
- Moos, R.H.; Moos, B.S. Family Environment Scale Manual; Consulting Psychologist Press: Palo Alto, CA, USA, 1981. [Google Scholar]
- Fernández-Ballesteros, R.; Sierra, B. Escalas de Clima Social FES, WES, CIES y CES.; TEA: Madrid, Spain, 1989. [Google Scholar]
- First, M.S.R.; Gibbon, M.; Williams, J. Structured Clinical Interview for DSM-IV Axis I Disorders; Administration booklet; American Psychiatric Press Inc.: Washington, DC, USA, 1994. [Google Scholar]
- Kay, S.R.; Fiszbein, A.; Opler, L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987, 13, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Peralta, V.; Cuesta, M.J. Psychometric properties of the positive and negative syndrome scale (PANSS) in schizophrenia. Psychiatry Res. 1994, 53, 31–40. [Google Scholar] [CrossRef]
- Colom, F.; Vieta, E.; Martinez-Aran, A.; Garcia-Garcia, M.; Reinares, M.; Torrent, C.; Goikolea, J.M.; Banus, S.; Salamero, M. Spanish version of a scale for the assessment of mania: Validity and reliability of the Young Mania Rating Scale. Med. Clin. 2002, 119, 366–371. [Google Scholar] [CrossRef]
- Young, R.C.; Biggs, J.T.; Ziegler, V.E.; Meyer, D.A. A rating scale for mania: Reliability, validity and sensitivity. Br. J. Psychiatry J. Ment. Sci. 1978, 133, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Lobo, A.; Chamorro, L.; Luque, A.; Dal-Re, R.; Badia, X.; Baro, E. Validation of the Spanish versions of the Montgomery-Asberg depression and Hamilton anxiety rating scales. Med. Clin. 2002, 118, 493–499. [Google Scholar] [CrossRef]
- Montgomery, S.A.; Asberg, M. A new depression scale designed to be sensitive to change. Br. J. Psychiatry J. Ment. Sci. 1979, 134, 382–389. [Google Scholar] [CrossRef]
- Cannon, M.; Jones, P.; Gilvarry, C.; Rifkin, L.; McKenzie, K.; Foerster, A.; Murray, R.M. Premorbid social functioning in schizophrenia and bipolar disorder: Similarities and differences. Am. J. Psychiatry 1997, 154, 1544–1550. [Google Scholar] [CrossRef]
- Rosa, A.; Reinares, M.; Amann, B.; Popovic, D.; Franco, C.; Comes, M.; Torrent, C.; Bonnin, C.; Sole, B.; Valenti, M.; et al. Six-month functional outcome of a bipolar disorder cohort in the context of a specialized-care program. Bipolar. Disord. 2011, 13, 679–686. [Google Scholar] [CrossRef]
- Bonnín, C.M.; Martínez-Arán, A.; Reinares, M.; Valentí, M.; Solé, B.; Jiménez, E.; Montejo, L.; Vieta, E.; Rosa, A.R. Thresholds for severity, remission and recovery using the functioning assessment short test (FAST) in bipolar disorder. J. Affect. Disord. 2018, 240, 57–62. [Google Scholar] [CrossRef]
- González-Ortega, I.; Rosa, A.; Alberich, S.; Barbeito, S.; Vega, P.; Echeburúa, E.; Vieta, E.; González-Pinto, A. Validation and use of the functioning assessment short test in first psychotic episodes. J. Nerv. Ment. Dis. 2010, 198, 836–840. [Google Scholar] [CrossRef] [PubMed]
- Bobes, J.; Calcedo-Barba, A.; Garcia, M.; Francois, M.; Rico-Villademoros, F.; Gonzalez, M.P.; Bascaran, M.T.; Bousono, M. Evaluation of the psychometric properties of the Spanish version of 5 questionnaires for the evaluation of post-traumatic stress syndrome. Actas Esp. De Psiquiatr. 2000, 28, 207–218. [Google Scholar]
- Davidson, J.; Smith, R. Traumatic experiences in psychiatric outpatients. J. Trauma. Stress 1990, 3, 459–475. [Google Scholar] [CrossRef]
- Perkins, D.O.; Leserman, J.; Jarskog, L.F.; Graham, K.; Kazmer, J.; Lieberman, J.A. Characterizing and dating the onset of symptoms in psychotic illness: The Symptom Onset in Schizophrenia (SOS) inventory. Schizophr. Res. 2000, 44, 1–10. [Google Scholar] [CrossRef]
- Nuechterlein, K.H.; Green, M.; Kern, R.S.; Kern, R.; Baade, L.E.; Baade, L.; Barch, D.M.; Barch, D.; Cohen, J.D.; Cohen, J.; et al. The MATRICS Consensus Cognitive Battery, part 1: Test selection, reliability, and validity. Am. J. Psychiatry 2008, 165, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, D. Wechsler Adult Intelligence Scale—III (WAIS-III); Psychological Corporation: San Antonio, TX, USA, 1997. [Google Scholar]
- Golden, C.J. Stroop Color and Word Test: A Manual for Clinical and Experimental Uses; Stoelting Co.: Chicago, IL, USA, 1978. [Google Scholar]
- Heaton, R.K. Wisconsin Card Sorting Test Manual; Psychological Assessment Resources: Odessa, FL, USA, 1981. [Google Scholar]
- Reitan, R.M. Validity of the Trail Making Test as an indicator of organic brain damage. Percept. Mot. Ski. 1958, 8, 271–276. [Google Scholar] [CrossRef]
- Conners, C.K. Conners’ Continuous Performance Test; Multi-Health System: Toronto, ON, Canada, 2002. [Google Scholar]
- Reitan, R.M.; Wolfson, D.W. The Halstead–ReitanNeuropsychological Test Battery: Theory and Clinical Interpretation; Neuropsychology Press: Tucson, AZ, USA, 1993. [Google Scholar]
- Benton, A.L.; Hamsher, K. Multilingual Aphasia Examination Manual; University of Iowa: Iowa City, IA, USA, 1976. [Google Scholar]
- Benedet, M. Test de Aprendizaje Verbal España-Complutense (TAVEC); Tea Ediciones: Madrid, Spain, 1998. [Google Scholar]
- Brackett, M.A.; Salovey, P. Measuring emotional intelligence with the Mayer-Salovery-Caruso Emotional Intelligence Test (MSCEIT). Psicothema 2006, 18, 34–41. [Google Scholar] [PubMed]
- Extremera, N.; Fernandez-Berrocal, P.; Salovey, P. Spanish version of the Mayer-Salovey-Caruso Emotional Intelligence Test (MSCEIT). Version 2.0: Reliabilities, age and gender differences. Psicothema 2006, 18, 42–48. [Google Scholar]
- Cuesta, M.J.; Sanchez-Torres, A.M.; Cabrera, B.; Bioque, M.; Merchan-Naranjo, J.; Corripio, I.; Gonzalez-Pinto, A.; Lobo, A.; Bombin, I.; de la Serna, E.; et al. Premorbid adjustment and clinical correlates of cognitive impairment in first-episode psychosis. The PEPsCog Study. Schizophr. Res. 2015, 164, 65–73. [Google Scholar] [CrossRef]
- Van de Schoot, R.; Sijbrandij, M.; Winter, S.D.; Depaoli, S.; Vermunt, J.K. The GRoLTS-Checklist: Guidelines for Reporting on Latent Trajectory Studies. Struct. Equ. Modeling A Multidiscip. J. 2017, 24, 451–467. [Google Scholar] [CrossRef]
- Celeux, G.; Soromenho, G. An entropy criterion for assessing the number of clusters in a mixture model. J. Classif. 1996, 13, 195–212. [Google Scholar] [CrossRef]
- Proust-Lima, C.; Philipps, V.; Liquet, B. Estimation of Extended Mixed Models Using Latent Classes and Latent Processes: The R Package lcmm. J. Stat. Softw. 2017, 78, 1–56. [Google Scholar] [CrossRef]
- Torrent, C.; Reinares, M.; Martinez-Arán, A.; Cabrera, B.; Amoretti, S.; Corripio, I.; Contreras, F.; Sarró, S.; González-Pinto, A.; Lobo, A.; et al. Affective versus non-affective first episode psychoses: A longitudinal study. J. Affect. Disord. 2018, 238, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Amoretti, S.; Cabrera, B.; Torrent, C.; Mezquida, G.; Lobo, A.; Gonzalez-Pinto, A.; Parellada, M.; Corripio, I.; Vieta, E.; de la Serna, E.; et al. Cognitive reserve as an outcome predictor: First-episode affective versus non-affective psychosis. Acta Psychiatr. Scand. 2018, 138, 441–455. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.B.; Karstoft, K.I.; Bertelsen, M.; Madsen, T. Latent trajectories of trauma symptoms and resilience: The 3-year longitudinal prospective USPER study of Danish veterans deployed in Afghanistan. J. Clin. Psychiatry 2014, 75, 1001–1008. [Google Scholar] [CrossRef]
- Shaunna, L.; Clark, B.M. Relating Latent Class Analysis Results to Variables not Included in the Analysis. 2009. Available online: https://www.statmodel.com/download/relatinglca.pdf (accessed on 6 August 2020).
- Jordan, G.; Veru, F.; Lepage, M.; Joober, R.; Malla, A.; Iyer, S.N. Pathways to functional outcomes following a first episode of psychosis: The roles of premorbid adjustment, verbal memory and symptom remission. Aust. N. Zealand J. Psychiatry 2018, 52, 793–803. [Google Scholar] [CrossRef]
- Preacher, K.J.; Hayes, A.F. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav. Res. Methods 2008, 40, 879–891. [Google Scholar] [CrossRef]
- Hayes, A.F. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach; Guilford Press: New York, NY, USA, 2013. [Google Scholar]
- Zhao, X.; Lynch, J.G., Jr.; Chen, Q. Reconsidering Baron and Kenny: Myths and truths about mediation analysis. J. Consum. Res. 2010, 37, 197–206. [Google Scholar] [CrossRef]
- Hower, H.; Lee, E.J.; Jones, R.N.; Birmaher, B.; Strober, M.; Goldstein, B.I.; Merranko, J.; Keller, M.B.; Goldstein, T.R.; Weinstock, L.M.; et al. Predictors of longitudinal psychosocial functioning in bipolar youth transitioning to adults. J. Affect. Disord. 2019, 246, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Milev, P.; Ho, B.C.; Arndt, S.; Andreasen, N.C. Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: A longitudinal first-episode study with 7-year follow-up. Am. J. Psychiatry 2005, 162, 495–506. [Google Scholar] [CrossRef]
- Chang, W.C.; Ho, R.W.H.; Tang, J.Y.M.; Wong, C.S.M.; Hui, C.L.M.; Chan, S.K.W.; Lee, E.M.H.; Suen, Y.N.; Chen, E.Y.H. Early-Stage Negative Symptom Trajectories and Relationships With 13-Year Outcomes in First-Episode Nonaffective Psychosis. Schizophr. Bull. 2019, 45, 610–619. [Google Scholar] [CrossRef]
- Stouten, L.H.; Veling, W.; Laan, W.; van der Helm, M.; van der Gaag, M. Psychotic symptoms, cognition and affect as predictors of psychosocial problems and functional change in first-episode psychosis. Schizophr. Res. 2014, 158, 113–119. [Google Scholar] [CrossRef]
- Bonnín, C.M.; Jiménez, E.; Solé, B.; Torrent, C.; Radua, J.; Reinares, M.; Grande, I.; Ruíz, V.; Sánchez-Moreno, J.; Martínez-Arán, A.; et al. Lifetime Psychotic Symptoms, Subthreshold Depression and Cognitive Impairment as Barriers to Functional Recovery in Patients with Bipolar Disorder. J. Clin. Med. 2019, 8, 1046. [Google Scholar] [CrossRef] [PubMed]
- Addington, J.; Addington, D. Patterns of premorbid functioning in first episode psychosis: Relationship to 2-year outcome. Acta Psychiatr. Scand. 2005, 112, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Velthorst, E.; Fett, A.J.; Reichenberg, A.; Perlman, G.; van Os, J.; Bromet, E.J.; Kotov, R. The 20-Year Longitudinal Trajectories of Social Functioning in Individuals With Psychotic Disorders. Am. J. Psychiatry 2017, 174, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- Wells, R.; Jacomb, I.; Swaminathan, V.; Sundram, S.; Weinberg, D.; Bruggemann, J.; Cropley, V.; Lenroot, R.K.; Pereira, A.M.; Zalesky, A.; et al. The Impact of Childhood Adversity on Cognitive Development in Schizophrenia. Schizophr. Bull. 2020, 46, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Grande, I.; Sanchez-Moreno, J.; Sole, B.; Jimenez, E.; Torrent, C.; Bonnin, C.M.; Varo, C.; Tabares-Seisdedos, R.; Balanzá-Martínez, V.; Valls, E.; et al. High cognitive reserve in bipolar disorders as a moderator of neurocognitive impairment. J. Affect. Disord. 2017, 208, 621–627. [Google Scholar] [CrossRef]
- Amoretti, S.; Rosa, A.R.; Mezquida, G.; Cabrera, B.; Ribeiro, M.; Molina, M.; Bioque, M.; Lobo, A.; González-Pinto, A.; Fraguas, D.; et al. The impact of cognitive reserve, cognition and clinical symptoms on psychosocial functioning in first-episode psychoses. Psychol. Med. 2020, 1–12. [Google Scholar] [CrossRef]
- Omer, S.; Finnegan, M.; Pringle, D.G.; Kinsella, A.; Fearon, P.; Russell, V.; O’Callaghan, E.; Waddington, J.L. Socioeconomic status at birth and risk for first episode psychosis in rural Ireland: Eliminating the features of urbanicity in the Cavan-Monaghan First Episode Psychosis Study (CAMFEPS). Schizophr. Res. 2016, 173, 84–89. [Google Scholar] [CrossRef]
- Xiang, L.; Su, Z.; Liu, Y.; Huang, Y.; Zhang, X.; Li, S.; Zhang, H. Impact of Family Socioeconomic Status on Health-Related Quality of Life in Children With Critical Congenital Heart Disease. J. Am. Heart Assoc. 2019, 8, e010616. [Google Scholar] [CrossRef]
- Arango, C.; Díaz-Caneja, C.M.; McGorry, P.D.; Rapoport, J.; Sommer, I.E.; Vorstman, J.A.; McDaid, D.; Marín, O.; Serrano-Drozdowskyj, E.; Freedman, R.; et al. Preventive strategies for mental health. Lancet. Psychiatry 2018, 5, 591–604. [Google Scholar] [CrossRef]
- Fusar-Poli, P.; McGorry, P.D.; Kane, J.M. Improving outcomes of first-episode psychosis: An overview. World Psychiatry Off. J. World Psychiatr. Assoc. (WPA) 2017, 16, 251–265. [Google Scholar] [CrossRef]
- Fu, S.; Czajkowski, N.; Rund, B.R.; Torgalsbøen, A.K. The relationship between level of cognitive impairments and functional outcome trajectories in first-episode schizophrenia. Schizophr. Res. 2017, 190, 144–149. [Google Scholar] [CrossRef]
- Tabarés-Seisdedos, R.; Balanzá-Martínez, V.; Sánchez-Moreno, J.; Martinez-Aran, A.; Salazar-Fraile, J.; Selva-Vera, G.; Rubio, C.; Mata, I.; Gómez-Beneyto, M.; Vieta, E. Neurocognitive and clinical predictors of functional outcome in patients with schizophrenia and bipolar I disorder at one-year follow-up. J. Affect. Disord. 2008, 109, 286–299. [Google Scholar] [CrossRef]
- Sanchez-Moreno, J.; Bonnin, C.M.; González-Pinto, A.; Amann, B.L.; Solé, B.; Balanzá-Martinez, V.; Arango, C.; Jiménez, E.; Tabarés-Seisdedos, R.; Garcia-Portilla, M.P.; et al. Factors associated with poor functional outcome in bipolar disorder: Sociodemographic, clinical, and neurocognitive variables. Acta Psychiatr. Scand. 2018, 138, 145–154. [Google Scholar] [CrossRef]
- Solé, B.; Bonnín, C.M.; Radua, J.; Montejo, L.; Hogg, B.; Jimenez, E.; Reinares, M.; Valls, E.; Varo, C.; Pacchiarotti, I.; et al. Long-term outcome predictors after functional remediation in patients with bipolar disorder. Psychol. Med. 2020, 1–9. [Google Scholar] [CrossRef]
- Albert, N.; Bertelsen, M.; Thorup, A.; Petersen, L.; Jeppesen, P.; Le Quack, P.; Krarup, G.; Jørgensen, P.; Nordentoft, M. Predictors of recovery from psychosis Analyses of clinical and social factors associated with recovery among patients with first-episode psychosis after 5 years. Schizophr. Res. 2011, 125, 257–266. [Google Scholar] [CrossRef]
- Gee, B.; Hodgekins, J.; Fowler, D.; Marshall, M.; Everard, L.; Lester, H.; Jones, P.B.; Amos, T.; Singh, P.S.; Sharma, V.; et al. The course of negative symptom in first episode psychosis and the relationship with social recovery. Schizophr. Res. 2016, 174, 165–171. [Google Scholar] [CrossRef]
- Bucci, P.; Mucci, A.; van Rossum, I.W.; Aiello, C.; Arango, C.; Baandrup, L.; Buchanan, R.W.; Dazzan, P.; Demjaha, A.; Díaz-Caneja, C.M.; et al. Persistent negative symptoms in recent-onset psychosis: Relationship to treatment response and psychosocial functioning. Eur. Neuropsychopharmacol. 2020, 34, 76–86. [Google Scholar] [CrossRef]
- Dickinson, D.; Coursey, R.D. Independence and overlap among neurocognitive correlates of community functioning in schizophrenia. Schizophr. Res. 2002, 56, 161–170. [Google Scholar] [CrossRef]
- Buck, G.; Lavigne, K.M.; Makowski, C.; Joober, R.; Malla, A.; Lepage, M. Sex Differences in Verbal Memory Predict Functioning Through Negative Symptoms in Early Psychosis. Schizophr. Bull. 2020, 46, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Simons, C.J.; Bartels-Velthuis, A.A.; Pijnenborg, G.H. Cognitive Performance and Long-Term Social Functioning in Psychotic Disorder: A Three-Year Follow-Up Study. PLoS ONE 2016, 11, e0151299. [Google Scholar] [CrossRef] [PubMed]
- Treen Calvo, D.; Giménez-Donoso, S.; Setién-Suero, E.; Toll Privat, A.; Crespo-Facorro, B.; Ayesa Arriola, R. Targeting recovery in first episode psychosis: The importance of neurocognition and premorbid adjustment in a 3-year longitudinal study. Schizophr. Res. 2018, 195, 320–326. [Google Scholar] [CrossRef]
- Seidman, L.J.; Nordentoft, M. New Targets for Prevention of Schizophrenia: Is It Time for Interventions in the Premorbid Phase? Schizophr. Bull. 2015, 41, 795–800. [Google Scholar] [CrossRef]
- Salagre, E.; Dodd, S.; Aedo, A.; Rosa, A.; Amoretti, S.; Pinzon, J.; Reinares, M.; Berk, M.; Kapczinski, F.P.; Vieta, E.; et al. Toward Precision Psychiatry in Bipolar Disorder: Staging 2.0. Front. Psychiatry 2018, 9, 641. [Google Scholar] [CrossRef] [PubMed]
- Bonnin, C.M.; Reinares, M.; Martínez-Arán, A.; Balanzá-Martínez, V.; Sole, B.; Torrent, C.; Tabarés-Seisdedos, R.; García-Portilla, M.P.; Ibáñez, A.; Amann, B.L.; et al. Effects of functional remediation on neurocognitively impaired bipolar patients: Enhancement of verbal memory. Psychol. Med. 2016, 46, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Bowie, C.R.; McGurk, S.R.; Mausbach, B.; Patterson, T.L.; Harvey, P.D. Combined cognitive remediation and functional skills training for schizophrenia: Effects on cognition, functional competence, and real-world behavior. Am. J. Psychiatry 2012, 169, 710–718. [Google Scholar] [CrossRef]
- Glenthøj, L.B.; Hjorthøj, C.; Kristensen, T.D.; Davidson, C.A.; Nordentoft, M. The effect of cognitive remediation in individuals at ultra-high risk for psychosis: A systematic review. Npj Schizophr. 2017, 3, 20. [Google Scholar] [CrossRef]
- Lyngstad, S.H.; Gardsjord, E.S.; Simonsen, C.; Engen, M.J.; Romm, K.L.; Melle, I.; Færden, A. Consequences of persistent depression and apathy in first-episode psychosis—A one-year follow-up study. Compr. Psychiatry 2018, 86, 60–66. [Google Scholar] [CrossRef]
- González-Ortega, I.; Alberich, S.; Echeburúa, E.; Aizpuru, F.; Millán, E.; Vieta, E.; Matute, C.; González-Pinto, A. Subclinical depressive symptoms and continued cannabis use: Predictors of negative outcomes in first episode psychosis. PLoS ONE 2015, 10, e0123707. [Google Scholar] [CrossRef]
- Edwards, C.J.; Garety, P.; Hardy, A. The relationship between depressive symptoms and negative symptoms in people with non-affective psychosis: A meta-analysis. Psychol. Med. 2019, 49, 2486–2498. [Google Scholar] [CrossRef] [PubMed]
- Clementz, B.A.; Trotti, R.L.; Pearlson, G.D.; Keshavan, M.S.; Gershon, E.S.; Keedy, S.K.; Ivleva, E.I.; McDowell, J.E.; Tamminga, C.A. Testing Psychosis Phenotypes From Bipolar-Schizophrenia Network for Intermediate Phenotypes for Clinical Application: Biotype Characteristics and Targets. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2020, 5, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, B.; Strauss, G.P.; Nguyen, L.; Fischer, B.A.; Daniel, D.G.; Cienfuegos, A.; Marder, S.R. The brief negative symptom scale: Psychometric properties. Schizophr. Bull. 2011, 37, 300–305. [Google Scholar] [CrossRef]
- Kring, A.M.; Gur, R.E.; Blanchard, J.J.; Horan, W.P.; Reise, S.P. The Clinical Assessment Interview for Negative Symptoms (CAINS): Final development and validation. Am. J. Psychiatry 2013, 170, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Amoretti, S.; Cabrera, B.; Torrent, C.; Bonnín, C.D.M.; Mezquida, G.; Garriga, M.; Jiménez, E.; Martínez-Arán, A.; Solé, B.; Reinares, M.; et al. Cognitive Reserve Assessment Scale in Health (CRASH): Its Validity and Reliability. J. Clin. Med. 2019, 8, 586. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Median (IQR)/n (%) |
|---|---|
| Age (Years) | 25.05 (9) |
| Sex (Female) | 87 (33.3) |
| Marital status (Single) | 222 (85.1) |
| Ethnicity (Caucasian) | 228 (87.4) |
| Parental socioeconomic status (Medium-high) | 141 (54.6) |
| Living situation (Living independently) | 55 (21.1) |
| Educational level (Higher education) | 115 (44.2) |
| Occupational status (Active *) | 136 (52.1) |
| Somatic comorbidity (Yes) | 73 (28.0) |
| Family history of psychiatric disorder (Yes) | 146 (55.9) |
| Fit Statistics a | % of the Sample in Each Class | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of Classes | Number of Parameters | AIC | BIC | aBIC | Entropy | Class 1 | Class 2 | Class 3 | Class 4 |
| 1 | 4 | 9029.81 | 9044.07 | 9031.39 | - | 100 | - | - | - |
| 2 | 8 | 8675.19 | 8703.71 | 8678.35 | 0.82 | 54.02 | 45.98 | - | - |
| 3 | 12 | 8639.13 | 8681.91 | 8643.86 | 0.71 | 41.00 | 32.18 | 26.82 | - |
| 4 | 16 | 8574.11 | 8631.14 | 8580.41 | 0.76 | 38.31 | 18.39 | 12.26 | 31.03 |
| Mi-I (1) n = 100 | Mo-S (2) n = 48 | Se-I (3) n = 32 | Se-S (4) n = 81 | Kruskal–Wallis/X2 | p-Value | Post-Hoc a | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 vs. 2 | 1 vs. 3 | 1 vs. 4 | 2 vs. 3 | 2 vs. 4 | 3 vs. 4 | |||||||
| Sociodemographic characteristics | ||||||||||||
| Age (years) b | 25.8 (10) | 24.9 (8) | 24.4 (8) | 24.8 (8) | 1.44 | 0.70 | ||||||
| Sex (Female) c | 29 (29.0) | 18 (37.5) | 10 (31.2) | 30 (37.0) | 1.78 | 0.62 | ||||||
| Ethnicity (Caucasian) c | 91 (91.0) | 41 (85.4) | 27 (84.4) | 69 (85.2) | 1.97 | 0.58 | ||||||
| Marital status (Single) c | 83 (83.0) | 42 (87.5) | 29 (90.6) | 68 (83.9) | 1.42 | 0.70 | ||||||
| Living situation (Living independent) c | 30 (30.0) | 4 (8.3) | 7 (21.9) | 14 (17.3) | 10.19 | 0.02 | <0.05 | |||||
| Educational level (Higher education) c | 55 (55.0) | 20 (41.7) | 16 (50.0) | 24 (29.6) | 12.71 | <0.01 | <0.05 | |||||
| Occupational status (Active d) c | 63 (63.0) | 26 (54.2) | 18 (56.2) | 29 (35.8) | 13.68 | <0.01 | <0.05 | |||||
| Socioeconomic status (Medium-high) c | 72 (72.0) | 22 (45.8) | 18 (56.2) | 29 (35.8) | 24.06 | <0.001 | <0.05 | <0.05 | ||||
| Family history of psychiatric disorders (Yes) c | 53 (53.0) | 33 (68.7) | 17 (53.1) | 43 (53.1) | 3.92 | 0.27 | ||||||
| Previous psychiatric diagnoses (Yes) c | 24 (24.0) | 13 (27.1) | 8 (25.0) | 29 (35.8) | 4.65 | 0.59 | ||||||
| Substance use c | ||||||||||||
| Tobacco | 70 (70.0) | 33 (68.7) | 24 (75.0) | 55 (67.9) | 0.57 | 0.90 | ||||||
| Alcohol | 60 (60.0) | 25 (52.1) | 24 (75.0) | 32 (39.5) | 14.05 | <0.01 | <0.05 | <0.05 | ||||
| Cannabis | 44 (44.0) | 22 (45.8) | 13 (40.6) | 36 (44.4) | 0.22 | 0.97 | ||||||
| Cocaine | 9 (9.0) | 8 (16.7) | 6 (18.7) | 12 (14.8) | 3.04 | 0.39 | ||||||
| Clinical measures | ||||||||||||
| DUP (days) b | 85.0 (165) | 133.0 (263) | 110.0 (168) | 162.0 (216) | 14.36 | <0.01 | <0.05 | |||||
| PANSS b | ||||||||||||
| PANSS positive | 14.0 (14) | 16.0 (10) | 23.5 (10) | 21.0 (9) | 32.14 | <0.001 | <0.05 | <0.05 | <0.05 | <0.05 | ||
| PANSS negative | 14.0 (11) | 19.0 (13) | 19.5 (11) | 23.0 (9) | 57.27 | <0.001 | <0.05 | <0.05 | <0.05 | <0.05 | ||
| PANSS general | 29.5 (19) | 34.5 (17) | 45.5 (19) | 43.0 (14) | 58.89 | <0.001 | <0.05 | <0.05 | <0.05 | <0.05 | ||
| PANSS total | 57.5 (39) | 72.0 (28) | 86.5 (29) | 88.0 (24) | 65.12 | <0.001 | <0.05 | <0.05 | <0.05 | <0.05 | ||
| Young total b | 2.0 (14) | 2.0 (14) | 13.5 (19) | 7.0 (18) | 15.26 | <0.01 | <0.05 | <0.05 | ||||
| MADRS total b | 6.0 (10) | 13.0 (12) | 16.0 (17) | 16.0 (12) | 39.05 | <0.001 | <0.05 | <0.05 | <0.05 | |||
| PAS total b | 30.0 (24) | 43.0 (27) | 36.0 (31) | 57.0 (33) | 51.58 | <0.001 | <0.05 | <0.05 | <0.05 | |||
| TQ b | 1.0 (2) | 0.0 (1) | 0.0 (2) | 0.0 (2) | 2.69 | 0.44 | ||||||
| FES b | ||||||||||||
| Cohesion | 52.0 (13) | 52.0 (13) | 52.0 (15) | 47.0 (17) | 5.30 | 0.15 | ||||||
| Expressiveness | 53.0 (16) | 50.0 (16) | 53.0 (14) | 47.0 (16) | 4.14 | 0.25 | ||||||
| Conflict | 49.0 (9) | 49.0 (9) | 49.0 (9) | 49.0 (17) | 7.19 | 0.07 | ||||||
| Independence | 51.0 (11) | 51.0 (11) | 51.0 (14) | 51.0 (17) | 3.50 | 0.32 | ||||||
| Achievement-orientation | 47.0 (10) | 47.0 (10) | 47.0 (15) | 47.0 (16) | 1.35 | 0.72 | ||||||
| Intellectual-cultural orientation | 51.0 (23) | 47.0 (14) | 47.0 (14) | 42.0 (19) | 5.78 | 0.12 | ||||||
| Active-recreational orientation | 53.0 (14) | 48.0 (19) | 48.0 (21) | 44.0 (4) | 19.69 | <0.001 | <0.05 | |||||
| Moral-religious emphasis | 44.0 (10) | 49.0 (15) | 44.0 (10) | 44.0 (15) | 3.34 | 0.34 | ||||||
| Organization | 54.0 (10) | 51.5 (19) | 49.0 (20) | 49.0 (15) | 4.51 | 0.21 | ||||||
| Control | 45.0 (14) | 49.0 (14) | 49.0 (14) | 49.0 (14) | 4.06 | 0.25 | ||||||
| Cognitive measures | ||||||||||||
| n = 93 | n = 44 | n = 28 | n = 76 | |||||||||
| IQ b | 100 (20) | 92.5 (24) | 93.5 (19) | 90.0 (20) | 10.18 | 0.02 | <0.05 | |||||
| n = 91 | n = 43 | n = 25 | n = 67 | |||||||||
| Verbal Fluency b | 0.20 (1.2) | −0.08 (1.4) | 0.25 (1.1) | −0.50 (0.9) | 20.69 | <0.001 | <0.05 | |||||
| n = 85 | n = 36 | n = 23 | n = 53 | |||||||||
| Attention b | 0.18 (0.4) | 0.03 (0.7) | 0.10 (0.6) | −0.11 (0.5) | 13.54 | <0.01 | <0.05 | |||||
| n = 94 | n = 43 | n = 28 | n = 73 | |||||||||
| Working memory b | 0.14 (1.1) | −0.01 (1.3) | 0.15 (0.9) | −0.17 (1.2) | 14.10 | <0.01 | <0.05 | <0.05 | ||||
| n = 92 | n = 40 | n = 25 | n = 67 | |||||||||
| Verbal Learning and Memory b | 0.38 (1.2) | 0.20 (1.3) | 0.25 (0.9) | −0.43 (1.4) | 24.18 | <0.001 | <0.05 | <0.05 | ||||
| n = 93 | n = 41 | n = 30 | n = 70 | |||||||||
| Processing Speed b | 0.32 (0.8) | 0.14 (0.9) | −0.11 (1.4) | −0.16 (1.0) | 14.78 | <0.01 | <0.05 | |||||
| n = 92 | n = 40 | n = 28 | n = 58 | |||||||||
| Executive function b | 0.29 (0.7) | −0.03 (0.8) | 0.14 (0.8) | 0.00 (1.0) | 13.26 | <0.01 | <0.05 | |||||
| n = 89 | n = 43 | n = 26 | n = 66 | |||||||||
| Social cognition b | −0.33 (1.3) | −0.01 (1.4) | 0.04 (1.3) | −0.07 (1.4) | 3.47 | 0.32 | ||||||
| B | SE | Wald c | Sig. | Adjusted Exp(B) b | 95% Interval Confidence Exp(B) | ||
|---|---|---|---|---|---|---|---|
| Lower Limit | Upper Limit | ||||||
| Mild impairment-improving trajectory * | |||||||
| Intersection | 4.95 | 1.62 | 9.35 | <0.01 | |||
| Parental SES (medium-high) | 1.42 | 0.47 | 9.14 | <0.01 | 4.14 | 1.65 | 10.42 |
| PANSS positive (baseline score) | −0.05 | 0.03 | 2.94 | 0.09 | 0.95 | 0.89 | 1.01 |
| PANSS negative (baseline score) | −0.12 | 0.04 | 9.75 | <0.01 | 0.89 | 0.83 | 0.96 |
| MADRS total (baseline score) | −0.06 | 0.03 | 4.44 | 0.03 | 0.94 | 0.89 | 0.99 |
| PAS total (baseline score) | −0.04 | 0.01 | 10.91 | <0.01 | 0.96 | 0.94 | 0.98 |
| Verbal learning & memory | 0.39 | 0.28 | 1.89 | 0.17 | 1.47 | 0.85 | 2.55 |
| Moderate impairment-stable trajectory * | |||||||
| Intersection | 3.58 | 1.65 | 4.71 | 0.03 | |||
| Parental SES (medium-high) | 0.58 | 0.48 | 1.48 | 0.22 | 1.78 | 0.70 | 4.54 |
| PANSS positive (baseline score) | −0.07 | 0.03 | 4.94 | 0.03 | 0.93 | 0.87 | 0.99 |
| PANSS negative (baseline score) | −0.07 | 0.05 | 2.44 | 0.12 | 0.93 | 0.85 | 1.02 |
| MADRS total (baseline score) | 0.06 | 0.03 | 2.99 | 0.08 | 1.06 | 0.99 | 1.13 |
| PAS total (baseline score) | −0.02 | 0.01 | 2.83 | 0.09 | 0.98 | 0.96 | 1.00 |
| Verbal learning & memory | 0.29 | 0.28 | 1.08 | 0.30 | 1.33 | 0.77 | 2.30 |
| Severe impairment-improving trajectory * | |||||||
| Intersection | −1.44 | 2.12 | 0.46 | 0.50 | |||
| Parental SES (medium-high) | 0.81 | 0.61 | 1.76 | 0.18 | 2.25 | 0.68 | 7.46 |
| PANSS positive (baseline score) | 0.05 | 0.04 | 1.76 | 0.18 | 1.06 | 0.97 | 1.14 |
| PANSS negative (baseline score) | −0.07 | 0.05 | 2.44 | 0.12 | 0.93 | 0.85 | 1.02 |
| MADRS total (baseline score) | 0.06 | 0.03 | 2.99 | 0.08 | 1.06 | 0.99 | 1.13 |
| PAS total (baseline score) | −0.04 | 0.01 | 7.56 | <0.01 | 0.96 | 0.93 | 0.99 |
| Verbal learning & memory | 1.13 | 0.42 | 7.25 | <0.01 | 3.09 | 1.36 | 7.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salagre, E.; Grande, I.; Solé, B.; Mezquida, G.; Cuesta, M.J.; Díaz-Caneja, C.M.; Amoretti, S.; Lobo, A.; González-Pinto, A.; Moreno, C.; et al. Exploring Risk and Resilient Profiles for Functional Impairment and Baseline Predictors in a 2-Year Follow-Up First-Episode Psychosis Cohort Using Latent Class Growth Analysis. J. Clin. Med. 2021, 10, 73. https://doi.org/10.3390/jcm10010073
Salagre E, Grande I, Solé B, Mezquida G, Cuesta MJ, Díaz-Caneja CM, Amoretti S, Lobo A, González-Pinto A, Moreno C, et al. Exploring Risk and Resilient Profiles for Functional Impairment and Baseline Predictors in a 2-Year Follow-Up First-Episode Psychosis Cohort Using Latent Class Growth Analysis. Journal of Clinical Medicine. 2021; 10(1):73. https://doi.org/10.3390/jcm10010073
Chicago/Turabian StyleSalagre, Estela, Iria Grande, Brisa Solé, Gisela Mezquida, Manuel J. Cuesta, Covadonga M. Díaz-Caneja, Silvia Amoretti, Antonio Lobo, Ana González-Pinto, Carmen Moreno, and et al. 2021. "Exploring Risk and Resilient Profiles for Functional Impairment and Baseline Predictors in a 2-Year Follow-Up First-Episode Psychosis Cohort Using Latent Class Growth Analysis" Journal of Clinical Medicine 10, no. 1: 73. https://doi.org/10.3390/jcm10010073
APA StyleSalagre, E., Grande, I., Solé, B., Mezquida, G., Cuesta, M. J., Díaz-Caneja, C. M., Amoretti, S., Lobo, A., González-Pinto, A., Moreno, C., Pina-Camacho, L., Corripio, I., Baeza, I., Bergé, D., Verdolini, N., Carvalho, A. F., Vieta, E., Bernardo, M., & PEPs Group. (2021). Exploring Risk and Resilient Profiles for Functional Impairment and Baseline Predictors in a 2-Year Follow-Up First-Episode Psychosis Cohort Using Latent Class Growth Analysis. Journal of Clinical Medicine, 10(1), 73. https://doi.org/10.3390/jcm10010073







