Smoking Is Related to Reduced Motivation, But Not Global Cognition, in the First Two Years of Treatment for First Episode Psychosis
Abstract
1. Introduction
2. Methods
2.1. Data Acquisition
2.2. Participants
2.3. Measures
2.4. Statistical Analysis
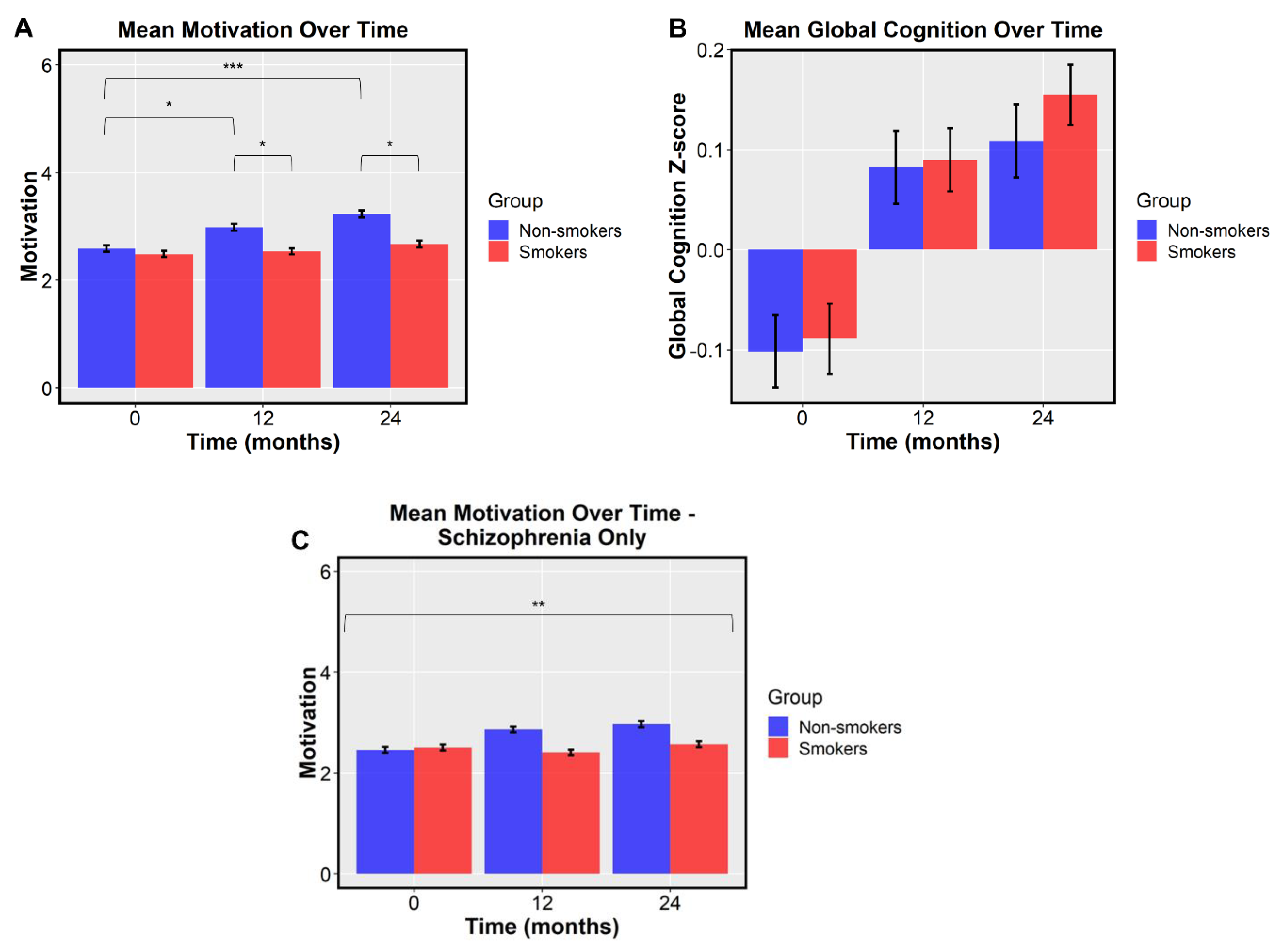
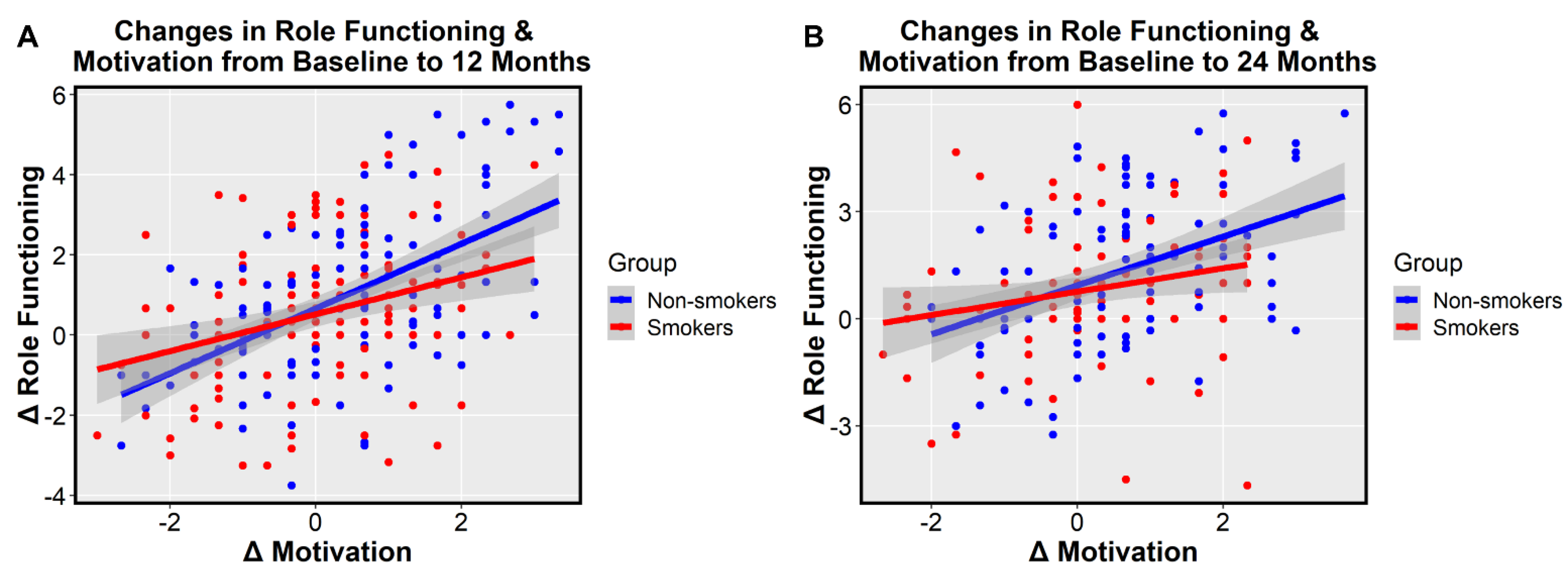
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vermeulen, J.; Schirmbeck, F.; Blankers, M.; van Tricht, M.; van den Brink, W.; de Haan, L.; Alizadeh, B.Z.; van Amelsvoort, T.; Bartels-Velthuis, A.A.; van Beveren, N.J.; et al. Smoking, symptoms, and quality of life in patients with psychosis, siblings, and healthy controls: A prospective, longitudinal cohort study. Lancet Psychiatry 2019, 6, 25–34. [Google Scholar] [CrossRef]
- Beratis, S.; Katrivanou, A.; Gourzis, P. Factors affecting smoking in schizophrenia. Compr. Psychiatry 2001, 42, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.; McCreadie, R.G. Smoking habits, current symptoms, and premorbid characteristics of schizophrenic patients in Nithsdale, Scotland. Am. J. Psychiatry 1999, 156, 1751–1757. [Google Scholar] [CrossRef] [PubMed]
- Caponnetto, P.; Polosa, R.; Robson, D.; Bauld, L. Tobacco smoking, related harm and motivation to quit smoking in people with schizophrenia spectrum disorders. Health Psychol. Res. 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Mackenbach, J.P.; Damhuis, R.A.M.; Been, J.V. The effects of smoking on health: Growth of knowledge reveals even grimmer picture. Ned. Tijdschr. Geneeskd. 2017, 160, D869. [Google Scholar] [PubMed]
- Colizzi, M.; Carra, E.; Fraietta, S.; Lally, J.; Quattrone, D.; Bonaccorso, S.; Mondelli, V.; Ajnakina, O.; Dazzan, P.; Trotta, A.; et al. Substance use, medication adherence and outcome one year following a first episode of psychosis. Schizophr. Res. 2016, 170, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Oluwoye, O.; Monroe-DeVita, M.; Burduli, E.; Chwastiak, L.; McPherson, S.; McClellan, J.M.; McDonell, M.G. Impact of tobacco, alcohol and cannabis use on treatment outcomes among patients experiencing first episode psychosis: Data from the national RAISE-ETP study. Early Interv. Psychiatry 2019, 13, 142–146. [Google Scholar] [CrossRef]
- Levin, E.D.; Wilson, W.; Rose, J.E.; McEvoy, J. Nicotine-haloperidol interactions and cognitive performance in schizophrenics. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 1996, 15, 429–436. [Google Scholar] [CrossRef]
- Šagud, M.; Mihaljević-Peleš, A.; Mück-Šeler, D.; Pivac, N.; Vuksan-Ćusa, B.; Brataljenović, T.; Jakovljević, M. Smoking and schizophrenia. Psychiatr. Danub. 2009, 21, 371–375. [Google Scholar]
- McCutcheon, R.A.; Abi-Dargham, A.; Howes, O.D. Schizophrenia, dopamine and the striatum: From biology to symptoms. Trends Neurosci. 2019, 42, 205–220. [Google Scholar] [CrossRef]
- Brewer, W.J.; Wood, S.J.; Phillips, L.J.; Francey, S.M.; Pantelis, C.; Yung, A.R.; Cornblatt, B.; McGorry, P.D. Generalized and specific cognitive performance in clinical high-risk cohorts: A review highlighting potential vulnerability markers for psychosis. Schizophr. Bull. 2006, 32, 538–555. [Google Scholar] [CrossRef]
- Carrión, R.E.; Goldberg, T.E.; McLaughlin, D.; Auther, A.M.; Correll, C.U.; Cornblatt, B.A. Impact of neurocognition on social and role functioning in individuals at clinical high risk for psychosis. Am. J. Psychiatry 2011, 168, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Haining, K.; Matrunola, C.; Mitchell, L.; Gajwani, R.; Gross, J.; Gumley, A.I.; Lawrie, S.M.; Schwannauer, M.; Schultze-Lutter, F.; Uhlhaas, P.J. Neuropsychological deficits in participants at clinical high risk for psychosis recruited from the community: Relationships to functioning and clinical symptoms. Psychol. Med. 2020, 50, 77–85. [Google Scholar] [CrossRef]
- Schlosser, D.A.; Fisher, M.; Gard, D.; Fulford, D.; Loewy, R.L.; Vinogradov, S. Motivational deficits in individuals at-risk for psychosis and across the course of schizophrenia. Schizophr. Res. 2014, 158, 52–57. [Google Scholar] [CrossRef]
- Najas-Garcia, A.; Gómez-Benito, J.; Huedo-Medina, T.B. The relationship of motivation and neurocognition with functionality in schizophrenia: A meta-analytic review. Community Ment. Health J. 2018, 54, 1019–1049. [Google Scholar] [CrossRef] [PubMed]
- Barr, R.S.; Culhane, M.A.; Jubelt, L.E.; Mufti, R.S.; Dyer, M.A.; Weiss, A.P.; Deckersbach, T.; Kelly, J.F.; Freudenreich, O.; Goff, D.C.; et al. The effects of transdermal nicotine on cognition in nonsmokers with schizophrenia and nonpsychiatric controls. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2008, 33, 480–490. [Google Scholar] [CrossRef]
- Dépatie, L.; O’Driscoll, G.A.; Holahan, A.-L.V.; Atkinson, V.; Thavundayil, J.X.; Kin, N.N.Y.; Lal, S. Nicotine and behavioral markers of risk for schizophrenia: A double-blind, placebo-controlled, cross-over study. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2002, 27, 1056–1070. [Google Scholar] [CrossRef]
- Harris, J.G.; Kongs, S.; Allensworth, D.; Martin, L.; Tregellas, J.; Sullivan, B.; Zerbe, G.; Freedman, R. Effects of nicotine on cognitive deficits in schizophrenia. Neuropsychopharmacology 2004, 29, 1378–1385. [Google Scholar] [CrossRef]
- Jacobsen, L.K.; D’Souza, D.C.; Mencl, W.E.; Pugh, K.R.; Skudlarski, P.; Krystal, J.H. Nicotine effects on brain function and functional connectivity in schizophrenia. Biol. Psychiatry 2004, 55, 850–858. [Google Scholar] [CrossRef]
- Hickling, L.M.; Perez-Iglesias, R.; Ortiz-García de la Foz, V.; Balanzá-Martínez, V.; McGuire, P.; Crespo-Facorro, B.; Ayesa-Arriola, R. Tobacco smoking and its association with cognition in first episode psychosis patients. Schizophr. Res. 2018, 192, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Gutiérrez, T.; García-Portilla, M.P.; Parellada, M.; Bobes, J.; Calvo, A.; Moreno-Izco, L.; González-Pinto, A.; Lobo, A.; de la Serna, E.; Cabrera, B.; et al. Smoking does not impact social and non-social cognition in patients with first episode psychosis. Schizophr. Res. 2018, 199, 64–74. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Chen, D.C.; Xiu, M.H.; Haile, C.N.; He, S.C.; Luo, X.; Zuo, L.; Rosenheck, R.; Kosten, T.A.; Kosten, T.R. Cigarette smoking, psychopathology and cognitive function in first-episode drug-naive patients with schizophrenia: A case-control study. Psychol. Med. 2013, 43, 1651–1660. [Google Scholar] [CrossRef] [PubMed]
- Zabala, A.; Eguiluz, J.I.; Segarra, R.; Enjuto, S.; Ezcurra, J.; Pinto, A.G.; Gutiérrez, M. Cognitive performance and cigarette smoking in first-episode psychosis. Eur. Arch. Psychiatry Clin. Neurosci. 2009, 259, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Segarra, R.; Zabala, A.; Eguíluz, J.I.; Ojeda, N.; Elizagarate, E.; Sánchez, P.; Ballesteros, J.; Gutiérrez, M. Cognitive performance and smoking in first-episode psychosis: The self-medication hypothesis. Eur. Arch. Psychiatry Clin. Neurosci. 2011, 261, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Grossman, M.; Bowie, C.R.; Lepage, M.; Malla, A.K.; Joober, R.; Iyer, S.N. Smoking status and its relationship to demographic and clinical characteristics in first episode psychosis. J. Psychiatr. Res. 2017, 85, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Kesby, J.; Eyles, D.; McGrath, J.; Scott, J. Dopamine, psychosis and schizophrenia: The widening gap between basic and clinical neuroscience. Transl. Psychiatry 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Smith, R.C.; Singh, A.; Infante, M.; Khandat, A.; Kloos, A. Effects of cigarette smoking and nicotine nasal spray on psychiatric symptoms and cognition in schizophrenia. Neuropsychopharmacology 2002, 27, 479–497. [Google Scholar] [CrossRef]
- Misiak, B.; Kiejna, A.; Frydecka, D. Assessment of cigarette smoking status with respect to symptomatic manifestation in first-episode schizophrenia patients. Compr. Psychiatry 2015, 58, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Kane, J.M.; Robinson, D.G.; Schooler, N.R.; Mueser, K.T.; Penn, D.L.; Rosenheck, R.A.; Addington, J.; Brunette, M.F.; Correll, C.U.; Estroff, S.E.; et al. Comprehensive versus usual community care for first episode psychosis: Two-year outcomes from the NIMH RAISE early treatment program. Am. J. Psychiatry 2016, 173, 362–372. [Google Scholar] [CrossRef]
- Heatherton, T.F.; Kozlowski, L.T.; Frecker, R.C.; Fagerström, K.O. The Fagerström test for nicotine dependence: A revision of the Fagerström tolerance questionnaire. Br. J. Addict. 1991, 86, 1119–1127. [Google Scholar] [CrossRef]
- Keefe, R.S.E.; Goldberg, T.E.; Harvey, P.D.; Gold, J.M.; Poe, M.P.; Coughenour, L. The brief assessment of cognition in schizophrenia: Reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr. Res. 2004, 68, 283–297. [Google Scholar] [CrossRef]
- Heinrichs, D.W.; Hanlon, T.E.; Carpenter, W.T. The quality of life scale: An instrument for rating the schizophrenic deficit syndrome. Schizophr. Bull. 1984, 10, 388–398. [Google Scholar] [CrossRef]
- Foussias, G.; Siddiqui, I.; Fervaha, G.; Mann, S.; McDonald, K.; Agid, O.; Zakzanis, K.K.; Remington, G. Motivated to do well: An examination of the relationships between motivation, effort, and cognitive performance in schizophrenia. Schizophr. Res. 2015, 166, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Fulford, D.; Piskulic, D.; Addington, J.; Kane, J.M.; Schooler, N.R.; Mueser, K.T. Prospective relationships between motivation and functioning in recovery after a first episode of schizophrenia. Schizophr. Bull. 2018, 44, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Nakagami, E.; Hoe, M.; Brekke, J.S. The prospective relationships among intrinsic motivation, neurocognition, and psychosocial functioning in schizophrenia. Schizophr. Bull. 2010, 36, 935–948. [Google Scholar] [CrossRef] [PubMed]
- Kay, S.R.; Fiszbein, A.; Opler, L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987, 13, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. nlme: Linear and Nonlinear Mixed Effects Models. R Package Version 3.1-147. 2020. Available online: https://CRAN.R-project.org/package=nlme (accessed on 29 October 2020).
- Fervaha, G.; Foussias, G.; Agid, O.; Remington, G. Motivational deficits in early schizophrenia: Prevalent, persistent, and key determinants of functional outcome. Schizophr. Res. 2015, 166, 9–16. [Google Scholar] [CrossRef]
- West, R.; McEwen, A.; Bolling, K.; Owen, L. Smoking cessation and smoking patterns in the general population: A 1-year follow-up. Addiction 2001, 96, 891–902. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Nonnemaker, J.; Sherrill, B.; Gilsenan, A.W.; Coste, F.; West, R. Attempts to quit smoking and relapse: Factors associated with success or failure from the ATTEMPT cohort study. Addict. Behav. 2009, 34, 365–373. [Google Scholar] [CrossRef]
- Boardman, T.; Catley, D.; Mayo, M.S.; Ahluwalia, J.S. Self-efficacy and motivation to quit during participation in a smoking cessation program. Int. J. Behav. Med. 2005, 12, 266–272. [Google Scholar] [CrossRef]
- Heppner, W.L.; Ji, L.; Reitzel, L.R.; Castro, Y.; Correa-Fernandez, V.; Vidrine, J.I.; Li, Y.; Dolan-Mullen, P.; Velasquez, M.M.; Cinciripini, P.M.; et al. The role of prepartum motivation in the maintenance of postpartum smoking abstinence. Health Psychol. Off. J. Div. Health Psychol. Am. Psychol. Assoc. 2011, 30, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, M.L.; Williams, J.M.; Stahl, N.F.; Budsock, P.D.; Cooperman, N.A. An adaptation of motivational interviewing increases quit attempts in smokers with serious mental illness. Nicotine Tob. Res. 2016, 18, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Tantirangsee, N.; Assanangkornchai, S.; Marsden, J. Effects of a brief intervention for substance use on tobacco smoking and family relationship functioning in schizophrenia and related psychoses: A Randomised Controlled Trial. J. Subst. Abus. Treat. 2015, 51, 30–37. [Google Scholar] [CrossRef]
- Schlosser, D.A.; Campellone, T.R.; Truong, B.; Etter, K.; Vergani, S.; Komaiko, K.; Vinogradov, S. Efficacy of PRIME, a Mobile App Intervention Designed to Improve Motivation in Young People With Schizophrenia. Schizophr. Bull. 2018, 44, 1010–1020. [Google Scholar] [CrossRef] [PubMed]
- Fulford, D.; Meyer-Kalos, P.S.; Mueser, K.T. Focusing on recovery goals improves motivation in first-episode psychosis. Soc. Psychiatry Psychiatr. Epidemiol. 2019, 55, 1629–1637. [Google Scholar] [CrossRef]
- Gard, D.E.; Fisher, M.; Garrett, C.; Genevsky, A.; Vinogradov, S. Motivation and its relationship to neurocognition, social cognition, and functional outcome in schizophrenia. Schizophr. Res. 2009, 115, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Nakagami, E.; Xie, B.; Hoe, M.; Brekke, J.S. Intrinsic motivation, neurocognition and psychosocial functioning in schizophrenia: Testing mediator and moderator effects. Schizophr. Res. 2008, 105, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Exley, R.; Cragg, S.J. Presynaptic nicotinic receptors: A dynamic and diverse cholinergic filter of striatal dopamine neurotransmission. Br. J. Pharmacol. 2008, 153, S283–S297. [Google Scholar] [CrossRef]
- Snyder, F.R.; Davis, F.C.; Henningfield, J.E. The tobacco withdrawal syndrome: Performance decrements assessed on a computerized test battery. Drug Alcohol Depend. 1989, 23, 259–266. [Google Scholar] [CrossRef]
- Lally, J.; Spaducci, G.; Gardner-Sood, P.; Atakan, Z.; Greenwood, K.; Di Forti, M.; Ismail, K.; Murphy, K.C.; Smith, S.; McNeill, A.; et al. Tobacco smoking and nicotine dependence in first episode and established psychosis. Asian J. Psychiatry 2019, 43, 125–131. [Google Scholar] [CrossRef]
- Mørch-Johnsen, L.; Nesvåg, R.; Faerden, A.; Haukvik, U.K.; Jørgensen, K.N.; Lange, E.H.; Andreassen, O.A.; Melle, I.; Agartz, I. Brain structure abnormalities in first-episode psychosis patients with persistent apathy. Schizophr. Res. 2015, 164, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Veselinović, T.; Scharpenberg, M.; Heinze, M.; Cordes, J.; Mühlbauer, B.; Juckel, G.; Habel, U.; Rüther, E.; Timm, J.; Gründer, G.; et al. Disparate effects of first and second generation antipsychotics on cognition in schizophrenia—Findings from the randomized NeSSy trial. Eur. Neuropsychopharmacol. 2019, 29, 720–739. [Google Scholar] [CrossRef] [PubMed]
| Baseline Characteristic | Smokers (N = 207) | Non-Smokers (N = 196) | t or X2 | Degrees of Freedom (df) | p |
|---|---|---|---|---|---|
| Age | 23.64 ± 5.15 | 22.6 ± 4.94 | −1.87 | 397 | 0.063 |
| Gender | 9.63 | 1 | 0.002 | ||
| Female | 43 (10.67%) | 68 (16.87%) | |||
| Male | 164 (40.69%) | 128 (31.76%) | |||
| Race | 4.25 | 4 | 0.374 | ||
| American Indian | 8 (1.99%) | 12 (2.98%) | |||
| Asian | 4 (0.99%) | 8 (1.99%) | |||
| Black | 77 (19.11%) | 75 (18.61%) | |||
| Pacific Islander | 0 (0%) | 1 (0.25%) | |||
| White | 118 (29.28%) | 100 (24.81%) | |||
| Ethnicity | 4.77 | 1 | 0.029 | ||
| Hispanic/Latino | 28 (6.97%) | 44 (10.95%) | |||
| Non-Hispanic/-Latino | 178 (44.17%) | 152 (37.72%) | |||
| Education | 14.983 | 8 | 0.059 | ||
| Advanced degree | 0 (0%) | 0 (0%) | |||
| Post-grad training, no degree | 1 (0.25%) | 4 (1.00%) | |||
| Completed 4-year degree | 4 (1.00%) | 11 (2.74%) | |||
| Some college, no 4-year degree | 47 (11.72%) | 58 (14.46%) | |||
| High school diploma | 72 (17.96%) | 60 (14.96%) | |||
| Attended high school, no diploma | 67 (16.71%) | 57 (14.21%) | |||
| Completed grade 8, no high school | 11 (2.74%) | 3 (0.75%) | |||
| Attended grade school, not through 8 | 4 (1.00%) | 2 (0.50%) | |||
| No schooling | 0 (0%) | 0 (0%) | |||
| Diagnosis | 13.496 | 6 | 0.036 | ||
| Schizophrenia | 119 (29.53%) | 94 (23.33%) | |||
| Schizoaffective Bipolar | 12 (2.98%) | 12 (2.98%) | |||
| Schizoaffective Depressive | 31 (7.69%) | 26 (6.45%) | |||
| Schizophreniform Provisional | 17 (4.22%) | 40 (9.93%) | |||
| Schizophreniform Definite | 4 (0.99%) | 6 (1.49%) | |||
| Brief Psychotic Disorder | 1 (0.25%) | 1 (0.25%) | |||
| Psychotic Disorder Not Otherwise Specified | 23 (5.71%) | 17 (4.22%) | |||
| Treatment Group | 0.57 | 1 | 0.452 | ||
| NAVIGATE | 110 (27.30%) | 112 (27.79%) | |||
| Community Care | 97 (24.07%) | 84 (20.84%) | |||
| Duration of Untreated Psychosis | 239.97 ± 297.59 | 144.62 ± 209.63 | −3.74 | 367 | 0.0002 |
| Quality of Life Scale Total | 51.93 ± 18.39 | 53.45 ± 19.18 | 0.84 | 396 | 0.4 |
| Positive and Negative Syndrome Scale (PANSS) Total | 77.5 ± 15.28 | 75.65 ± 19.19 | −1.26 | 399 | 0.209 |
| PANSS Positive | 19.39 ± 5.29 | 18.11 ± 5.1 | −2.52 | 399 | 0.012 |
| PANSS Negative | 20.03 ± 5.61 | 20.32 ± 4.98 | 0.55 | 397 | 0.582 |
| PANSS General | 38.08 ± 7.97 | 37.22 ± 8.13 | −1.08 | 397 | 0.279 |
| Brief Assessment of Cognition in Schizophrenia Composite Z-Score | −0.09 ± 0.71 | −0.10 ± 0.73 | −0.1 | 391 | 0.859 |
| Smokers (N = 207) | Non-Smokers (N = 196) | Baseline to 12-Month Effect Size (N = 255/250) | Baseline to 24-Month Effect Size (N = 203/173) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | Baseline Mean (SD) | 12-Month Mean (SD) | 24-Month Mean (SD) | Baseline Mean (SD) | 12-Month Mean (SD) | 24-Month Mean (SD) | F | p | d | Lower | Upper | d | Lower | Upper |
| Motivation | 2.49 (1.20) | 2.54 (1.13) | 2.67 (1.20) | 2.59 (1.16) | 2.98 (1.29) | 3.23 (1.29) | 5.21 | 0.023 | −0.2 | −0.45 | 0.05 | −0.27 | −0.55 | 0.01 |
| Global Cognition | −0.09 (0.71) | 0.09 (0.64) | 0.15 (0.60) | −0.10 (0.73) | 0.08 (0.73) | 0.11 (0.73) | 0.44 | 0.507 | 0.08 | −0.17 | 0.33 | 0.08 | −0.23 | 0.38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schermitzler, B.; Miley, K.; Vinogradov, S.; Ramsay, I.S. Smoking Is Related to Reduced Motivation, But Not Global Cognition, in the First Two Years of Treatment for First Episode Psychosis. J. Clin. Med. 2021, 10, 1619. https://doi.org/10.3390/jcm10081619
Schermitzler B, Miley K, Vinogradov S, Ramsay IS. Smoking Is Related to Reduced Motivation, But Not Global Cognition, in the First Two Years of Treatment for First Episode Psychosis. Journal of Clinical Medicine. 2021; 10(8):1619. https://doi.org/10.3390/jcm10081619
Chicago/Turabian StyleSchermitzler, Brandon, Kathleen Miley, Sophia Vinogradov, and Ian S. Ramsay. 2021. "Smoking Is Related to Reduced Motivation, But Not Global Cognition, in the First Two Years of Treatment for First Episode Psychosis" Journal of Clinical Medicine 10, no. 8: 1619. https://doi.org/10.3390/jcm10081619
APA StyleSchermitzler, B., Miley, K., Vinogradov, S., & Ramsay, I. S. (2021). Smoking Is Related to Reduced Motivation, But Not Global Cognition, in the First Two Years of Treatment for First Episode Psychosis. Journal of Clinical Medicine, 10(8), 1619. https://doi.org/10.3390/jcm10081619






