Repeatability of Spectral Domain Optical Coherence Tomography Measurements of Bruch’s Membrane Opening-Minimum Rim Width in Epiretinal Membrane Patients with Peripapillary Involvement
Abstract
:1. Introduction
2. Materials and Methods
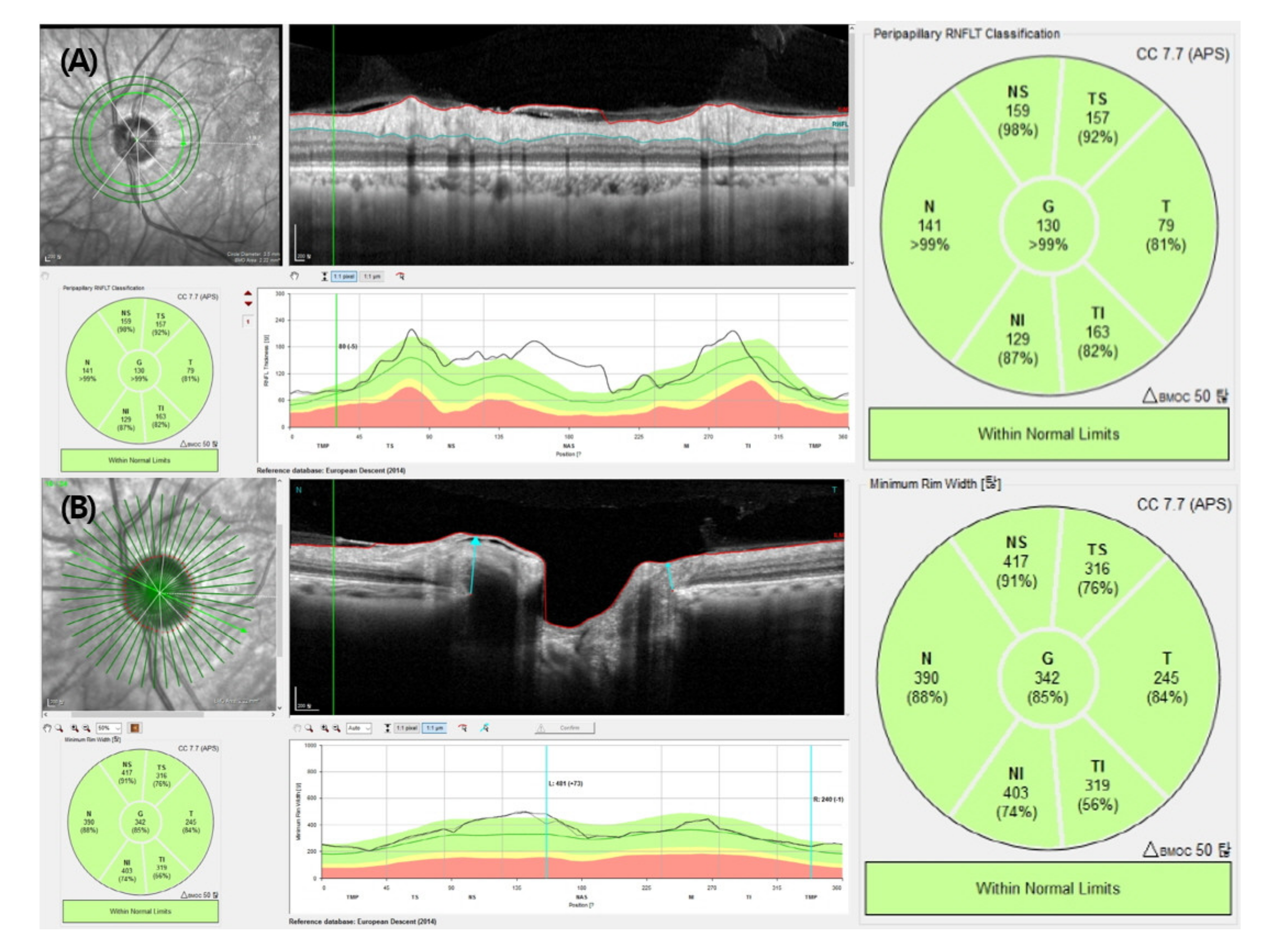
2.1. Optical Coherence Tomography Measurements
2.2. Statistics
3. Results
3.1. Baseline Characteristics
3.2. Average and Sectoral BMO-MRW and RNFL Thickness
3.3. Repeatability of Two Consecutive BMO-MRW and RNFL Thickness Measurements
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reis, A.S.; Sharpe, G.P.; Yang, H.; Nicolela, M.T.; Burgoyne, C.F.; Chauhan, B.C. Optic Disc Margin Anatomy in Patients with Glaucoma and Normal Controls with Spectral Domain Optical Coherence Tomography. Ophthalmology 2012, 119, 738–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chauhan, B.C.; O’Leary, N.; AlMobarak, F.A.; Reis, A.S.; Yang, H.; Sharpe, G.P.; Hutchison, D.M.; Nicolela, M.T.; Burgoyne, C.F. Enhanced detection of open-angle glaucoma with an anatomically accurate optical coherence tomography-derived neuroretinal rim parameter. Ophthalmology 2013, 120, 535–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zako, M.; Gosho, M.; Mizumoto, K. Correlation between optic nerve head structural parameters and glaucomatous visual field indices. Clin. Ophthalmol. 2014, 8, 1203–1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pollet-Villard, F.; Chiquet, C.; Romanet, J.-P.; Noel, C.; Aptel, F. Structure-Function Relationships with Spectral-Domain Optical Coherence Tomography Retinal Nerve Fiber Layer and Optic Nerve Head Measurements. Investig. Opthalmology Vis. Sci. 2014, 55, 2953–2962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gardiner, S.K.; Ren, R.; Yang, H.; Fortune, B.; Burgoyne, C.F.; Demirel, S. A Method to Estimate the Amount of Neuroretinal Rim Tissue in Glaucoma: Comparison with Current Methods for Measuring Rim Area. Am. J. Ophthalmol. 2014, 157, 540–549.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danthurebandara, V.M.; Sharpe, G.P.; Hutchison, D.M.; Denniss, J.; Nicolela, M.T.; McKendrick, A.M.; Turpin, A.; Chauhan, B.C. Enhanced Structure-Function Relationship in Glaucoma With an Anatomically and Geometrically Accurate Neuroretinal Rim Measurement. Investig. Opthalmology Vis. Sci. 2014, 56, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.; Smith, W.; Attebo, K.; Healey, P.R. Prevalence of Open-angle Glaucoma in Australia. Opthalmology 1996, 103, 1661–1669. [Google Scholar] [CrossRef]
- Iwase, A.; Suzuki, Y.; Araie, M.; Yamamoto, T.; Abe, H.; Shirato, S.; Kuwayama, Y.; Mishima, H.K.; Shimizu, H.; Tomita, G. The prevalence of primary open-angle glaucoma in Japanese*1The Tajimi Study. Ophthalmology 2004, 111, 1641–1648. [Google Scholar] [CrossRef]
- Dielemans, I.; Vingerling, J.R.; Wolfs, R.C.; Hofman, A.; Grobbee, D.E.; de Jong, P.T. The prevalence of primary open-angle glaucoma in a population-based study in The Netherlands. The Rotterdam Study. Ophthalmology 1994, 101, 1851–1855. [Google Scholar] [CrossRef]
- Mitchell, P.; Smith, W.; Chey, T.; Wang, J.J.; Chang, A. Prevalence and Associations of Epiretinal Membranes. Ophthalmology 1997, 104, 1033–1040. [Google Scholar] [CrossRef]
- McCarty, D.J.; Mukesh, B.N.; Chikani, V.; Wang, J.J.; Mitchell, P.; Taylor, H.R.; McCarty, C.A. Prevalence and associations of epi-retinal membranes in the visual impairment project. Am. J. Ophthalmol. 2005, 140, 288–294. [Google Scholar] [CrossRef] [PubMed]
- akimoto, S.; Okazaki, T.; Usui, S.; Ishibashi, T.; Oura, Y.; Nishida, K.; Miki, A.; Kawasaki, R.; Matsushita, K.; Sakaguchi, H.; et al. Cross-Sectional Imaging Analysis of Epiretinal Membrane Involvement in Unilat-eral Open-Angle Glaucoma Severity. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5745–5751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enders, P.; Bremen, A.; Schaub, F.; Hermann, M.M.; Diestelhorst, M.; Dietlein, T.; Cursiefen, C.; Heindl, L.M. Intraday Repeatability of Bruch’s Membrane Opening-Based Neuroretinal Rim Measurements. Investig. Opthalmology Vis. Sci. 2017, 58, 5195–5200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, K.; Kim, J.; Lee, J. Reproducibility of Bruch Membrane Opening-Minimum Rim Width Measurements With Spectral Domain Optical Coherence Tomography. J. Glaucoma 2017, 26, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G*Power 3.1: Tests for correlation and regres-sion analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartlett, J.W.; Frost, C. Reliability, repeatability and reproducibility: Analysis of measurement errors in continuous varia-bles. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2008, 31, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Altman, D.G. Statistics Notes: Measurement error proportional to the mean. BMJ 1996, 313, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.J.; Kim, M.S.; Jo, Y.J.; Kim, J.Y. Thickness of the Macula, Retinal Nerve Fiber Layer, and Ganglion Cell Layer in the Epi-retinal Membrane: The Repeatability Study of Optical Coherence Tomography. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4554–4559. [Google Scholar] [CrossRef] [PubMed]
- Portney, L.G.; Watkins, M.P. Foundations of Clinical Research: Applications to Practice; Pearson/Prentice Hall: Upper Saddle River, NJ, USA, 2009. [Google Scholar]
| Diseased Eyes (n = 52) | Normal Eyes (n = 62) | p-Value | |
|---|---|---|---|
| Mean age (years) | 59.2 (±13.5) | 58.6 (±10.0) | 0.843 † |
| Sex | |||
| Male (n,%) | 30 (57.7%) | 41 (66.1%) | 0.355 ‡ |
| Female (n,%) | 22 (42.3%) | 21 (33.9%) | |
| Laterality of eye | |||
| Right (n,%) | 33 (63.5%) | 23 (37.1%) | 0.005 ‡ |
| Left (n,%) | 19 (36.5%) | 39 (62.9%) | |
| Initial mean BCVA (logMAR) | 0.15 (±0.4) | 0.28 (±0.32) | 0.111 † |
| Mean spherical equivalent | −0.66 (±2.09) | −0.41 (±1.66) | 0.647 † |
| Mean CMT | 333.4 (±88.7) μm | 275.6 (±45.2) | <0.001 † |
| BMO-MRW | RNFL Thickness | ||||||
|---|---|---|---|---|---|---|---|
| Sector | Measurement 1 | Measurement 2 | p-Value † | Measurement 1 | Measurement 2 | p-Value † | |
| Diseased Eyes (n = 52) | Average | 321.6 (±107.0) | 320.8 (±104.2) | 0.449 | 124.3 (±42.5) | 124.8 (±43.1) | 0.729 |
| TS | 327.3 (±105.8) | 324.8 (±102.5) | 0.206 | 165.3 (±58.3) | 164.4 (±56.9) | 0.830 | |
| T | 247.0 (±97.1) | 245.6 (±96.0) | 0.541 | 106.4 (±45.5) | 109.9 (±49.8) | 0.272 | |
| TI | 355.9 (±104.1) | 351.5 (±95.8) | 0.174 | 177.2 (±63.3) | 176.6 (±63.8) | 0.604 | |
| NI | 390.9 (±118.2) | 392.0 (±119.1) | 0.506 | 127.1 (±52.7) | 129.6 (±54.5) | 0.142 | |
| N | 329.1 (±126.2) | 329.3 (±125.4) | 0.814 | 96.4 (±42.2) | 95.8 (±41.3) | 0.469 | |
| NS | 360.0 (±124.2) | 359.6 (±118.4) | 0.888 | 144.6 (±69.7) | 145.8 (±61.1) | 0.732 | |
| Normal Eyes (n = 62) | Average | 265.9 (±49.4) | 266.0 (±49.6) | 0.770 | 101.3 (±13.6) | 101.4 (±13.5) | 0.240 |
| TS | 266.0 (±43.9) | 266.9 (±42.0) | 0.482 | 139.2 (±22.8) | 139.4 (±22.7) | 0.409 | |
| T | 189.9 (±43.7) | 189.6 (±44.5) | 0.674 | 76.4 (±10.4) | 76.5 (±10.2) | 0.201 | |
| TI | 299.3 (±69.1) | 298.9 (±70.4) | 0.810 | 153.9 (±29.6) | 154.2 (±29.5) | 0.101 | |
| NI | 320.0 (±73.7) | 323.4 (±71.9) | 0.052 | 111.1 (±29.6) | 111.5 (±29.8) | 0.057 | |
| N | 280.7 (±60.4) | 280.3 (±60.5) | 0.515 | 79.6 (±17.6) | 79.6 (±17.7) | 0.907 | |
| NS | 308.6 (±56.2) | 307.6 (±56.0) | 0.318 | 117.4 (±25.5) | 117.3 (±25.8) | 0.701 | |
| Diseased Eyes | Normal Eyes | |||||||
|---|---|---|---|---|---|---|---|---|
| BMO-MRW | RNFL Thickness | BMO-MRW | RNFL Thickness | |||||
| Sector | ICC | 95% CI | ICC | 95% CI | ICC | 95% CI | ICC | 95% CI |
| Average | 0.999 | 0.997–0.999 | 0.985 | 0.975–0.992 | 0.999 | 0.998–0.999 | 0.999 | 0.999–1.000 |
| TS | 0.995 | 0.992–0.997 | 0.917 | 0.855–0.952 | 0.988 | 0.980–0.993 | 0.999 | 0.998–0.999 |
| T | 0.993 | 0.987–0.996 | 0.939 | 0.894–0.965 | 0.994 | 0.991–0.997 | 0.998 | 0.997–0.999 |
| TI | 0.986 | 0.976–0.992 | 0.995 | 0.992–0.997 | 0.993 | 0.989–0.996 | 0.999 | 0.999–1.000 |
| NI | 0.998 | 0.996–0.999 | 0.986 | 0.976–0.992 | 0.991 | 0.985–0.995 | 0.999 | 0.999–1.000 |
| N | 0.999 | 0.999–1.000 | 0.995 | 0.991–0.997 | 0.998 | 0.997–0.999 | 0.999 | 0.998–0.999 |
| NS | 0.991 | 0.984–0.995 | 0.968 | 0.945–0.982 | 0.995 | 0.992–0.997 | 0.998 | 0.997–0.999 |
| Diseased Eyes | Normal Eyes | |||||||
|---|---|---|---|---|---|---|---|---|
| BMO-MRW | RNFL Thickness | BMO-MRW | RNFL Thickness | |||||
| Sector | RC (μm) | CV (%) | RC (μm) | CV (%) | RC (μm) | CV (%) | RC (μm) | CV (%) |
| Average | 9.00 | 0.91 | 6.25 | 1.45 | 4.61 | 0.63 | 0.92 | 0.33 |
| TS | 15.41 | 1.63 | 15.67 | 2.26 | 12.04 | 1.58 | 2.21 | 0.60 |
| T | 14.38 | 1.79 | 12.39 | 3.18 | 7.83 | 1.49 | 1.23 | 0.59 |
| TI | 22.45 | 2.01 | 5.01 | 1.11 | 11.88 | 1.51 | 1.83 | 0.46 |
| NI | 12.47 | 1.12 | 7.46 | 2.28 | 15.57 | 2.09 | 2.21 | 0.76 |
| N | 8.43 | 0.94 | 5.24 | 1.67 | 7.11 | 0.95 | 1.48 | 0.72 |
| NS | 22.90 | 2.41 | 11.52 | 2.78 | 10.01 | 1.19 | 2.75 | 0.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nam, K.Y.; Kim, B.J.; Lee, W.H.; Han, Y.S. Repeatability of Spectral Domain Optical Coherence Tomography Measurements of Bruch’s Membrane Opening-Minimum Rim Width in Epiretinal Membrane Patients with Peripapillary Involvement. J. Clin. Med. 2021, 10, 2240. https://doi.org/10.3390/jcm10112240
Nam KY, Kim BJ, Lee WH, Han YS. Repeatability of Spectral Domain Optical Coherence Tomography Measurements of Bruch’s Membrane Opening-Minimum Rim Width in Epiretinal Membrane Patients with Peripapillary Involvement. Journal of Clinical Medicine. 2021; 10(11):2240. https://doi.org/10.3390/jcm10112240
Chicago/Turabian StyleNam, Ki Yup, Bum Jun Kim, Woo Hyuk Lee, and Yong Seop Han. 2021. "Repeatability of Spectral Domain Optical Coherence Tomography Measurements of Bruch’s Membrane Opening-Minimum Rim Width in Epiretinal Membrane Patients with Peripapillary Involvement" Journal of Clinical Medicine 10, no. 11: 2240. https://doi.org/10.3390/jcm10112240
APA StyleNam, K. Y., Kim, B. J., Lee, W. H., & Han, Y. S. (2021). Repeatability of Spectral Domain Optical Coherence Tomography Measurements of Bruch’s Membrane Opening-Minimum Rim Width in Epiretinal Membrane Patients with Peripapillary Involvement. Journal of Clinical Medicine, 10(11), 2240. https://doi.org/10.3390/jcm10112240






