Evaluation of the Feasibility and Effectiveness of Placement of Fully Covered Self-Expandable Metallic Stents via Various Insertion Routes for Benign Biliary Strictures
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Methods
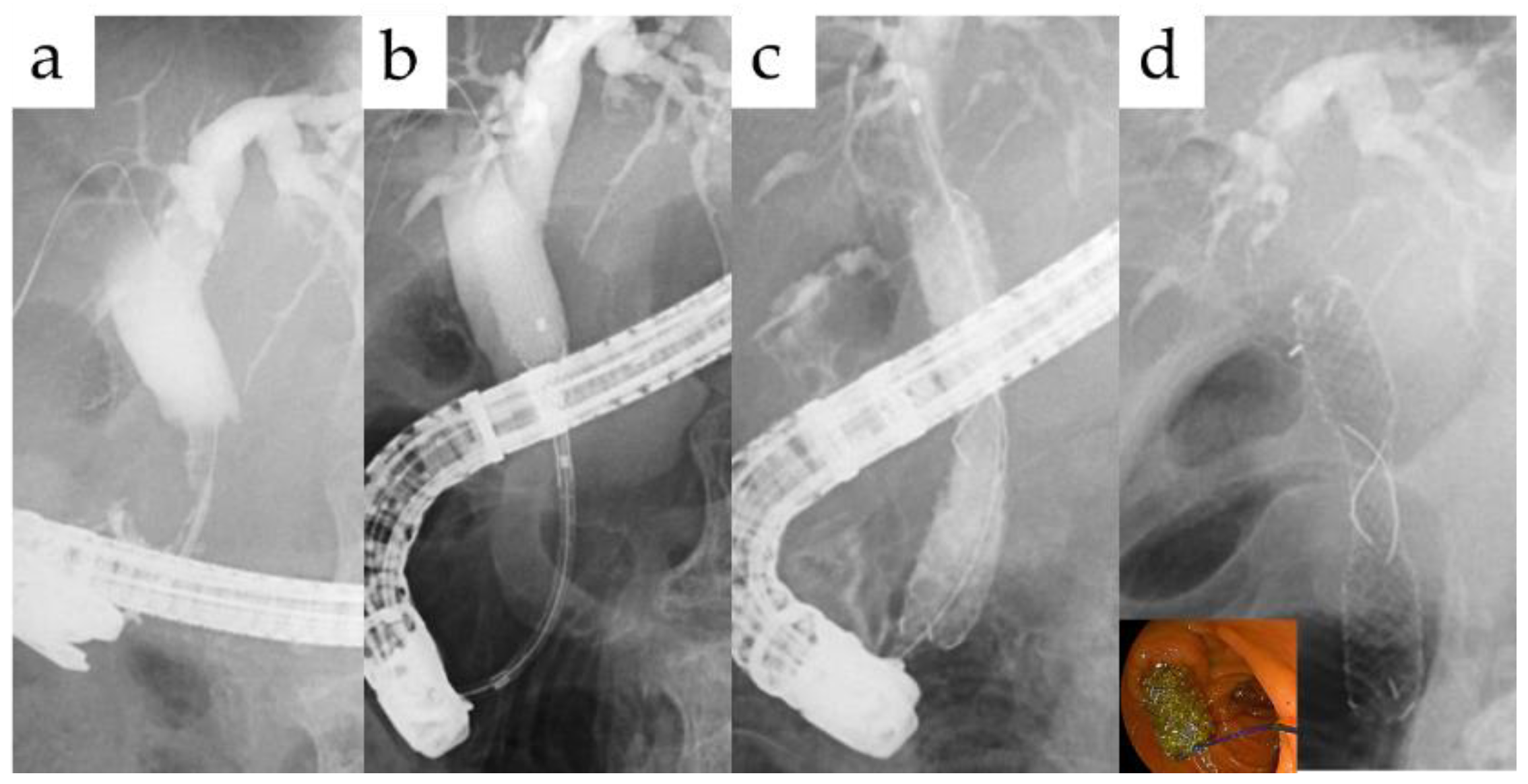
2.3. Endoscopic and Percutaneous Procedure
2.4. Outcome Measurement and Statistical Analyses
3. Results
3.1. Patient Characteristics and Etiology of BBSs
3.2. Procedure
3.3. Adverse Events
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Costamagna, G.; Pandolfi, M.; Mutignani, M.; Spada, C.; Perri, V. Long-term results of endoscopic management of postoperative bile duct strictures with increasing numbers of stents. Gastrointest. Endosc. 2001, 54, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Draganov, P.; Hoffman, B.; Marsh, W.; Cotton, P.; Cunningham, J. Long-term outcome in patients with benign biliary strictures treated endoscopically with multiple stents. Gastrointest. Endosc. 2002, 55, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Dumonceau, J.M.; Tringali, A.; Papanikolaou, I.S.; Blero, D.; Mangiavillano, B.; Schmidt, A.; Vanbiervliet, G.; Costamagna, G.; Devière, J.; García-Cano, J.; et al. Endoscopic biliary stenting: Indications, choice of stents, and results: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline—Updated October 2017. Endoscopy 2018, 50, 910–930. [Google Scholar] [CrossRef]
- Coté, G.A.; Slivka, A.; Tarnasky, P.; Mullady, D.K.; Elmunzer, B.J.; Elta, G.; Fogel, E.; Lehman, G.; McHenry, L.; Romagnuolo, J.; et al. Effect of Covered Metallic Stents Compared With Plastic Stents on Benign Biliary Stricture Resolution: A Randomized Clinical Trial. JAMA 2016, 315, 1250–1257. [Google Scholar] [CrossRef]
- Inamdar, S.; Slattery, E.; Sejpal, D.V.; Miller, L.S.; Pleskow, D.K.; Berzin, T.M.; Trindade, A.J. Systematic review and meta-analysis of single-balloon enteroscopy-assisted ERCP in patients with surgically altered GI anatomy. Gastrointest. Endosc. 2015, 82, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.J.; Lee, J.K.; Moon, J.H.; Lee, Y.N.; Park, J.K.; Lee, K.T.; Lee, K.H.; Lee, W.J.; Woo, S.M.; Lee, T.H.; et al. Intraductal placement of non-flared fully covered metallic stent for refractory anastomotic biliary strictures after living donor liver transplantation: Long-term results of prospective multicenter trial. J. Gastroenterol. Hepatol. 2020, 35, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Isayama, H.; Hamada, T.; Yasuda, I.; Itoi, T.; Ryozawa, S.; Nakai, Y.; Kogure, H.; Koike, K. TOKYO criteria 2014 for transpapillary biliary stenting. Dig. Endosc. 2015, 27, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Familiari, P.; Boškoski, I.; Bove, V.; Costamagna, G. ERCP for biliary strictures associated with chronic pancreatitis. Gastrointest. Endosc. Clin. 2013, 23, 833–845. [Google Scholar] [CrossRef]
- Lakhtakia, S.; Reddy, N.; Dolak, W.; Ponchon, T.; Bruno, M.J.; Bourke, M.J.; Neuhaus, H.; Roy, A.; González-Huix Lladó, F.; Kortan, P.P.; et al. Long-term outcomes after temporary placement of a self-expanding fully covered metal stent for benign biliary strictures secondary to chronic pancreatitis. Gastrointest. Endosc. 2020, 91, 361–369.e363. [Google Scholar] [CrossRef]
- Shibuya, H.; Hara, K.; Mizuno, N.; Hijioka, S.; Imaoka, H.; Tajika, M.; Tanaka, T.; Ishihara, M.; Hirayama, Y.; Yoshida, T.; et al. Treatment of biliary strictures with fully covered self-expandable metal stents after pancreaticoduodenectomy. Endoscopy 2017, 49, 75–79. [Google Scholar] [CrossRef]
- Sato, T.; Kogure, H.; Nakai, Y.; Ishigaki, K.; Hakuta, R.; Saito, K.; Saito, T.; Takahara, N.; Hamada, T.; Mizuno, S.; et al. A prospective study of fully covered metal stents for different types of refractory benign biliary strictures. Endoscopy 2020, 52, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, I.; Traina, M.; Mocciaro, F.; Barresi, L.; Curcio, G.; Di Pisa, M.; Granata, A.; Volpes, R.; Gridelli, B. Fully covered metallic stents in biliary stenosis after orthotopic liver transplantation. Endoscopy 2012, 44, 246–250. [Google Scholar] [CrossRef]
- Walter, D.; Laleman, W.; Jansen, J.M.; van Milligen de Wit, A.W.; Weusten, B.L.; van Boeckel, P.G.; Hirdes, M.M.; Vleggaar, F.P.; Siersema, P.D. A fully covered self-expandable metal stent with antimigration features for benign biliary strictures: A prospective, multicenter cohort study. Gastrointest. Endosc. 2015, 81, 1197–1203. [Google Scholar] [CrossRef] [PubMed]
- Mukai, S.; Itoi, T.; Sofuni, A.; Tsuchiya, T.; Tanaka, R.; Tonozuka, R.; Honjo, M.; Fujita, M.; Yamamoto, K.; Nagakawa, Y. EUS-guided antegrade intervention for benign biliary diseases in patients with surgically altered anatomy (with videos). Gastrointest. Endosc. 2019, 89, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Mukai, S.; Itoi, T.; Sofuni, A.; Tsuchiya, T.; Ishii, K.; Tanaka, R.; Tonozuka, R. Percutaneous endoscopic removal of a biliary metal stent retained in the jejunum using a digital cholangioscope. Endoscopy 2020. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, J.M.; Webber, G.R.; Knechtle, S.J.; Spivey, J.R.; Xing, M.; Kim, H.S. Percutaneous Management of Benign Biliary Strictures with Large-Bore Catheters: Comparison between Patients with and without Orthotopic Liver Transplantation. J. Vasc. Interv. Radiol. 2016, 27, 219–225.e211. [Google Scholar] [CrossRef]
- Haapamäki, C.; Kylänpää, L.; Udd, M.; Lindström, O.; Grönroos, J.; Saarela, A.; Mustonen, H.; Halttunen, J. Randomized multicenter study of multiple plastic stents vs. covered self-expandable metallic stent in the treatment of biliary stricture in chronic pancreatitis. Endoscopy 2015, 47, 605–610. [Google Scholar] [CrossRef]
- Mahajan, A.; Ho, H.; Sauer, B.; Phillips, M.S.; Shami, V.M.; Ellen, K.; Rehan, M.; Schmitt, T.M.; Kahaleh, M. Temporary placement of fully covered self-expandable metal stents in benign biliary strictures: Midterm evaluation (with video). Gastrointest. Endosc. 2009, 70, 303–309. [Google Scholar] [CrossRef]
- Moon, J.H.; Choi, H.J.; Koo, H.C.; Han, S.H.; Lee, T.H.; Cho, Y.D.; Park, S.H.; Kim, S.J. Feasibility of placing a modified fully covered self-expandable metal stent above the papilla to minimize stent-induced bile duct injury in patients with refractory benign biliary strictures (with videos). Gastrointest. Endosc. 2012, 75, 1080–1085. [Google Scholar] [CrossRef]
- Sauer, P.; Chahoud, F.; Gotthardt, D.; Stremmel, W.; Weiss, K.H.; Büchler, M.; Schemmer, P.; Weitz, J.; Schaible, A. Temporary placement of fully covered self-expandable metal stents in biliary complications after liver transplantation. Endoscopy 2012, 44, 536–538. [Google Scholar] [CrossRef]
- Poley, J.W.; van Tilburg, A.J.; Kuipers, E.J.; Bruno, M.J. Breaking the barrier: Using extractable fully covered metal stents to treat benign biliary hilar strictures. Gastrointest. Endosc. 2011, 74, 916–920. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Moon, J.H.; Lee, Y.N.; Jo, S.J.; Park, J.K.; Yang, J.K.; Cha, S.W.; Cho, Y.D.; Park, S.H. Efficacy of a modified short fully covered self-expandable metal stent for perihilar benign biliary strictures. J. Gastroenterol. Hepatol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Lee, S.S.; Song, T.J.; Park, D.H.; Seo, D.W.; Lee, S.K.; Han, S.; Kim, M.H. Long-term outcomes of covered self-expandable metal stents for treating benign biliary strictures. Endoscopy 2016, 48, 440–447. [Google Scholar] [CrossRef] [PubMed]

| Cases | 26 |
|---|---|
| Male/Female | 19/7 |
| Age, mean ± SD | 66.3 ± 14.3 |
| Mean overall follow-up period, months | 43.3 ± 30.7 |
| Mean period of plastic stent placement, month | 22.2 ± 23.5 |
| Mean period after SEMS insertion, month | 21.9 ± 13.2 |
| Etiology | Number (%) |
|---|---|
| Postoperative | 12 (46) |
| Hepaticojejunostomy anastomosis stricture | 4 |
| Perihilar stricture after cholecystectomy | 4 |
| Choledochoduodenostomy anastomosis stricture | 2 |
| Perihilar stricture after hemihepatectomy | 2 |
| Inflammatory | 8 (31) |
| Hepatolithiasis | 2 |
| Iatrogenicity | 2 |
| IgG4-related sclerosing cholangitis | 1 |
| Caroli’s disease | 1 |
| Walled-off necrosis after pancreatitis | 1 |
| Unknown | 1 |
| Chronic pancreatitis | 6 (23) |
| FCSEMS Insertion Method | N | |
|---|---|---|
| Duodenoscopy | 20 | |
| Trans-papillary | 18 | |
| Trans-anastomotic | 2 | |
| BAE | Trans-anastomotic | 4 |
| Percutaneous | 1 | |
| EUS-guided | 1 | |
| The Number of Endoscopic Sessions | |
|---|---|
| Mean PS exchange period, month | 2.3 |
| Mean FCSEMS exchange period, month | 5.3 |
| Withdrawn by death due to other causes | 3 |
| Remove the FCSEMS, (%) | 19 (73) |
| Mean time to removal, month | 7.3 ± 4.9 |
| Mean stent free period after FCSEMS removal | 13.6 ± 9.9 |
| Re-intervention for RBO after FCSEMS removal, (%) | 2 (11) |
| TRBO after FCSEMS removal, m | 1.2/17 |
| Total Number of Procedures | 48 | |
|---|---|---|
| Within 30 Days | 31 Days or Later | |
| Dislocation, (%) | 0 | 11 (23) |
| Disappeared | 0 | 5 |
| Migration | 0 | 6 |
| Broken of the FCSEMS, (%) | 0 | 2 (4.2) |
| Placement duration until FCSEMS broken, m | N/A | 7.2/10.3 |
| Cholangitis, (%) | 3 (6.3) | 0 |
| Hyperamylasemia, (%) | 1 (2.1) | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomishima, K.; Ishii, S.; Fujisawa, T.; Ikemura, M.; Ushio, M.; Takahashi, S.; Yamagata, W.; Takasaki, Y.; Suzuki, A.; Ito, K.; et al. Evaluation of the Feasibility and Effectiveness of Placement of Fully Covered Self-Expandable Metallic Stents via Various Insertion Routes for Benign Biliary Strictures. J. Clin. Med. 2021, 10, 2397. https://doi.org/10.3390/jcm10112397
Tomishima K, Ishii S, Fujisawa T, Ikemura M, Ushio M, Takahashi S, Yamagata W, Takasaki Y, Suzuki A, Ito K, et al. Evaluation of the Feasibility and Effectiveness of Placement of Fully Covered Self-Expandable Metallic Stents via Various Insertion Routes for Benign Biliary Strictures. Journal of Clinical Medicine. 2021; 10(11):2397. https://doi.org/10.3390/jcm10112397
Chicago/Turabian StyleTomishima, Ko, Shigeto Ishii, Toshio Fujisawa, Muneo Ikemura, Mako Ushio, Sho Takahashi, Wataru Yamagata, Yusuke Takasaki, Akinori Suzuki, Koichi Ito, and et al. 2021. "Evaluation of the Feasibility and Effectiveness of Placement of Fully Covered Self-Expandable Metallic Stents via Various Insertion Routes for Benign Biliary Strictures" Journal of Clinical Medicine 10, no. 11: 2397. https://doi.org/10.3390/jcm10112397
APA StyleTomishima, K., Ishii, S., Fujisawa, T., Ikemura, M., Ushio, M., Takahashi, S., Yamagata, W., Takasaki, Y., Suzuki, A., Ito, K., Haga, K., Ochiai, K., Nomura, O., Saito, H., Shibuya, T., Nagahara, A., & Isayama, H. (2021). Evaluation of the Feasibility and Effectiveness of Placement of Fully Covered Self-Expandable Metallic Stents via Various Insertion Routes for Benign Biliary Strictures. Journal of Clinical Medicine, 10(11), 2397. https://doi.org/10.3390/jcm10112397







