Mitochondrial DNA Instability Is Common in HIV-Exposed Uninfected Newborns
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Sample Collection and Processing
2.3. Detection and Quantification of Mitochondrial DNA Deletion: The eKLIPse High-Throughput Computational Pipeline
2.4. Statistical Analysis
3. Results
3.1. Study Population
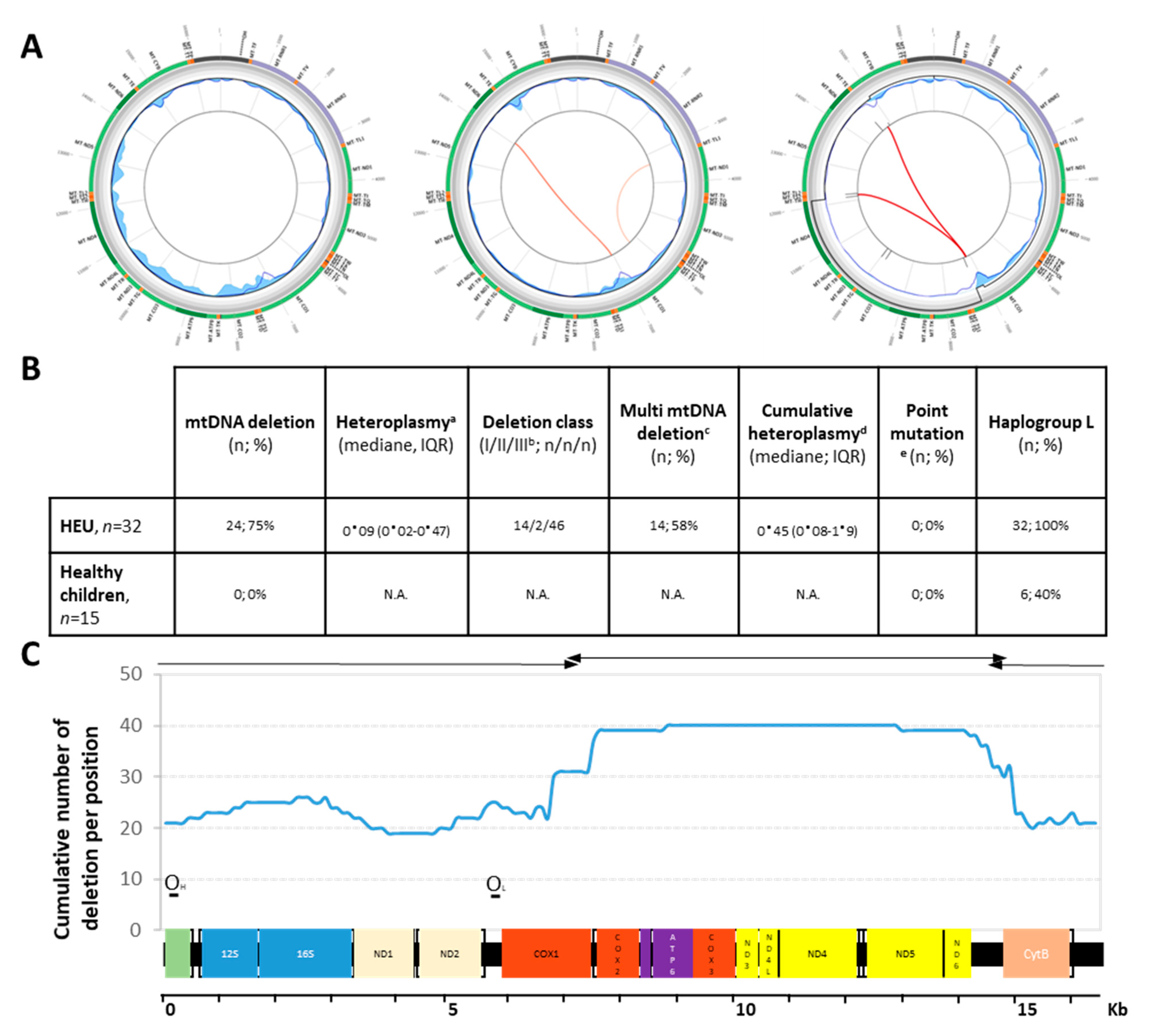
3.2. Deletion Profile among HEU Children
3.3. Predictive Factors for mtDNA Instability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- AIDSinfo. UNAIDS [Internet]. Available online: http://aidsinfo.unaids.org/ (accessed on 10 December 2020).
- Newell, M.-L.; Coovadia, H.; Cortina-Borja, M.; Rollins, N.; Gaillard, P.; Dabis, F. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: A pooled analysis. Lancet 2004, 364, 1236–1243. [Google Scholar] [CrossRef]
- Arikawa, S.; Rollins, N.; Newell, M.; Becquet, R. Mortality risk and associated factors in HIV-exposed, uninfected children. Trop. Med. Int. Health 2016, 21, 720–734. [Google Scholar] [CrossRef] [PubMed]
- Brennan, A.T.; Bonawitz, R.; Gill, C.J.; Thea, D.; Kleinman, M.; Useem, J.; Garrison, L.; Ceccarelli, R.; Udokwu, C.; Long, L.; et al. A meta-analysis assessing all-cause mortality in HIV-exposed uninfected compared with HIV-unexposed uninfected infants and children. AIDS 2016, 30, 2351–2360. [Google Scholar] [CrossRef] [Green Version]
- Taron-Brocard, C.; Chenadec, L.J.; Faye, A.; Dollfus, C.; Goetghebuer, T.; Gajdos, V. Increased risk of serious bacterial infec-tions due to maternal immunosuppression in HIV-exposed uninfected infants in a European country. Clin. Infect. Dis. 2014, 59, 1332–1345. [Google Scholar] [CrossRef]
- Omoni, A.O.; Ntozini, R.; Evans, C.; Prendergast, A.J.; Moulton, L.H.; Christian, P.S.; Humphrey, J.H. Child growth according to maternal and child HIV status in Zimbabwe. Pediatr. Infect. Dis. J. 2017, 36, 869–876. [Google Scholar] [CrossRef]
- Uthman, O.; Nachega, J.B.; Anderson, J.; Kanters, S.; Mills, E.J.; Renaud, F.; Essajee, S.; Doherty, M.C.; Mofenson, L.M. Timing of initiation of antiretroviral therapy and adverse pregnancy outcomes: A systematic review and meta-analysis. Lancet HIV 2017, 4, e21–e30. [Google Scholar] [CrossRef] [Green Version]
- le Roux, S.M.; Abrams, E.J.; Donald, K.A.; Brittain, K.; Phillips, T.K.; Nguyen, K.K. Growth trajectories of breastfed HIV-exposed uninfected and HIV-unexposed children under conditions of universal maternal antiretroviral therapy: A prospective study. Lancet Child Adolesc. Health 2019, 3, 234–244. [Google Scholar] [CrossRef]
- Blanche, S.; Tylleskär, T.; Peries, M.; Kankasa, C.; Engebretsen, I.; Meda, N.; Tumwine, J.K.; Singata-Madliki, M.; Mwiya, M.; Van de Perre, P.; et al. Growth in HIV-1-exposed but uninfected infants treated with lopinavir-ritonavir versus lamivudine: A secondary analysis of the ANRS 12174 trial. Lancet. HIV 2019, 5, e307–e314. Available online: https://pubmed-ncbi-nlm-nih-gov.proxy.insermbiblio.inist.fr/30814028/ (accessed on 10 December 2020). [CrossRef]
- Wedderburn, C.J.; Evans, C.; Yeung, S.; Gibb, D.M.; Donald, K.A.; Prendergast, A.J. Growth and neurodevelopment of HIV-exposed uninfected children: A conceptual framework. Curr. HIV/AIDS Rep. 2019, 16, 501–513. [Google Scholar] [CrossRef] [Green Version]
- Bunders, M.; Pembrey, L.; Kuijpers, T.; Newell, M.-L. Evidence of impact of maternal HIV infection on immunoglobulin levels in HIV-exposed uninfected children. AIDS Res. Hum. Retrovir. 2010, 26, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Reikie, B.A.; Adams, R.C.M.; Leligdowicz, A.; Ho, K.; Naidoo, S.; Ruck, C.E.; de Beer, C.; Preiser, W.; Cotton, M.F.; Speert, D.P.; et al. Altered innate immune development in HIV-exposed uninfected infants. J. Acquir. Immune Defic. Syndr. 2014, 66, 245–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abu-Raya, B.; Kollmann, T.R.; Marchant, A.; MacGillivray, D.M. The immune system of HIV-exposed uninfected infants. Front. Immunol. 2016, 7, 383. [Google Scholar] [CrossRef] [Green Version]
- Williams, P.L.; Hazra, R.; Van Dyke, R.B.; Yildirim, C.; Crain, M.J.; Seage, G.R.; Civitello, L.; Ellis, A.; Butler, L.; Rich, K. Antiretroviral exposure during pregnancy and adverse outcomes in HIV-exposed uninfected infants and children using a trigger-based design. AIDS 2016, 30, 133–144. [Google Scholar] [CrossRef] [Green Version]
- Simon, A.; Warszawski, J.; Kariyawasam, D.; Le Chenadec, J.; Benhammou, V.; Czernichow, P.; Foissac, F.; Laborde, K.; Treluyer, J.-M.; Firtion, G.; et al. Association of prenatal and postnatal exposure to lopinavir-ritonavir and adrenal dysfunction among uninfected infants of HIV-infected mothers. JAMA 2011, 306, 70–78. [Google Scholar] [CrossRef] [Green Version]
- Kariyawasam, D.; Peries, M.; Foissac, F.; Eymard-Duvernay, S.; Tylleskär, T.; Singata-Madliki, M.; Kankasa, C.; Meda, N.; Tumwine, J.; Mwiya, M.; et al. Lopinavir-ritonavir impairs adrenal function in infants. Clin Infect Dis. 2019, 71, 1031–1039. [Google Scholar] [CrossRef]
- Margolis, A.M.; Heverling, H.; Pham, P.A.; Stolbach, A. A review of the toxicity of HIV medications. J. Med. Toxicol. 2014, 10, 26–39. [Google Scholar] [CrossRef] [Green Version]
- Koczor, A.C.; Lewis, W. Nucleoside reverse transcriptase inhibitor toxicity and mitochondrial DNA. Expert Opin. Drug Metab. Toxicol. 2010, 6, 1493–1504. [Google Scholar] [CrossRef]
- Johnson, A.A.; Johnson, K.A. Exonuclease proofreading by human mitochondrial DNA polymerase. J. Biol. Chem. 2001, 276, 38097–38107. [Google Scholar] [CrossRef] [PubMed]
- Longley, M.J.; Nguyen, D.; Kunkel, T.A.; Copeland, W.C. The fidelity of human DNA polymerase gamma with and without exo-nucleolytic proofreading and the p55 accessory subunit. J. Biol. Chem. 2001, 42, 38555–38562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, A.P.; Luo, X.; Russell, W.; Yin, Y.W. Oxidative damage diminishes mitochondrial DNA polymerase replication fidelity. Nucleic Acids Res. 2020, 48, 817–829. [Google Scholar] [CrossRef] [PubMed]
- Rozzi, S.J.; Avdoshina, V.; Fields, J.A.; Trejo, M.; Ton, H.T.; Ahern, G.P.; Neurotoxicity Research. Human immunodeficiency virus promotes mitochon-drial toxicity. Neurotox Res. 2017, 32, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Aldrovandi, G.M.; Chu, C.; Shearer, W.T.; Li, D.; Walter, J.; Thompson, B.; McIntosh, K.; Foca, M.; Meyer, W.A.; Ha, B.F.; et al. Antiretroviral exposure and lymphocyte mtDNA content among uninfected infants of HIV-1-infected women. Pediatrics 2009, 124, e1189–e1197. [Google Scholar] [CrossRef] [Green Version]
- Hernàndez, S.; Morén, C.; López, M.; Coll, O.; Cardellach, F.; Gratacos, E.; Òscar, M.; Garrabou, G. Perinatal outcomes, mitochondrial toxicity and apoptosis in HIV-treated pregnant women and in-utero-exposed newborn. AIDS 2012, 26, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Ajaykumar, A.; Zhu, M.; Kakkar, F.; Brophy, J.; Bitnun, A.; Alimenti, A. Blood mitochondrial DNA levels remain elevated from birth to early life in children HIV-exposed uninfected exposed to combination antiretroviral therapy in utero. J. Infect. Dis. 2020, 223, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Qiao, L.; Liu, K.; Zang, Y.; Sun, Y.; Dong, Y.; Liu, D.; Guo, X.; Wei, F.; Lin, M.; et al. Mitochondrial DNA mutations in blood samples from HIV-1-infected children undergoing long-term antiretroviral therapy. Mutat. Res. Toxicol. Environ. Mutagen. 2016, 805, 1–6. [Google Scholar] [CrossRef]
- Ouyang, Y.; Wei, F.; Qiao, L.; Liu, K.; Dong, Y.; Guo, X.; Wang, S.; Pang, L.; Lin, M.; Zhang, F.; et al. Mitochondrial DNA mutations accumulated in HIV-1-infected chil-dren who have an excellent virological response when exposed to long-term antiretroviral therapy. J. Antimicrob. Chemother. 2018, 73, 3114–3121. [Google Scholar] [CrossRef]
- Jitratkosol, M.H.; Sattha, B.; Maan, E.J.; Gadawski, I.; Harrigan, P.R.; Forbes, J.C.; Alimenti, A.; Van Schalkwyk, J.; Money, D.M.; Côté, H.C. Blood mitochondrial DNA mutations in HIV-infected women and their infants exposed to HAART during pregnancy. AIDS 2012, 26, 675–683. [Google Scholar] [CrossRef] [Green Version]
- Nagot, N.; Kankasa, C.; Tumwine, J.K.; Meda, N.; Hofmeyr, G.J.; Vallo, R.; Mwiya, M.; Kwagala, M.; Traore, H.; Sunday, A.; et al. Extended pre-exposure prophylaxis with lop-inavir-ritonavir versus lamivudine to prevent HIV-1 transmission through breastfeeding up to 50 weeks in infants in Africa (ANRS 12174): A randomised controlled trial. Lancet 2016, 387, 566–573. [Google Scholar] [CrossRef]
- Boucret, L.; Bris, C.; Seegers, V.; Goudenège, D.; Desquiret-Dumas, V.; Domin-Bernhard, M.; Ferré-L’Hotellier, V.; Bouet, P.; Descamps, P.; Reynier, P.; et al. Deep sequencing shows that oocytes are not prone to accumulate mtDNA heteroplasmic mutations during ovarian ageing. Hum. Reprod. 2017, 32, 2101–2109. [Google Scholar] [CrossRef]
- Bris, C.; Goudenege, D.; Desquiret-Dumas, V.; Charif, M.; Colin, E.; Bonneau, D.; Amati-Bonneau, P.; Lenaers, G.; Reynier, P.; Procaccio, V. Bioinformatics tools and databases to assess the pathogenicity of mitochondrial DNA variants in the field of next generation sequencing. Front. Genet. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Kauppila, T.E.; Kauppila, J.H.; Larsson, N.-G. Mammalian mitochondria and aging: An update. Cell Metab. 2017, 25, 57–71. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.-H.; Kao, S.-H.; Lee, H.-C. Simultaneous increase of mitochondrial DNA deletions and lipid peroxidation in human aging. Ann. N. Y. Acad. Sci. 1996, 786, 24–43. [Google Scholar] [CrossRef]
- Goldstein, A.; Falk, M.J. Mitochondrial DNA deletion syndromes. In GeneReviews®; Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J., Stephens, K., Eds.; University of Washington: Seattle, WA, USA, 1993; Available online: http://www.ncbi.nlm.nih.gov/books/NBK1203/ (accessed on 29 January 2020).
- Damas, J.; Samuels, D.C.; Carneiro, J.; Amorim, A.; Pereira, F. Mitochondrial DNA rearrangements in health and disease—A com-prehensive study. Hum. Mutat. 2014, 35, 1–14. [Google Scholar] [CrossRef]
- Lujan, S.A.; Longley, M.J.; Humble, M.H.; Lavender, C.A.; Burkholder, A.; Blakely, E.L.; Alston, C.L.; Gorman, G.S.; Turnbull, D.M.; McFarland, R.; et al. Ultrasensitive deletion detection links mitochondrial DNA replication, disease, and aging. Genome Biol. 2020, 21, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Xie, X.; Uhler, J.P.; Hedberg-Oldfors, C.; Milenkovic, D.; Baris, O.R. Accurate mapping of mitochondrial DNA dele-tions and duplications using deep sequencing. PLoS Genet. 2020, 16, e1009242. [Google Scholar] [CrossRef] [PubMed]
- Lawless, C.; Greaves, L.; Reeve, A.K.; Turnbull, D.M.; Vincent, A.E. The rise and rise of mitochondrial DNA mutations. Open Biol. 2020, 10, 200061. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Song, F.; Gao, Z.; Ding, W.; Qiao, L.; Yang, S. Long-term exposure of mice to nucleoside analogues disrupts mito-chondrial DNA maintenance in cortical neurons. PLoS ONE 2014, 9, e85637. [Google Scholar]
- Wessels, J.; Sherman, G.; Bamford, L.; Makua, M.; Ntloana, M.; Nuttall, J.; Pillay, Y.; Goga, A.; Feucht, U. The updated South African national guideline for the prevention of mother to child transmission of communicable infections (2019). S. Afr. J. HIV Med. 2020, 21, 8. [Google Scholar] [CrossRef]
- Republic of Zambia, Ministry of Health. Zambia Consolidated Guidelines for Treatment and Prevention of HIV Infection; Republic of Zambia, Ministry of Health: Lusaka, Zambia, 2020.
- Lee-Huang, S.; Lin Huang, P.; Lee Huang, P. Live-cell real-time imaging reveals role of mitochondria in cell-to-cell transmission of HIV-1. Biochem. Biophys. Res. Commun. 2011, 415, 384–389. [Google Scholar] [CrossRef]
- Òscar, M.; López, S.; Martínez, E.; Pedrol, E.; Milinkovic, A.; Deig, E.; Garrabou, G.; Casademont, J.; Gatell, J.M.; Cardellach, F. Mitochondrial effects of HIV infection on the peripheral blood mononuclear cells of HIV-infected patients who were never treated with antiretrovirals. Clin. Infect. Dis. 2004, 39, 710–716. [Google Scholar] [CrossRef]
- Jang, J.Y.; Blum, A.; Liu, J.; Finkel, T. The role of mitochondria in aging. J. Clin. Investig. 2018, 128, 3662–3670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipshultz, S.E.; Sasaki, N.; Thompson, B.; Eidem, B.W.; Cheng, I.; Colan, S.D.; O’Brien, S.E.; Amdani, S.; Shearer, W.T.; Orav, E.J.; et al. Left ventricular diastolic dysfunction in HIV-uninfected infants exposed in utero to antiretroviral therapy. AIDS 2020, 34, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Guerra, V.; Leister, E.C.; Williams, P.L.; Starc, T.J.; Lipshultz, S.E.; Wilkinson, J.D.; Van Dyke, R.B.; Hazra, R.; Colan, S.D.; For the pediatric HIV/AIDS cohort study (PHACS). Long-term effects of in utero antiretroviral exposure: Systolic and diastolic function in HIV-exposed uninfected youth. AIDS Res. Hum. Retrovir. 2016, 32, 621–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noguera, A.; Fortuny, C.; Muñoz-Almagro, C.; Sanchez, E.; Vilaseca, M.A.; Artuch, R. Hyperlactatemia in human immunode-ficiency virus-uninfected infants who are exposed to antiretrovirals. Pediatrics 2004, 114, e598–e603. [Google Scholar] [CrossRef] [Green Version]
- Kogachi, K.; Ter-Zakarian, A.; Asanad, S.; Sadun, A.; Karanjia, R. Toxic medications in Leber’s hereditary optic neuropathy. Mitochondrion 2019, 46, 270–277. [Google Scholar] [CrossRef]
| Children | n = 32 |
|---|---|
| Sociodemographic | |
| Site; n (%) | |
| Burkina Faso | 19 (59.4) |
| South Africa | 4 (12.5) |
| Zambia | 9 (28.1) |
| Gender; n (%) | |
| Boy | 17 (53.1) |
| Anthropometry | |
| Weight (kg); mean ± SD | 3.1 ± 0.4 |
| Height (cm); mean ± SD | 49.1 ± 2.0 |
| WAZ; mean ± SD | −0.7 ± 0.8 |
| HAZ; mean ± SD | −0.9 ± 1.0 |
| WHZ; mean ± SD | −0.4 ± 1.2 † |
| Gestational age (week); median [IQR] | 38.0 [37.0;39.0] |
| Preterm birth (week); n (%) | |
| No prematurity ≥ 37 | 28 (87.5) |
| Prematurity < 37 | 4 (12.5) |
| Hematology | |
| Hemoglobin (g/dL); mean ± SD | 15.6 ± 2.0 |
| Hemoglobin (g/dL); n (%) | |
| Normal > 13 | 28 (87.5) |
| Anemia ≤ 13 | 4 (12.5) |
| Mild [12;13] | 4 (12.5) |
| Platelet count (103/mm3); n (%) | |
| Normal ≥ 125 | 32 (100.0) |
| White cell count (103/mm3); n (%) | |
| Normal > 2.5 | 32 (100.0) |
| Neutrophil count (103/mm3); n (%) | |
| Normal > 1.5 | 30 (93.8) † |
| Neutropenia ≤ 1.5 | 1 (3.2) † |
| Mild [1.25;1.5] | 1 (3.2) † |
| Biochemistry | |
| ALAT (U/L); n (%) | |
| Normal ≤ 40 | 31 (96.9) |
| Abnormal > 40 | 1 (3.1) |
| Mild [40;100] | 1 (3.1) |
| Mother | n = 32 |
| Sociodemographic characteristics | |
| Age (year); mean ± SD | 29.0 ± 5.3 |
| Parity; median [IQR] | 3.0 [2.0;3.5] |
| Education | |
| Mother/caregiver ever attended school; n (%) | |
| Yes | 23 (82.1) ‡ |
| Clinical and biological characteristics | |
| BMI; median [IQR] | 23.5 [21.6;26.2] |
| CD4 cells count (cells/mm3); median [IQR] | 532.5 [432.5;734.0] |
| HIV viral load control (copies/mL); n (%) | |
| <1000 | 20 (62.5) |
| ≥1000 | 12 (37.5) |
| WHO HIV staging; n (%) | |
| Stage 1 | 32 (100.0) |
| Maternal prophylaxis during pregnancy | |
| ARV regimen; n (%) | |
| ZDV | 32 (100.0) |
| Duration of ARV prophylaxis (week); median [IQR] | 8.8 [5.0;10.5] |
| Maternal lifestyle during pregnancy | |
| Smoking during pregnancy; n (%) | |
| No | 24 (100.0) § |
| Alcohol consumption during pregnancy; n (%) | |
| Yes | 12 (50.0) § |
| Deletion Yes vs No (n = 32) | Cumulative Heteroplasmy Rate ≥ 0.5 (n = 32) | |||||
|---|---|---|---|---|---|---|
| PR (95%CI) | p Value | PR (95%CI) | p Value | |||
| Site (ref. = Zambia) | ||||||
| Burkina Faso | 1.61 (0.88–2.95) | 0.12 | ||||
| South Africa | 0.90 (0.29–2.82) | 0.86 | ||||
| Sex (ref. = Female) | ||||||
| Male | 1.04 (0.70–1.56) | 0.84 | 0.63 (0.25–1.57) | 0.32 | ||
| Age of the mother (per one year) | 1.03 (0.99–1.07) | 0.16 | 1.03 (0.95–1.12) | 0.47 | ||
| Age of the mother (ref. ≤ 30 years | ||||||
| ≥30 years | 1.46 (1.00–2.13) | 0.05 | 2.05 (0.83–5.01) | 0.12 | ||
| Mother viral load at D7 (Log copies/mL) | 1.27 (1.01–1.58) | 0.03 | 1.54 (1.02–2.34) | 0.04 | ||
| Duration of maternal prophylaxis (per week) | 0.99 (0.95–1.04) | 0.77 | 0.95 (0.87–1.04) | 0.27 | ||
| Gestational age (per week) | 1.02 (0.95–1.10) | 0.54 | 0.89 (0.69–1.15) | 0.37 | ||
| Weight of the child at D7 (per 500 g) | 1.07 (0.85–1.34) | 0.59 | 0.92 (0.49–1.75) | 0.81 | ||
| Alcohol consumption during pregnancy (ref. = No) | 0.89 (0.53–1.49) | 0.65 | 1.00 (0.39–2.58) | 1.00 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monnin, A.; Desquiret-Dumas, V.; Méda, N.; Goudenège, D.; Bris, C.; Kankasa, C.; Singata-Madliki, M.; Tylleskar, T.; Procaccio, V.; Nagot, N.; et al. Mitochondrial DNA Instability Is Common in HIV-Exposed Uninfected Newborns. J. Clin. Med. 2021, 10, 2399. https://doi.org/10.3390/jcm10112399
Monnin A, Desquiret-Dumas V, Méda N, Goudenège D, Bris C, Kankasa C, Singata-Madliki M, Tylleskar T, Procaccio V, Nagot N, et al. Mitochondrial DNA Instability Is Common in HIV-Exposed Uninfected Newborns. Journal of Clinical Medicine. 2021; 10(11):2399. https://doi.org/10.3390/jcm10112399
Chicago/Turabian StyleMonnin, Audrey, Valérie Desquiret-Dumas, Nicolas Méda, David Goudenège, Céline Bris, Chipepo Kankasa, Mandisa Singata-Madliki, Thorkild Tylleskar, Vincent Procaccio, Nicolas Nagot, and et al. 2021. "Mitochondrial DNA Instability Is Common in HIV-Exposed Uninfected Newborns" Journal of Clinical Medicine 10, no. 11: 2399. https://doi.org/10.3390/jcm10112399
APA StyleMonnin, A., Desquiret-Dumas, V., Méda, N., Goudenège, D., Bris, C., Kankasa, C., Singata-Madliki, M., Tylleskar, T., Procaccio, V., Nagot, N., Van de Perre, P., Reynier, P., & Molès, J.-P. (2021). Mitochondrial DNA Instability Is Common in HIV-Exposed Uninfected Newborns. Journal of Clinical Medicine, 10(11), 2399. https://doi.org/10.3390/jcm10112399






