Time in Range for Closed-Loop Systems versus Standard of Care during Physical Exercise in People with Type 1 Diabetes: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
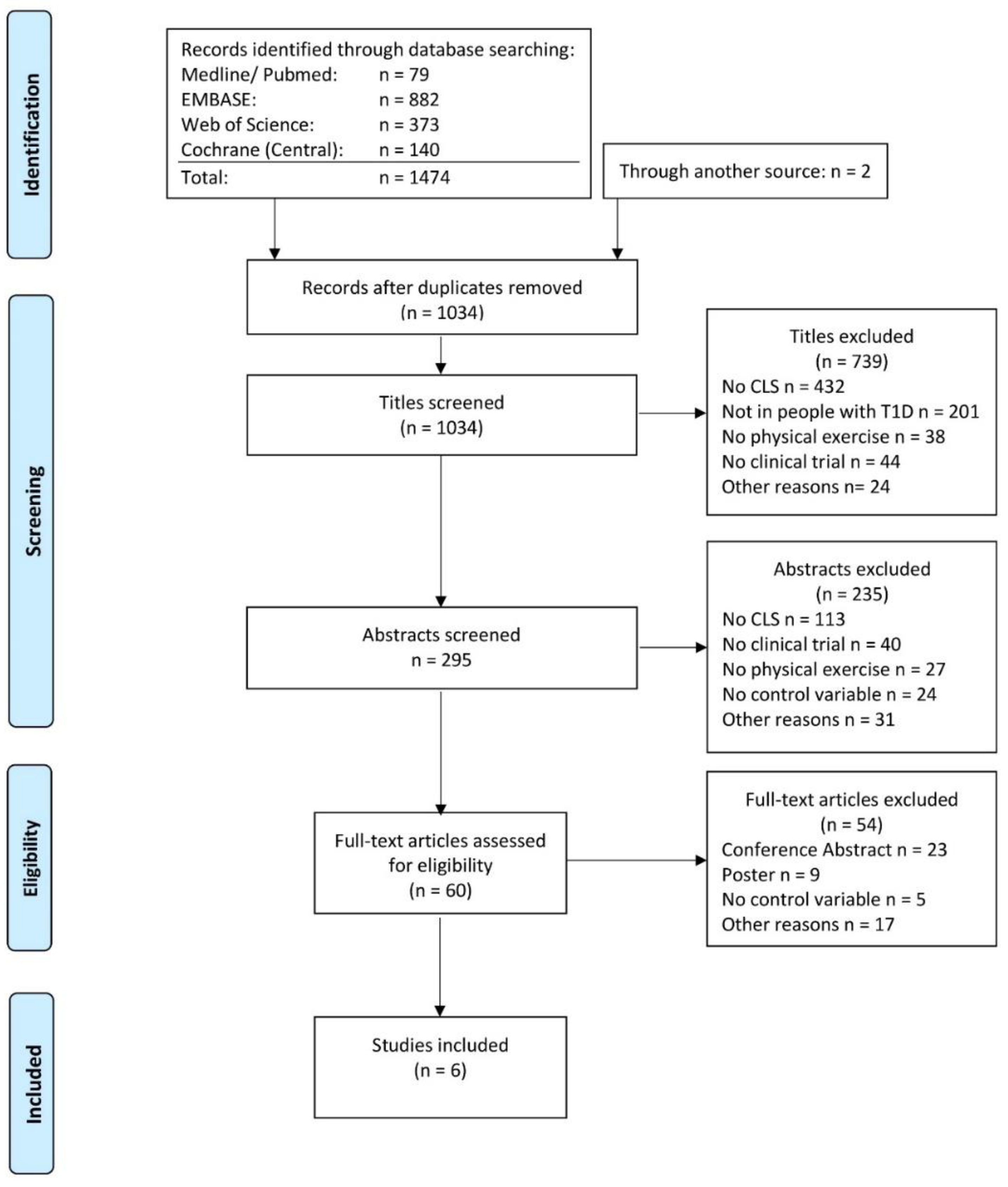
2.1. Search Strategy and Selection Criteria
2.2. Data Extraction and Quality Assessment
2.3. Data Synthesis and Analysis
2.4. Meta-Analysis
3. Results
3.1. Subgroup Analysis
3.2. Secondary Outcomes
3.3. Assessment of Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shah, V.N.; Grimsmann, J.M.; Foster, N.C.; Dost, A.; Miller, K.M.; Pavel, M.; Weinstock, R.S.; Karges, W.; Maahs, D.M.; Holl, R.W. Undertreatment of cardiovascular risk factors in the type 1 diabetes exchange clinic network (United States) and the prospective diabetes follow-up (Germany/Austria) registries. Diabetes Obes. Metab. 2020, 22, 1577–1585. [Google Scholar] [CrossRef]
- Moser, O.; Riddell, M.C.; Eckstein, M.L.; Adolfsson, P.; Rabasa-Lhoret, R.; van den Boom, L.; Gillard, P.; Nørgaard, K.; Oliver, N.S.; Zaharieva, D.P.; et al. Glucose management for exercise using continuous glucose monitoring (CGM) and intermittently scanned CGM (isCGM) systems in type 1 diabetes: Position statement of the European Association for the Study of Diabetes (EASD) and of the International Society for Pediatric and Adolescent Diabetes (ISPAD) endorsed by JDRF and supported by the American Diabetes Association (ADA). Diabetologia 2020, 63, 2501–2520. [Google Scholar] [CrossRef] [PubMed]
- Moser, O.; Riddell, M.C.; Eckstein, M.L.; Adolfsson, P.; Rabasa-Lhoret, R.; van den Boom, L.; Gillard, P.; Nørgaard, K.; Oliver, N.S.; Zaharieva, D.P.; et al. ISPAD GUIDELINES Glucose management for exercise using continuous glucose monitoring (CGM) and intermittently scanned CGM (isCGM) systems in type 1 diabetes: Position statement of the European Association for the Study of Diabetes (EASD) and of the International Society for Pediatric and Adolescent Diabetes (ISPAD) endorsed by JDRF and supported by the American Diabetes Association (ADA). Pediatr. Diabetes 2020, 21, 1375–1393. [Google Scholar] [CrossRef] [PubMed]
- Sherr, J.; Hermann, J.M.; Campbell, F.; Foster, N.C.; Hofer, S.E.; Allgrove, J.; Maahs, D.M.; Kapellen, T.M.; Holman, N.; Tamborlane, W.V.; et al. Use of insulin pump therapy in children and adolescents with type 1 diabetes and its impact on metabolic control: Comparison of results from three large, transatlantic paediatric registries. Diabetologia 2016, 59, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Phillip, M.; Battelino, T.; Rodriguez, H.; Danne, T.; Kaufman, F.; for the Consensus Forum Participants. Use of Insulin Pump Therapy in the Pediatric Age-Group: Consensus statement from the European Society for Paediatric Endocrinology, the Lawson Wilkins Pediatric Endocrine Society, and the International Society for Pediatric and Adolescent Diabetes, endorsed by the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2007, 30, 1653–1662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peyser, T.; Dassau, E.; Breton, M.; Skyler, J.S. The artificial pancreas: Current status and future prospects in the management of diabetes. Ann. N. Y. Acad. Sci. 2014, 1311, 102–123. [Google Scholar] [CrossRef] [PubMed]
- Kovatchev, B. The year of transition from research to clinical practice. Nat. Rev. Endocrinol. 2017, 14, 74–76. [Google Scholar] [CrossRef]
- Breton, M.; Farret, A.; Bruttomesso, D.; Anderson, S.; Magni, L.; Patek, S.; Man, C.D.; Place, J.; DeMartini, S.; Del Favero, S.; et al. Fully Integrated Artificial Pancreas in Type 1 Diabetes: Modular Closed-Loop Glucose Control Maintains Near Normoglycemia. Diabetes 2012, 61, 2230–2237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haidar, A.; Legault, L.; Matteau-Pelletier, L.; Messier, V.; Dallaire, M.; Ladouceur, M.; Rabasa-Lhoret, R. Outpatient overnight glucose control with dual-hormone artificial pancreas, single-hormone artificial pancreas, or conventional insulin pump therapy in children and adolescents with type 1 diabetes: An open-label, randomised controlled trial. Lancet Diabetes Endocrinol. 2015, 3, 595–604. [Google Scholar] [CrossRef]
- Tauschmann, M.; Allen, J.M.; Wilinska, M.E.; Thabit, H.; Stewart, Z.; Cheng, P.; Kollman, C.; Acerini, C.L.; Dunger, D.B.; Hovorka, R. Day-and-Night Hybrid Closed-Loop Insulin Delivery in Adolescents with Type 1 Diabetes: A Free-Living, Randomized Clinical Trial. Diabetes Care 2016, 39, 1168–1174. [Google Scholar] [CrossRef] [Green Version]
- Weinzimer, S.A.; Steil, G.M.; Swan, K.L.; Dziura, J.; Kurtz, N.; Tamborlane, W.V. Fully Automated Closed-Loop Insulin Delivery Versus Semiautomated Hybrid Control in Pediatric Patients with Type 1 Diabetes Using an Artificial Pancreas. Diabetes Care 2008, 31, 934–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forlenza, G.P.; Cameron, F.M.; Ly, T.T.; Lam, D.; Howsmon, D.P.; Baysal, N.; Kulina, G.; Messer, L.; Clinton, P.; Levister, C.; et al. Fully Closed-Loop Multiple Model Probabilistic Predictive Controller Artificial Pancreas Performance in Adolescents and Adults in a Supervised Hotel Setting. Diabetes Technol. Ther. 2018, 20, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Renard, E.; Farret, A.; Kropff, J.; Bruttomesso, D.; Messori, M.; Place, J.; Visentin, R.; Calore, R.; Toffanin, C.; Di Palma, F.; et al. Day-and-Night Closed-Loop Glucose Control in Patients with Type 1 Diabetes Under Free-Living Conditions: Results of a Single-Arm 1-Month Experience Compared With a Previously Reported Feasibility Study of Evening and Night at Home. Diabetes Care 2016, 39, 1151–1160. [Google Scholar] [CrossRef] [Green Version]
- Huyett, L.M.; Ly, T.T.; Forlenza, G.P.; Reuschel-Di-Virgilio, S.; Messer, L.H.; Wadwa, R.P.; Gondhalekar, R.; Doyle, F.J.; Pinsker, J.; Maahs, D.M.; et al. Outpatient Closed-Loop Control with Unannounced Moderate Exercise in Adolescents Using Zone Model Predictive Control. Diabetes Technol. Ther. 2017, 19, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Bekiari, E.; Kitsios, K.; Thabit, H.; Tauschmann, M.; Athanasiadou, E.; Karagiannis, T.; Haidich, A.-B.; Hovorka, R.; Tsapas, A. Artificial pancreas treatment for outpatients with type 1 diabetes: Systematic review and meta-analysis. BMJ 2018, 361, k1310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moser, O.; Eckstein, M.L.; West, D.J.; Goswami, N.; Sourij, H.; Hofmann, P. Type 1 Diabetes and Physical Exercise: Moving (forward) as an Adjuvant Therapy. Curr. Pharm. Des. 2020, 26, 946–957. [Google Scholar] [CrossRef] [PubMed]
- Adolfsson, P.; Riddell, M.C.; Taplin, C.E.; Davis, E.A.; Fournier, P.A.; Annan, F.; Scaramuzza, A.E.; Hasnani, D.; Hofer, S.E. ISPAD Clinical Practice Consensus Guidelines 2018 Compendium Exercise in children and adolescents with diabetes ISPAD CLINICAL PRACTICE CONSENSUS GUIDELINES ISPAD Clinical Practice Consensus Guidelines 2018: Exercise in children and adolescents with Diabetes. Pediatr. Diabetes 2018, 19, 205–226. [Google Scholar] [CrossRef]
- Aberer, F.; Hajnsek, M.; Rumpler, M.; Zenz, S.; Baumann, P.M.; Elsayed, H.; Puffing, A.; Treiber, G.; Pieber, T.R.; Sourij, H.; et al. Evaluation of subcutaneous glucose monitoring systems under routine environmental conditions in patients with type 1 diabetes. Diabetes Obes. Metab. 2017, 19, 1051–1055. [Google Scholar] [CrossRef] [Green Version]
- Moser, O.; Mader, J.K.; Tschakert, G.; Mueller, A.; Groeschl, W.; Pieber, T.R.; Koehler, G.; Messerschmidt, J.; Hofmann, P. Accuracy of Continuous Glucose Monitoring (CGM) during Continuous and High-Intensity Interval Exercise in Patients with Type 1 Diabetes Mellitus. Nutrients 2016, 8, 489. [Google Scholar] [CrossRef] [Green Version]
- Majeed, W.; Thabit, H. Closed-loop insulin delivery: Current status of diabetes technologies and future prospects. Expert Rev. Med. Devices 2018, 15, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Moser, O.; Yardley, J.E.; Bracken, R.M. Interstitial Glucose and Physical Exercise in Type 1 Diabetes: Integrative Physiology, Technology, and the Gap In-Between. Nutrients 2018, 10, 93. [Google Scholar] [CrossRef] [Green Version]
- Evans, M.; Wilkinson, M.; Giannpolou, A. Fast-Acting Insulin Aspart: The Rationale for a New Mealtime Insulin. Diabetes Ther. 2019, 10, 1793–1800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battelino, T.; Danne, T.; Bergenstal, R.M.; Amiel, S.A.; Beck, R.; Biester, T.; Bosi, E.; Buckingham, B.A.; Cefalu, W.T.; Close, K.L.; et al. Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations from the International Consensus on Time in Range. Diabetes Care 2019, 42, 1593–1603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steil, G.M.; Rebrin, K.; Darwin, C.; Hariri, F.; Saad, M.F. Feasibility of Automating Insulin Delivery for the Treatment of Type 1 Diabetes. Diabetes 2006, 55, 3344–3350. [Google Scholar] [CrossRef] [Green Version]
- Minimed 670G System—P160017/S031 | FDA. Available online: https://www.fda.gov/medical-devices/recently-approved-devices/minimed-670g-system-p160017s031 (accessed on 19 March 2020).
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- Dovc, K.; MacEdoni, M.; Phillip, M.; Battelino, T.; Bratina, N.; Lepej, D.; Nimri, R.; Atlas, E.; Muller, I.; Kordonouri, O.; et al. Closed-loop glucose control in young people with type 1 diabetes during and after unannounced physical activity: A randomised controlled crossover trial. Diabetologia 2017, 60, 2157–2167. [Google Scholar] [CrossRef]
- Jacobs, P.G.; El Youssef, J.; Reddy, R.; Resalat, N.; Branigan, D.; Condon, J.; Preiser, N.; Ramsey, K.; Jones, M.; Edwards, C.; et al. Randomized trial of a dual-hormone artificial pancreas with dosing adjustment during exercise compared with no adjustment and sensor-augmented pump therapy. Diabetes Obes. Metab. 2016, 18, 1110–1119. [Google Scholar] [CrossRef]
- Castle, J.R.; El Youssef, J.; Wilson, L.M.; Reddy, R.; Resalat, N.; Branigan, D.; Ramsey, K.; Leitschuh, J.; Rajhbeharrysingh, U.; Senf, B.; et al. Randomized Outpatient Trial of Single- and Dual-Hormone Closed-Loop Systems That Adapt to Exercise Using Wearable Sensors. Diabetes Care 2018, 41, 1471–1477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elleri, D.; Allen, J.M.; Kumareswaran, K.; Leelarathna, L.; Nodale, M.; Caldwell, K.; Cheng, P.; Kollman, C.; Haidar, A.; Murphy, H.R.; et al. Closed-loop basal insulin delivery over 36 hours in adolescents with type 1 diabetes: Randomized clinical trial. Diabetes Care 2013, 36, 838–844. [Google Scholar] [CrossRef] [Green Version]
- Breton, M.D.; Cherñavvsky, D.R.; Forlenza, G.P.; De Boer, M.D.; Robic, J.; Wadwa, R.P.; Messer, L.H.; Kovatchev, B.P.; Maahs, D.M. Closed-Loop Control During Intense Prolonged Outdoor Exercise in Adolescents with Type 1 Diabetes: The Artificial Pancreas Ski Study. Diabetes Care 2017, 40, 1644–1650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekhlaspour, L.; Forlenza, G.P.; Chernavvsky, D.; Maahs, D.M.; Wadwa, R.P.; DeBoer, M.D.; Messer, L.H.; Town, M.; Rn, J.P.; Kruse, G.; et al. Closed loop control in adolescents and children during winter sports: Use of the Tandem Control-IQ AP system. Pediatr. Diabetes 2019, 20, 759–768. [Google Scholar] [CrossRef]
- Schreiver, C.; Jacoby, U.; Watzer, B.; Thomas, A.; Haffner, D.; Fischer, D.-C. Glycaemic variability in paediatric patients with type 1 diabetes on continuous subcutaneous insulin infusion (CSII) or multiple daily injections (MDI): A cross-sectional cohort study. Clin. Endocrinol. 2013, 79, 641–647. [Google Scholar] [CrossRef]
- Moser, O.; Eckstein, M.L.; McCarthy, O.; Deere, R.; Pitt, J.; Williams, D.M.; Hayes, J.; Sourij, H.; Bain, S.C.; Bracken, R.M. Performance of the Freestyle Libre flash glucose monitoring (flash GM) system in individuals with type 1 diabetes: A secondary outcome analysis of a randomized crossover trial. Diabetes Obes. Metab. 2019, 21, 2505–2512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moser, O.; Eckstein, M.L.; Mueller, A.; Birnbaumer, P.; Aberer, F.; Koehler, G.; Sourij, C.; Kojzar, H.; Holler, P.; Simi, H.; et al. Impact of physical exercise on sensor performance of the FreeStyle Libre intermittently viewed continuous glucose monitoring system in people with Type 1 diabetes: A randomized crossover trial. Diabet. Med. 2019, 36, 606–611. [Google Scholar] [CrossRef]
- Zaharieva, D.P.; Turksoy, K.; McGaugh, S.M.; Pooni, R.; Vienneau, T.; Ly, T.; Riddell, M.C. Lag Time Remains with Newer Real-Time Continuous Glucose Monitoring Technology During Aerobic Exercise in Adults Living with Type 1 Diabetes. Diabetes Technol. Ther. 2019, 21, 313–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spendier, F.; Müller, A.; Korinek, M.; Hofmann, P. Intensity Thresholds and Maximal Lactate Steady State in Small Muscle Group Exercise. Sports 2020, 8, 77. [Google Scholar] [CrossRef]
- Moser, O.; Riddell, M.C.; Eckstein, M.L.; Adolfsson, P.; Rabasa-Lhoret, R.; Boom, L.V.D.; Gillard, P.; Nørgaard, K.; Oliver, N.S.; Zaharieva, D.P.; et al. Glucose management for exercise using continuous glucose monitoring: Should sex and prandial state be additional considerations? Reply to Yardley JE and Sigal RJ [letter]. Diabetology 2021, 64, 935–938. [Google Scholar] [CrossRef]
- Fahey, A.J.; Paramalingam, N.; Davey, R.J.; Davis, E.A.; Jones, T.W.; Fournier, P.A. The Effect of a Short Sprint on Postexercise Whole-Body Glucose Production and Utilization Rates in Individuals with Type 1 Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2012, 97, 4193–4200. [Google Scholar] [CrossRef] [Green Version]
- García-García, F.; Kumareswaran, K.; Hovorka, R.; Hernando, M.E. Quantifying the Acute Changes in Glucose with Exercise in Type 1 Diabetes: A Systematic Review and Meta-Analysis. Sports Med. 2015, 45, 587–599. [Google Scholar] [CrossRef] [Green Version]
- Viñals, C.; Beneyto, A.; Martín-SanJosé, J.-F.; Furió-Novejarque, C.; Bertachi, A.; Bondia, J.; Vehi, J.; Conget, I.; Giménez, M. Artificial Pancreas with Carbohydrate Suggestion Performance for Unannounced and Announced Exercise in Type 1 Diabetes. J. Clin. Endocrinol. Metab. 2021, 106, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Karageorgiou, V.; Papaioannou, T.G.; Bellos, I.; Alexandraki, K.; Tentolouris, N.; Stefanadis, C.; Chrousos, G.P.; Tousoulis, D. Effectiveness of artificial pancreas in the non-adult population: A systematic review and network meta-analysis. Metabolism 2019, 90, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Shaw, D.; Crabtree, T.S.J.; Hammond, P.; McLay, A.; Wilmot, E.G. The DIY artificial pancreas system: An ethical dilemma for doctors. Diabet. Med. 2020, 37, 1951–1953. [Google Scholar] [CrossRef] [PubMed]
- Bally, L.; Thabit, H. Closing the Loop on Exercise in Type 1 Diabetes. Curr. Diabetes Rev. 2018, 14, 257–265. [Google Scholar] [CrossRef]
- Quirk, H.; Blake, H.; Dee, B.; Glazebrook, C. “You can’t just jump on a bike and go”: A qualitative study exploring parents’ perceptions of physical activity in children with type 1 diabetes. BMC Pediatr. 2014, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]


| Year | Study Type | n | Age (years) | % TIR CLS | % TIR Control | Device Intervention | Algorithm | Device Control | Exercise Type | Exercise Duration | Exercise intensity | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [33] (a) | 2013 | Crossover RCT | 12 | 15.0 ± 1.4 | 79 (54–99) | 56 (24–70) | Animas 2020 Dexcom Seven Plus CGM | model predictive control | Animas 2020 Pump, Dexcom Seven Plus CGM | Cycling | 60 min | Moderate-intensity |
| [33] (b) | 2013 | Crossover RCT | 12 | 15.0 ± 1.4 | 60 (54–88) | 71 (18–95) | Animas 2020 Dexcom Seven Plus CGM | model predictive control | Animas 2020 Pump, Dexcom Seven Plus CGM | Cycling | 60 min | Moderate-intensity |
| [31] (a) | 2016 | Crossover RCT | 21 | 32 ± 7 | 67 (60–75) | 68 (60–76) | Tandem t:slim Dexcom G4 CGM (dual hormone) | Proportional-integral-derivative | Sensor augmented pump; Dexcom G4 CGM | Running | 45 min | Moderate-intensity |
| [31] (b) | 2016 | Crossover RCT | 21 | 32 ± 7 | 72 (66–78) | 68 (60–76) | Tandem t:slim Dexcom G4 CGM (dual hormone) | Proportional-integral-derivative | Sensor augmented pump; Dexcom G4 CGM | Running | 45 min | Moderate-intensity |
| [30] (a) | 2017 | Crossover RCT | 20 | 14.2 ± 2.0 | 80.9 (64.3–92.2) | 68.1 (59.1–83.6) | Paradigm Veo Enlite II sensor | fuzzy-logic | Medtronic Paradigm Veo; Enlite II sensor | Cycling | 40 min | Moderate-intensity |
| [30] (b) | 2017 | Crossover RCT | 20 | 14.2 ± 2.0 | 75.5 (66.6–92.9) | 68.4 (52.1–77.2) | Paradigm Veo Enlite II sensor | fuzzy-logic | Medtronic Paradigm Veo; Enlite II sensor | Cycling | 40 min | Moderate-intensity + Sprint |
| [34] | 2017 | RCT | 32 | 13.2 ± 1.7 | 63.2 ± 31.1 | 62.8 ± 31.4 | Tandem t:AP pump/ Roche-Accu-Check Spirit Combo pump Dexcom G4 CGM | DiA | Sensor-augmented pump; Dexcom G4 CGM | Skiing | 330 min | Moderate-intensity |
| [32] (a) | 2018 | Crossover RCT | 20 | 34.5 ± 4.7 | 84.3 ± 16.7 | 78.2 ± 26.2 | Tandem t:slim pump Dexcom G5 CGM (dual hormone) | Fading memory proportional-derivative | “standard of care” | Running | 45 min | Moderate-intensity |
| [32] (b) | 2018 | Crossover RCT | 20 | 34.5 ± 4.7 | 83.3 ± 16.7 | 78.2 ± 26.2 | Tandem t:slim pump Dexcom G5 CGM (single hormone) | Fading memory proportional-derivative | “standard of care” | Running | 45 min | Moderate-intensity |
| [32] (c) | 2018 | Crossover RCT | 20 | 34.5 ± 4.7 | 84.3 ± 16.7 | 78.3 ± 18.9 | Tandem t:slim pump Dexcom G5 CGM (dual hormone) | Fading memory proportional-derivative | Predictive low glucose suspend: t:slim pump, Dexcom G5 CGM | Running | 45 min | Moderate-intensity |
| [32] (d) | 2018 | Crossover RCT | 20 | 34.5 ± 4.7 | 83.3 ± 16.7 | 78.3 ± 18.9 | Tandem t:slim pump Dexcom G5 CGM (single hormone) | Fading memory proportional-derivative | Predictive low glucose suspend: t:slim pump, Dexcom G5 CGM | Running | 45 min | Moderate-intensity |
| [35] | 2019 | RCT | 48 | 12.3 ± 3.2 | 57.8 ± 27.3 | 55.9 ± 31.1 | Tandem t:slimX2 Dexcom G6 CGM | DiA | Sensor-augmented pump; Dexcom G5 CGM | Skiing | 240 min | Moderate-intensity |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eckstein, M.L.; Weilguni, B.; Tauschmann, M.; Zimmer, R.T.; Aziz, F.; Sourij, H.; Moser, O. Time in Range for Closed-Loop Systems versus Standard of Care during Physical Exercise in People with Type 1 Diabetes: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 2445. https://doi.org/10.3390/jcm10112445
Eckstein ML, Weilguni B, Tauschmann M, Zimmer RT, Aziz F, Sourij H, Moser O. Time in Range for Closed-Loop Systems versus Standard of Care during Physical Exercise in People with Type 1 Diabetes: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2021; 10(11):2445. https://doi.org/10.3390/jcm10112445
Chicago/Turabian StyleEckstein, Max L., Benjamin Weilguni, Martin Tauschmann, Rebecca T. Zimmer, Faisal Aziz, Harald Sourij, and Othmar Moser. 2021. "Time in Range for Closed-Loop Systems versus Standard of Care during Physical Exercise in People with Type 1 Diabetes: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 10, no. 11: 2445. https://doi.org/10.3390/jcm10112445
APA StyleEckstein, M. L., Weilguni, B., Tauschmann, M., Zimmer, R. T., Aziz, F., Sourij, H., & Moser, O. (2021). Time in Range for Closed-Loop Systems versus Standard of Care during Physical Exercise in People with Type 1 Diabetes: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 10(11), 2445. https://doi.org/10.3390/jcm10112445







