Cerebral and Somatic Oxygen Saturation in Neonates with Congenital Heart Disease before Surgery
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Regional Oximetry
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
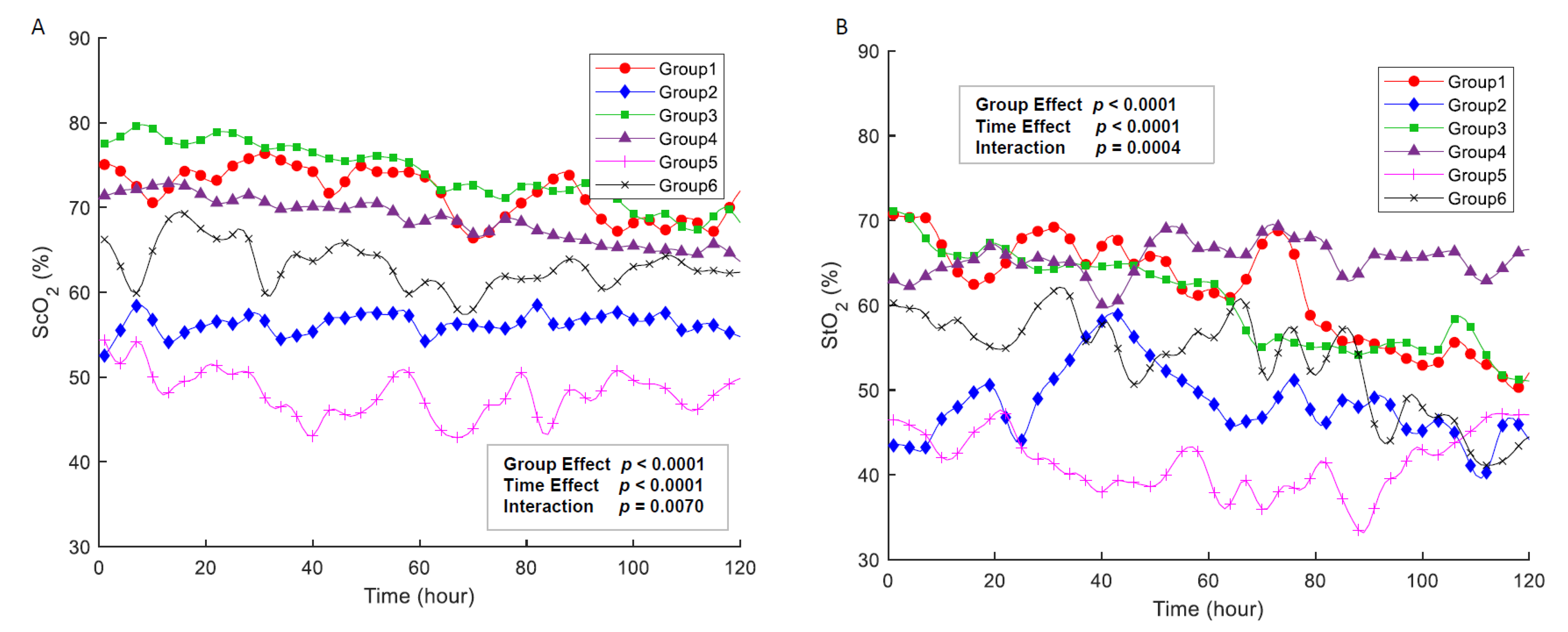
3.2. Cerebral and Somatic Oxygenation
3.3. Association with Regional NIRS and Physiologic Parameters
3.4. Changes in Regional Oxygenation in Patients with Adverse Events
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Glossary
| AVVR | Atrioventricular valvar regurgitation |
| CHD | Congenital heart disease |
| CoA | Coarctation of aorta |
| FSV | Functional single ventricle |
| IAA | Interrupted aortic arch |
| NEC | Necrotizing enterocolitis |
| NICU | Neonatal intensive care unit |
| NIRS | Near-infrared spectroscopy |
| PA | Pulmonary atresia |
| PS | Pulmonary stenosis |
| Qp/Qs | Ratio of pulmonary-to-systemic flow |
| ScO2 | Cerebral oxygenation |
| StO2 | Somatic oxygenation |
| TAPVR | Total anomalous pulmonary venous return |
| TGA | Transposition of the great arteries |
| VSD | Ventricular septal defect |
References
- Campbell, M.J.; Ziviani, J.M.; Stocker, C.F.; Khan, A.; Sakzewski, L. Neuromotor performance in infants before and after early open-heart surgery and risk factors for delayed development at 6 months of age. Cardiol. Young. 2019, 29, 100–109. [Google Scholar] [CrossRef]
- Bellinger, D.C.; Wypij, D.; duPlessis, A.J.; Rappaport, L.A.; Jonas, R.A.; Wernovsky, G.; Newburger, J.W. Neurodevelopmental status at eight years in children with dextro-transposition of the great arteries: The Boston Circulatory Arrest Trial. J. Thorac. Cardiovasc. Surg. 2003, 126, 1385–1396. [Google Scholar] [CrossRef]
- Limperopoulos, C.; Majnemer, A.; Shevell, M.I.; Rohlicek, C.; Rosenblatt, B.; Tchervenkov, C.; Darwish, H.Z. Predictors of developmental disabilities after open heart surgery in young children with congenital heart defects. J. Pediatr. 2002, 141, 51–58. [Google Scholar] [CrossRef]
- Bellinger, D.C.; Wypij, D.; Kuban, K.C.; Rappaport, L.A.; Hickey, P.R.; Wernovsky, G.; Jonas, R.A.; Newburger, J.W. Developmental and neurological status of children at 4 years of age after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. Circulation 1999, 100, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Eggli, K.D.; Contant, C.; Baylen, B.G.; Myers, J.L. Postoperative neurologic complications after open heart surgery on young infants. Arch. Pediatr. Adolesc. Med. 1995, 149, 764–768. [Google Scholar] [CrossRef]
- Bellinger, D.C.; Jonas, R.A.; Rappaport, L.A.; Wypij, D.; Wernovsky, G.; Kuban, K.C.; Barnes, P.D.; Holmes, G.L.; Hickey, P.R.; Strand, R.D.; et al. Developmental and neurologic status of children after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. N. Engl. J. Med. 1995, 332, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Gaynor, J.W.; Stopp, C.; Wypij, D.; Andropoulos, D.B.; Atallah, J.; Atz, A.M.; Beca, J.; Donofrio, M.T.; Duncan, K.; Ghanayem, N.S.; et al. Neurodevelopmental outcomes after cardiac surgery in infancy. Pediatrics 2015, 135, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Uzark, K.; Smith, C.; Donohue, J.; Yu, S.; Romano, J.C. Infant Motor Skills After a Cardiac Operation: The Need for Developmental Monitoring and Care. Ann. Thorac. Surg. 2017, 104, 681–686. [Google Scholar] [CrossRef][Green Version]
- Naef, N.; Liamlahi, R.; Beck, I.; Bernet, V.; Dave, H.; Knirsch, W.; Latal, B. Neurodevelopmental Profiles of Children with Congenital Heart Disease at School Age. J. Pediatr. 2017, 188, 75–81. [Google Scholar] [CrossRef]
- Bellinger, D.C.; Wypij, D.; Rivkin, M.J.; DeMaso, D.R.; Robertson, R.L., Jr.; Dunbar-Masterson, C.; Rappaport, L.A.; Wernovsky, G.; Jonas, R.A.; Newburger, J.W. Adolescents with d-transposition of the great arteries corrected with the arterial switch procedure: Neuropsychological assessment and structural brain imaging. Circulation 2011, 124, 1361–1369. [Google Scholar] [CrossRef]
- Miller, S.P.; McQuillen, P.S.; Vigneron, D.B.; Glidden, D.V.; Barkovich, A.J.; Ferriero, D.M.; Hamrick, S.E.G.; Azakie, A.; Karl, T.R. Preoperative brain injury in newborns with transposition of the great arteries. Ann. Thorac. Surg. 2004, 77, 1698–1706. [Google Scholar] [CrossRef]
- Licht, D.J.; Wang, J.J.; Silvestre, D.W.; Nicolson, S.C.; Montenegro, L.M.; Wernovsky, G.; Tabbutt, S.; Durning, S.M.; Shera ScD, D.M.; Gaynor, W.; et al. Preoperative cerebral blood flow is diminished in neonates with severe congenital heart defects. J. Thorac. Cardiov. Sur. 2004, 128, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, J.; Almodovar, M.C.; Zuk, J.; Friesen, R.H. Correlation of abdominal site near-infrared spectroscopy with gastric tonometry in infants following surgery for congenital heart disease. Pediatr. Crit. Care Med. 2008, 9, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Kurth, C.D.; Steven, J.L.; Montenegro, L.M.; Watzman, H.M.; Gaynor, J.W.; Spray, T.L.; Nicolson, S.C. Cerebral oxygen saturation before congenital heart surgery. Ann. Thorac. Surg. 2001, 72, 187–192. [Google Scholar] [CrossRef]
- Toet, M.C.; Flinterman, A.; van de Laar, I.; de Vries, J.W.; Bennink, G.B.; Uiterwaal, C.S.; van Bel, F. Cerebral oxygen saturation and electrical brain activity before, during, and up to 36 hours after arterial switch procedure in neonates without pre-existing brain damage: Its relationship to neurodevelopmental outcome. Exp. Brain Res. 2005, 165, 343–350. [Google Scholar] [CrossRef]
- Uebing, A.; Furck, A.K.; Hansen, J.H.; Nufer, E.; Scheewe, J.; Dutschke, P.; Jung, O.; Kramer, H.-H. Perioperative cerebral and somatic oxygenation in neonates with hypoplastic left heart syndrome or transposition of the great arteries. J. Thorac. Cardiovasc. Surg. 2011, 142, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.M.; Ko, T.; Busch, D.R.; Newland, J.J.; Winters, M.E.; Mensah-Brown, K.; Boorady, T.W.; Xiao, R.; Nicolson, S.C.; Montenegro, L.M.; et al. Preoperative cerebral hemodynamics from birth to surgery in neonates with critical congenital heart disease. J. Thorac. Cardiovasc. Surg. 2018, 156, 1657–1664. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.M.; Buckley, E.M.; Schwab, P.J.; McCarthy, A.L.; Winters, M.E.; Busch, D.R.; Xiao, R.; Goff, D.A.; Nicolson, S.C.; Montenegro, L.M.; et al. Time to surgery and preoperative cerebral hemodynamics predict postoperative white matter injury in neonates with hypoplastic left heart syndrome. J. Thorac. Cardiovasc. Surg. 2014, 148, 2181–2188. [Google Scholar] [CrossRef]
- Ghanayem, N.S.; Hoffman, G.M. Near Infrared Spectroscopy as a Hemodynamic Monitor in Critical Illness. Pediatr. Crit. Care Med. 2016, 17, S201–S206. [Google Scholar] [CrossRef]
- Hoffman, G.M.; Ghanayem, N.S.; Scott, J.P.; Tweddell, J.S.; Mitchell, M.E.; Mussatto, K.A. Postoperative Cerebral and Somatic Near-Infrared Spectroscopy Saturations and Outcome in Hypoplastic Left Heart Syndrome. Ann. Thorac. Surg. 2017, 103, 1527–1535. [Google Scholar] [CrossRef]
- Newburger, J.W.; Jonas, R.A.; Wernovsky, G.; Wypij, D.; Hickey, P.R.; Kuban, K.C.; Farrell, D.M.; Holmes, G.L.; Helmers, S.L.; Constantinou, E.; et al. A comparison of the perioperative neurologic effects of hypothermic circulatory arrest versus low-flow cardiopulmonary bypass in infant heart surgery. N. Engl. J. Med. 1993, 329, 1057–1064. [Google Scholar] [CrossRef]
- Bernal, N.P.; Hoffman, G.M.; Ghanayem, N.S.; Arca, M.J. Cerebral and somatic near-infrared spectroscopy in normal newborns. J. Pediatr. Surg. 2010, 45, 1306–1310. [Google Scholar] [CrossRef]
- McNeill, S.; Gatenby, J.C.; McElroy, S.; Engelhardt, B. Normal cerebral, renal and abdominal regional oxygen saturations using near-infrared spectroscopy in preterm infants. J. Perinatol. 2011, 31, 51–57. [Google Scholar] [CrossRef]
- Zaleski, K.L.; Kussman, B.D. Near-Infrared Spectroscopy in Pediatric Congenital Heart Disease. J. Cardiothorac. Vasc. Anesth. 2020, 34, 489–500. [Google Scholar] [CrossRef]
- Mebius, M.J.; du Marchie Sarvaas, G.J.; Wolthuis, D.W.; Bartelds, B.; Kneyber, M.C.J.; Bos, A.F.; Kooi, E.M.W. Near-infrared spectroscopy as a predictor of clinical deterioration: A case report of two infants with duct-dependent congenital heart disease. BMC Pediatr. 2017, 17, 79. [Google Scholar] [CrossRef] [PubMed]
- Arnold, D.M.; Burns, K.E.; Adhikari, N.K.; Kho, M.E.; Meade, M.O.; Cook, D.J.; MSc (Epid) for the McMaster Critical Care Interest Group. The design and interpretation of pilot trials in clinical research in critical care. Crit. Care Med. 2009, 37, S69–S74. [Google Scholar] [CrossRef]
- Chakravarti, S.B.; Mittnacht, A.J.; Katz, J.C.; Nguyen, K.; Joashi, U.; Srivastava, S. Multisite near-infrared spectroscopy predicts elevated blood lactate level in children after cardiac surgery. J. Cardiothorac. Vasc. Anesth. 2009, 23, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Karaaslan, P.; Unlukaplan, A.; Vural, G.B.; Alkan, B.T.; Akcevin, A. Cerebral perfusion correlations between NIRS and lactate levels during CPB in complex cardiac pathology children: 4AP3-9. Eur. J. Anaesthesiol. 2013, 30, 63. [Google Scholar] [CrossRef]
- Karaaslan, P.; Unlukaplan, A.; Gokay, B.V.; Darçin, K.; H1zard1, B.; Bozkaya, T.; Ozyuksel, A.; Akcevin, A. Correlation between blood lactate and regional cerebral oxygen saturation in complex cardiac pathology neonates and infants: The effect on extubation time and ICU stay. Biomed. Res. 2017, 28, 3101–3107. [Google Scholar]

| Variable | Value (n = 37) |
|---|---|
| Gestational age, weeks | 38.8 ± 1.1 |
| Birth weight, Kg | 3.2 ± 0.5 |
| Birth height (cm) | 49.4 ± 2.6 |
| Male, n (%) | 20 (54) |
| Apgar 5 min | 8.4 ± 1.0 |
| Diagnosis, n (%) | |
| Duct-dependent pulmonary circulation (TOF with PA or PS, FSV with PA or PS) | 11 (29.7) |
| TGA with IVS | 4 (8.1) |
| Mixed lesion (Truncus arteriosus, TAPVR) | 8 (21.6) |
| Duct-dependent systemic circulation (CoA with VSD, IAA with VSD, FSV with CoA) | 11 (29.7) |
| Severe valve regurgitation (TV dysplasia, Ebstein’s anomaly) | 2 (5.4) |
| Normal heart | 3 (5.4) |
| Respiratory support, n (%) | 25 (67.5) |
| Length of stay in the NICU, days | 12.9 ± 10.7 |
| Group 1 (N = 3, n = 479) Mean ± SD | Group 2 (N = 4, n = 466) Mean ± SD | Group 3 (N = 10, n = 1173) Mean ± SD | Group 4 (N = 13, n = 1151) Mean ± SD | Group 5 (N = 2, n = 198) Mean ± SD | Group 6 (N = 5, n = 253) Mean ± SD | p-Value | |
|---|---|---|---|---|---|---|---|
| SpO2 (%) | 92.8 ± 4.8 a,d | 84.5 ± 7.3 b | 93.5 ±5.8 d | 91.8± 7.3 a | 87.9± 6.9 c | 93.0± 4.0 d | 0.000 |
| Cerebral oximetry (ScO2) (%) | 71.7 ± 10.3 a | 56.3 ±11.3 b | 74.8± 7.9 c | 68.7± 7.5 d | 48.1± 8.0 e | 63.2 ± 10.6 f | 0.000 |
| Somatic oximetry (StO2) (%) | 61.7 ± 16.2 a | 48.6 ±15.8 b | 61.7 ± 13.8 a | 65.4± 14.2 c | 51.3± 7.5 d | 53.7± 13.9 e | 0.000 |
| p-value (between ScO2 and StO2) | 0.000 | 0.000 | 0.000 | 0.000 | 0.715 | 0.000 |
| ScO2 | StO2 | ||||||
|---|---|---|---|---|---|---|---|
| Effect | Estimate | SE | p-Value | Estimate | SE | p-Value | |
| Intercept | 74.917 | 1.648 | <0.0001 | 70.962 | 2.961 | <0.0001 | |
| Time point | −0.051 | 0.023 | 0.0290 | −0.150 | 0.042 | 0.0004 | |
| Group | 1 | 0.000 | 0.000 | ||||
| 2 | −19.490 | 2.343 | <0.0001 | −21.411 | 4.209 | <0.0001 | |
| 3 | 4.937 | 1.921 | 0.0106 | −1.273 | 3.451 | 0.7125 | |
| 4 | −1.985 | 1.937 | 0.3061 | −6.230 | 3.480 | 0.0743 | |
| 5 | −24.653 | 2.934 | <0.0001 | −28.278 | 5.268 | <0.0001 | |
| 6 | −9.793 | 2.678 | 0.0003 | −9.601 | 4.799 | 0.0463 | |
| Time point X Group | 1 | 0.000 | 0.000 | ||||
| 2 | 0.064 | 0.034 | 0.0606 | 0.130 | 0.060 | 0.0328 | |
| 3 | −0.045 | 0.028 | 0.1093 | 0.000 | 0.051 | 0.9939 | |
| 4 | −0.021 | 0.028 | 0.4460 | 0.161 | 0.050 | 0.0013 | |
| 5 | 0.014 | 0.046 | 0.7598 | 0.122 | 0.082 | 0.1374 | |
| 6 | 0.019 | 0.039 | 0.6202 | 0.017 | 0.070 | 0.8018 | |
| 1 | 0.000 | 0.000 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.J.; Baek, J.S.; Kim, J.A.; Cha, S.G.; Yu, J.J. Cerebral and Somatic Oxygen Saturation in Neonates with Congenital Heart Disease before Surgery. J. Clin. Med. 2021, 10, 2455. https://doi.org/10.3390/jcm10112455
Kim MJ, Baek JS, Kim JA, Cha SG, Yu JJ. Cerebral and Somatic Oxygen Saturation in Neonates with Congenital Heart Disease before Surgery. Journal of Clinical Medicine. 2021; 10(11):2455. https://doi.org/10.3390/jcm10112455
Chicago/Turabian StyleKim, Mi Jin, Jae Suk Baek, Jung A Kim, Seul Gi Cha, and Jeong Jin Yu. 2021. "Cerebral and Somatic Oxygen Saturation in Neonates with Congenital Heart Disease before Surgery" Journal of Clinical Medicine 10, no. 11: 2455. https://doi.org/10.3390/jcm10112455
APA StyleKim, M. J., Baek, J. S., Kim, J. A., Cha, S. G., & Yu, J. J. (2021). Cerebral and Somatic Oxygen Saturation in Neonates with Congenital Heart Disease before Surgery. Journal of Clinical Medicine, 10(11), 2455. https://doi.org/10.3390/jcm10112455






