Nasal Delivery of Acute Medications for Migraine: The Upper Versus Lower Nasal Space
Abstract
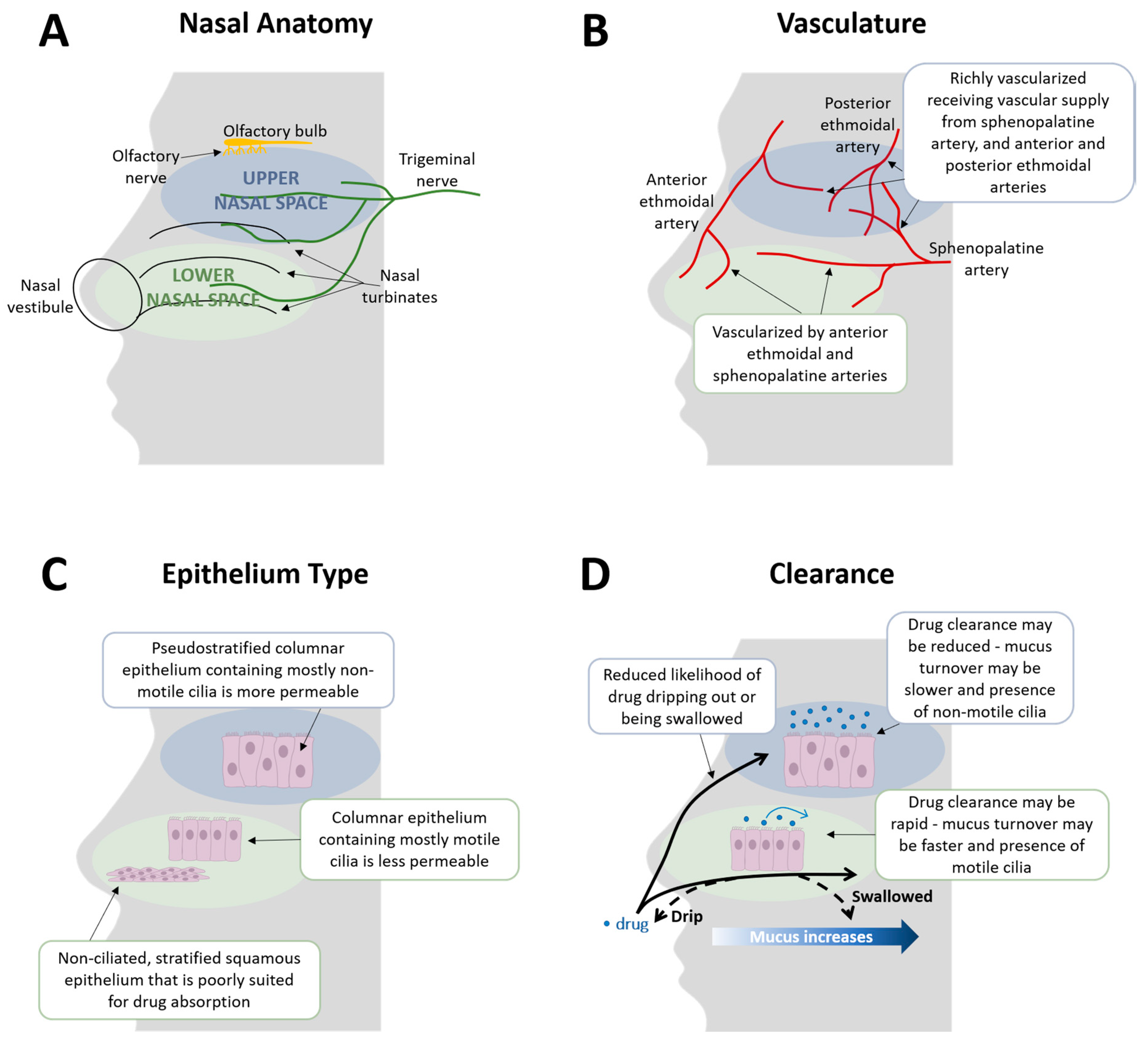
:1. Introduction
2. Nasal Delivery: All Parts of the Nose Are Not the Same
2.1. Lower Nasal Space
2.2. Upper Nasal Space
2.3. Additional Factors That May Impact Nasal Drug Delivery
3. Overview of Nasal Products That Are in Development or Approved, Including Efficacy, Safety, and Bioavailability
3.1. Approved Products
3.1.1. IMITREX®
3.1.2. MIGRANAL®
3.1.3. ZOMIG®
3.1.4. ONZETRA® Xsail®
3.1.5. TOSYMRA™
3.2. Comparator Studies between Nasal Routes of Delivery and Oral Tablets
3.3. Products in Development
3.3.1. STS101
3.3.2. INP104
4. Does Nasal Delivery Address Patient Needs?
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Leonardi, M.; Raggi, A. A narrative review on the burden of migraine: When the burden is the impact on people’s life. J. Headache Pain 2019, 20, 41. [Google Scholar] [CrossRef] [Green Version]
- Raggi, A.; Giovannetti, A.M.; Quintas, R.; D’Amico, D.; Cieza, A.; Sabariego, C.; Bickenbach, J.E.; Leonardi, M. A systematic review of the psychosocial difficulties relevant to patients with migraine. J. Headache Pain 2012, 13, 595–606. [Google Scholar] [CrossRef] [Green Version]
- Buse, D.C.; Scher, A.I.; Dodick, D.W.; Reed, M.L.; Fanning, K.M.; Manack Adams, A.; Lipton, R.B. Impact of Migraine on the Family: Perspectives of People With Migraine and Their Spouse/Domestic Partner in the CaMEO Study. Mayo Clin. Proc. 2016. [Google Scholar] [CrossRef]
- Global, regional, and national burden of migraine and tension-type headache, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 954–976. [CrossRef] [Green Version]
- Lipton, R.B.; Munjal, S.; Alam, A.; Buse, D.C.; Fanning, K.M.; Reed, M.L.; Schwedt, T.J.; Dodick, D.W. Migraine in America Symptoms and Treatment (MAST) Study: Baseline Study Methods, Treatment Patterns, and Gender Differences. Headache 2018, 58, 1408–1426. [Google Scholar] [CrossRef]
- Migraine Research Foundation. Migraine Facts. Available online: https://migraineresearchfoundation.org/about-migraine/migraine-facts (accessed on 27 July 2020).
- Cooke, L.J.; Becker, W.J. Migraine prevalence, treatment and impact: The Canadian women and migraine study. Can. J. Neurol. Sci. 2010, 37, 580–587. [Google Scholar] [CrossRef] [Green Version]
- Holland, S.; Fanning, K.M.; Serrano, D.; Buse, D.C.; Reed, M.L.; Lipton, R.B. Rates and reasons for discontinuation of triptans and opioids in episodic migraine: Results from the American Migraine Prevalence and Prevention (AMPP) study. J. Neurol. Sci. 2013, 326, 10–17. [Google Scholar] [CrossRef]
- Lipton, R.B.; Hutchinson, S.; Ailani, J.; Reed, M.L.; Fanning, K.M.; Manack Adams, A.; Buse, D.C. Discontinuation of Acute Prescription Medication for Migraine: Results from the Chronic Migraine Epidemiology and Outcomes (CaMEO) Study. Headache 2019, 59, 1762–1772. [Google Scholar] [CrossRef]
- Homayun, B.; Lin, X.; Choi, H.J. Challenges and Recent Progress in Oral Drug Delivery Systems for Biopharmaceuticals. Pharmaceutics 2019, 11, 129. [Google Scholar] [CrossRef] [Green Version]
- Djupesland, P.G.; Messina, J.C.; Mahmoud, R.A. Breath powered nasal delivery: A new route to rapid headache relief. Headache 2013, 53 (Suppl. 2), 72–84. [Google Scholar] [CrossRef] [PubMed]
- Price, G.; Patel, D.A. Drug Bioavailability; StatPearls: Treasure Island, FL, USA, 2020. [Google Scholar]
- Becker, D.E. Drug therapy in dental practice: General principles. Part 1—Pharmacokinetic considerations. Anesth. Prog. 2006, 53, 140–145. [Google Scholar] [CrossRef] [Green Version]
- Volans, G.N. Migraine and drug absorption. Clin. Pharm. 1978, 3, 313–318. [Google Scholar] [CrossRef]
- Brazzell, R.K.; Colburn, W.A. Controversy I: Patients or healthy volunteers for pharmacokinetic studies? J. Clin Pharmacol. 1986, 26, 242–247. [Google Scholar] [CrossRef]
- Aurora, S.K.; Kori, S.H.; Barrodale, P.; McDonald, S.A.; Haseley, D. Gastric stasis in migraine: More than just a paroxysmal abnormality during a migraine attack. Headache 2006, 46, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Aurora, S.K.; Rozen, T.D.; Kori, S.H.; Shrewsbury, S.B. A randomized, double blind, placebo-controlled study of MAP0004 in adult patients with migraine. Headache 2009, 49, 826–837. [Google Scholar] [CrossRef]
- Becker, W.J. Acute migraine treatment in adults. Headache 2015, 55, 778–793. [Google Scholar] [CrossRef]
- Rapoport, A.M.; Freitag, F.; Pearlman, S.H. Innovative delivery systems for migraine: The clinical utility of a transdermal patch for the acute treatment of migraine. CNS Drugs 2010, 24, 929–940. [Google Scholar] [CrossRef]
- Marmura, M.J.; Silberstein, S.D.; Schwedt, T.J. The acute treatment of migraine in adults: The American Headache Society Evidence Assessment of Migraine Pharmacotherapies. Headache 2015, 55, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.F.; Zhu, L.L.; Chen, M.; Xu, H.M.; Wang, H.F.; Feng, X.Q.; Zhu, X.P.; Zhou, Q. The optimal choice of medication administration route regarding intravenous, intramuscular, and subcutaneous injection. Patient Prefer. Adherence 2015, 9, 923–942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musumeci, T.; Bonaccorso, A.; Puglisi, G. Epilepsy Disease and Nose-to-Brain Delivery of Polymeric Nanoparticles: An Overview. Pharmaceutics 2019, 11, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silberstein, S.D.; Shrewsbury, S.B.; Hoekman, J. Dihydroergotamine (DHE)—Then and Now: A Narrative Review. Headache 2020, 60, 40–57. [Google Scholar] [CrossRef] [PubMed]
- Arslan, H.H.; Tokgoz, E.; Yildizoglu, U.; Durmaz, A.; Bek, S.; Gerek, M. Evaluation of the changes in the nasal cavity during the migraine attack. J. Craniofac. Surg. 2014, 25, e446–e449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ku, M.; Silverman, B.; Prifti, N.; Ying, W.; Persaud, Y.; Schneider, A. Prevalence of migraine headaches in patients with allergic rhinitis. Ann. Allergy Asthma Immunol. 2006, 97, 226–230. [Google Scholar] [CrossRef]
- Martin, V.T.; Fanning, K.M.; Serrano, D.; Buse, D.C.; Reed, M.L.; Bernstein, J.A.; Lipton, R.B. Chronic rhinitis and its association with headache frequency and disability in persons with migraine: Results of the American Migraine Prevalence and Prevention (AMPP) Study. Cephalalgia 2014, 34, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Barbanti, P.; Aurilia, C.; Dall’Armi, V.; Egeo, G.; Fofi, L.; Bonassi, S. The phenotype of migraine with unilateral cranial autonomic symptoms documents increased peripheral and central trigeminal sensitization. A case series of 757 patients. Cephalalgia 2016, 36, 1334–1340. [Google Scholar] [CrossRef]
- Fornazieri, M.A.; Neto, A.R.; de Rezende Pinna, F.; Gobbi Porto, F.H.; de Lima Navarro, P.; Voegels, R.L.; Doty, R.L. Olfactory symptoms reported by migraineurs with and without auras. Headache 2016, 56, 1608–1616. [Google Scholar] [CrossRef]
- IMITREX® Nasal Spray [Package Insert]. GlaxoSmithKline, Inc., 2017. Available online: https://gskpro.com/content/dam/global/hcpportal/en_US/Prescribing_Information/Imitrex_Nasal_Spray/pdf/IMITREX-NASAL-SPRAY-PI-PIL.PDF (accessed on 11 March 2021).
- Rapoport, A.M.; Bigal, M.E.; Tepper, S.J.; Sheftell, F.D. Intranasal medications for the treatment of migraine and cluster headache. CNS Drugs 2004, 18, 671–685. [Google Scholar] [CrossRef]
- Schönbach, C. Respiratory tract, upper and lower. In Encyclopedia of Systems Biology; Werner Dubitzky, O.W., Cho, K.-H., Yokota, H., Eds.; Springer Link: Berlin/Heidelberg, Germany, 2013; pp. 1–3. [Google Scholar] [CrossRef]
- Person, A.; Mintz, M. Current Clinical Practice: Disorders of the Respiratory Tract: Common Challenges in Primary Care; Humana Press: Totowa, NJ, USA, 2006. [Google Scholar]
- Hoekman, J.; Brunelle, A.; Hite, M.; Kim, P.; Fuller, C. SPECT imaging of direct nose-to-brain transfer of MAG-3 in man. In Proceedings of the American Association of Pharmaceutical Scientists Annual Meeting, San Antonio, TX, USA, 10–14 September 2013. W4009. [Google Scholar]
- Hoekman, J.; Ray, S.; Aurora, S.K.; Shrewsbury, S.B. The Upper Nasal Space—A Novel Delivery Route Ideal for Central Nervous System Drugs. US Neurol. 2020, 16, 25–31. [Google Scholar] [CrossRef]
- Crowe, T.P.; Greenlee, M.H.W.; Kanthasamy, A.G.; Hsu, W.H. Mechanism of intranasal drug delivery directly to the brain. Life Sci. 2018, 195, 44–52. [Google Scholar] [CrossRef]
- Djupesland, P.G.; Messina, J.C.; Mahmoud, R.A. The nasal approach to delivering treatment for brain diseases: An anatomic, physiologic, and delivery technology overview. Ther. Deliv. 2014, 5, 709–733. [Google Scholar] [CrossRef] [Green Version]
- Ganger, S.; Schindowski, K. Tailoring Formulations for Intranasal Nose-to-Brain Delivery: A Review on Architecture, Physico-Chemical Characteristics and Mucociliary Clearance of the Nasal Olfactory Mucosa. Pharmaceutics 2018, 10, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edvinsson, J.C.A.; Vigano, A.; Alekseeva, A.; Alieva, E.; Arruda, R.; De Luca, C.; D’Ettore, N.; Frattale, I.; Kurnukhina, M.; Macerola, N.; et al. The fifth cranial nerve in headaches. J. Headache Pain 2020, 21, 65. [Google Scholar] [CrossRef]
- Noseda, R.; Jakubowski, M.; Kainz, V.; Borsook, D.; Burstein, R. Cortical projections of functionally identified thalamic trigeminovascular neurons: Implications for migraine headache and its associated symptoms. J. Neurosci. 2011, 31, 14204–14217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashina, M.; Hansen, J.M.; Do, T.P.; Melo-Carrillo, A.; Burstein, R.; Moskowitz, M.A. Migraine and the trigeminovascular system—40 years and counting. Lancet Neurol. 2019, 18, 795–804. [Google Scholar] [CrossRef]
- Noseda, R.; Burstein, R. Migraine pathophysiology: Anatomy of the trigeminovascular pathway and associated neurological symptoms, cortical spreading depression, sensitization, and modulation of pain. Pain 2013, 154 (Suppl. 1), S44–S53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chadha, D.S.; Anadure, R.K. Handbook of Medical Emergencies, 2019th ed.; Department of Medicine Command Hospital (AF): Bangalore, India; Karnataka, India, 2019. [Google Scholar]
- Helwany, M.; Bordoni, B. Neuroanatomy, Cranial Nerve 1 (Olfactory). In StatPearls; StatPearls Publishing Copyright© 2020; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2020. [Google Scholar]
- Lochhead, J.J.; Thorne, R.G. Intranasal delivery of biologics to the central nervous system. Adv. Drug Deliv. Rev. 2012, 64, 614–628. [Google Scholar] [CrossRef]
- Ladel, S.; Schlossbauer, P.; Flamm, J.; Luksch, H.; Mizaikoff, B.; Schindowski, K. Improved In Vitro Model for Intranasal Mucosal Drug Delivery: Primary Olfactory and Respiratory Epithelial Cells Compared with the Permanent Nasal Cell Line RPMI 2650. Pharmaceutics 2019, 11, 367. [Google Scholar] [CrossRef] [Green Version]
- Harkema, J.R.; Carey, S.A.; Wagner, J.G. The nose revisited: A brief review of the comparative structure, function, and toxicologic pathology of the nasal epithelium. Toxicol. Pathol. 2006, 34, 252–269. [Google Scholar] [CrossRef] [PubMed]
- Harkema, J.R.; Plopper, C.G.; Hyde, D.M.; St George, J.A. Regional differences in quantities of histochemically detectable mucosubstances in nasal, paranasal, and nasopharyngeal epithelium of the bonnet monkey. J. Histochem. Cytochem. 1987, 35, 279–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mogensen, C.; Tos, M. Density of goblet cells on normal adult nasal turbinates. Anat. Anz. 1977, 142, 322–330. [Google Scholar] [PubMed]
- Morgan, K.T.; Jiang, X.Z.; Patterson, D.L.; Gross, E.A. The nasal mucociliary apparatus. Correlation of structure and function in the rat. Am. Rev. Respir. Dis. 1984, 130, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Hoekman, J.D.; Ho, R.J. Enhanced analgesic responses after preferential delivery of morphine and fentanyl to the olfactory epithelium in rats. Anesth. Analg. 2011, 113, 641–651. [Google Scholar] [CrossRef] [Green Version]
- Shrewsbury, S.B.; Jeleva, M.; Satterly, K.H.; Lickliter, J.; Hoekman, J. STOP 101: A Phase 1, Randomized, Open-Label, Comparative Bioavailability Study of INP104, Dihydroergotamine Mesylate (DHE) Administered Intranasally by a I123 Precision Olfactory Delivery (POD) Device, in Healthy Adult Subjects. Headache 2019, 59, 394–409. [Google Scholar] [CrossRef] [PubMed]
- Watelet, J.B.; Van Cauwenberge, P. Applied anatomy and physiology of the nose and paranasal sinuses. Allergy 1999, 54 (Suppl. 57), 14–25. [Google Scholar] [CrossRef]
- Rusznak, C.; Devalia, J.L.; Lozewicz, S.; Davies, R.J. The assessment of nasal mucociliary clearance and the effect of drugs. Respir. Med. 1994, 88, 89–101. [Google Scholar] [CrossRef] [Green Version]
- Escada, P.A.; Lima, C.; da Silva, J.M. The human olfactory mucosa. Eur. Arch. Otorhinolaryngol. 2009, 266, 1675–1680. [Google Scholar] [CrossRef]
- Merkus, F.W.; Verhoef, J.C.; Schipper, N.G.; Marttin, E. Nasal mucociliary clearance as a factor in nasal drug delivery. Adv. Drug Deliv. Rev. 1998, 29, 13–38. [Google Scholar] [CrossRef]
- Ugwoke, M.I.; Agu, R.U.; Verbeke, N.; Kinget, R. Nasal mucoadhesive drug delivery: Background, applications, trends and future perspectives. Adv. Drug Deliv. Rev. 2005, 57, 1640–1665. [Google Scholar] [CrossRef]
- Schipper, N.G.; Verhoef, J.C.; Merkus, F.W. The nasal mucociliary clearance: Relevance to nasal drug delivery. Pharm. Res. 1991, 8, 807–814. [Google Scholar] [CrossRef]
- Upadhyay, S.; Parikh, A.; Joshi, P.; Upadhyay, U.; Chotai, N. Intranasal drug delivery system—A glimpse to become maestro. J. Appl. Pharm. Sci. 2011, 1, 34–44. [Google Scholar]
- Gao, M.; Shen, X.; Mao, S. Factors influencing drug deposition in the nasal cavity upon delivery via nasal sprays. J. Pharm. Investig. 2020, 50, 251–259. [Google Scholar] [CrossRef]
- Djupesland, P.G. Nasal drug delivery devices: Characteristics and performance in a clinical perspective—A review. Drug Deliv. Transl. Res. 2013, 3, 42–62. [Google Scholar] [CrossRef] [Green Version]
- Drug Approval Package. IMITREX (sumatriptan) Nasal Spray. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/97/020626ap-1.pdf (accessed on 11 March 2021).
- MIGRANAL® [Package Insert]. Bausch Health Companies, Inc., 2019. Available online: https://www.bauschhealth.com/Portals/25/Pdf/PI/Migranal-PI.pdf (accessed on 24 February 2021).
- Gallagher, R.M.; Dihydroergotamine Working Group. Acute treatment of migraine with dihydroergotamine nasal spray. Arch. Neurol. 1996, 53, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- ZOMIG® Nasal Spray [Package Insert]. Amneal Pharmaceuticals LLC, 2019. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021450s007lbl.pdf (accessed on 16 March 2021).
- Drug Approval Package. ZOMIG (Zolmitriptan) Nasal Spray. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2003/21-450_Zomig.cfm (accessed on 16 November 2020).
- Charlesworth, B.R.; Dowson, A.J.; Purdy, A.; Becker, W.J.; Boes-Hansen, S.; Färkkilä, M. Speed of onset and efficacy of zolmitriptan nasal spray in the acute treatment of migraine: A randomised, double-blind, placebo-controlled, dose-ranging study versus zolmitriptan tablet. CNS Drugs 2003, 17, 653–667. [Google Scholar] [CrossRef] [PubMed]
- Winner, P.; Farkas, V.; Štillová, H.; Woodruff, B.; Liss, C.; Lillieborg, S.; Raines, S. Efficacy and tolerability of zolmitriptan nasal spray for the treatment of acute migraine in adolescents: Results of a randomized, double-blind, multi-center, parallel-group study (TEENZ). Headache 2016, 56, 1107–1119. [Google Scholar] [CrossRef]
- Dowson, A.J.; Charlesworth, B.R.; Green, J.; Farkkila, M.; Diener, H.C.; Hansen, S.B.; Gawel, M.; Index Study Group. Zolmitriptan nasal spray exhibits good long-term safety and tolerability in migraine: Results of the INDEX trial. Headache 2005, 45, 17–24. [Google Scholar] [CrossRef]
- ONZETRA Xsail (Sumatriptan) Nasal Powder. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/206099Orig1s000TOC.cfm (accessed on 16 November 2020).
- ONZETRA® Xsail® [Package Insert]. Currax™ Pharmaceuticals LLC, 2019. Available online: https://www.onzetra.com/sites/default/files/onzetra_xsail_prescribing_information.pdf (accessed on 10 November 2020).
- Tepper, S.J.; Cady, R.K.; Silberstein, S.; Messina, J.; Mahmoud, R.A.; Djupesland, P.G.; Shin, P.; Siffert, J. AVP-825 breath-powered intranasal delivery system containing 22 mg sumatriptan powder vs 100 mg oral sumatriptan in the acute treatment of migraines (The COMPASS study): A comparative randomized clinical trial across multiple attacks. Headache 2015, 55, 621–635. [Google Scholar] [CrossRef] [PubMed]
- Cady, R.K.; McAllister, P.J.; Spierings, E.L.; Messina, J.; Carothers, J.; Djupesland, P.G.; Mahmoud, R.A. A randomized, double-blind, placebo-controlled study of breath powered nasal delivery of sumatriptan powder (AVP-825) in the treatment of acute migraine (The TARGET Study). Headache 2015, 55, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Silberstein, S.; Winner, P.K.; McAllister, P.J.; Tepper, S.J.; Halker, R.; Mahmoud, R.A.; Siffert, J. Early Onset of Efficacy and Consistency of Response Across Multiple Migraine Attacks from the Randomized COMPASS Study: AVP-825 Breath Powered® Exhalation Delivery System (Sumatriptan Nasal Powder) vs Oral Sumatriptan. Headache 2017, 57, 862–876. [Google Scholar] [CrossRef]
- TOSYMRA™ Nasal Spray [Package Insert]. Upsher-Smith Laboratories, LLC, 2019. Available online: http://www.upsher-smith.com/wp-content/uploads/TOS-MI.pdf (accessed on 31 March 2021).
- Dr. Reddy’s Laboratories and Its U.S. Subsidiary, Promius Pharma, Announce FDA Approval for TOSYMRA™ (Sumatriptan Nasal Spray) 10 mg, in the U.S. Market. Available online: https://www.biospace.com/article/releases/dr-reddy-s-laboratories-and-its-u-s-subsidiary-promius-pharma-announce-fda-approval-for-tosymra-sumatriptan-nasal-spray-10-mg-in-the-u-s-market/ (accessed on 16 November 2020).
- Munjal, S.; Gautam, A.; Offman, E.; Brand-Schieber, E.; Allenby, K.; Fisher, D.M. A Randomized Trial Comparing the Pharmacokinetics, Safety, and Tolerability of DFN-02, an Intranasal Sumatriptan Spray Containing a Permeation Enhancer, With Intranasal and Subcutaneous Sumatriptan in Healthy Adults. Headache 2016, 56, 1455–1465. [Google Scholar] [CrossRef]
- Munjal, S.; Brand-Schieber, E.; Allenby, K.; Spierings, E.L.H.; Cady, R.K.; Rapoport, A.M. A multicenter, open-label, long-term safety and tolerability study of DFN-02, an intranasal spray of sumatriptan 10 mg plus permeation enhancer DDM, for the acute treatment of episodic migraine. J. Headache Pain 2017, 18, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipton, R.B.; Munjal, S.; Brand-Schieber, E.; Rapoport, A.M. DFN-02 (Sumatriptan 10 mg With a Permeation Enhancer) Nasal Spray vs Placebo in the Acute Treatment of Migraine: A Double-Blind, Placebo-Controlled Study. Headache 2018, 58, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Duquesnoy, C.; Mamet, J.P.; Sumner, D.; Fuseau, E. Comparative clinical pharmacokinetics of single doses of sumatriptan following subcutaneous, oral, rectal and intranasal administration. Eur. J. Pharm. Sci. 1998, 6, 99–104. [Google Scholar] [CrossRef]
- IMITREX Product Monograph. Available online: https://ca.gsk.com/media/527922/imitrex.pdf (accessed on 24 February 2021).
- MIGRANAL Product Monograph. Available online: http://sterimaxinc.com/wp-content/uploads/2016/03/1.3.1-Migranal-SteriMax-English-PM-February-9-2016.pdf (accessed on 24 February 2021).
- Yates, R.; Sorensen, J.; Bergstrom, M.; Antoni, G.; Nairn, K.; Kemp, J.; Langstrom, B.; Dane, A. Distribution of intranasal C-zolmitriptan assessed by positron emission tomography. Cephalalgia 2005, 25, 1103–1109. [Google Scholar] [CrossRef]
- Seaber, E.; On, N.; Dixon, R.M.; Gibbens, M.; Leavens, W.J.; Liptrot, J.; Chittick, G.; Posner, J.; Rolan, P.E.; Pack, R.W. The absolute bioavailability and metabolic disposition of the novel antimigraine compound zolmitriptan (311C90). Br. J. Clin. Pharmacol. 1997, 43, 579–587. [Google Scholar] [CrossRef] [Green Version]
- Dowson, A.J.; Charlesworth, B.R.; Purdy, A.; Becker, W.J.; Boes-Hansen, S.; Farkkila, M. Tolerability and consistency of effect of zolmitriptan nasal spray in a long-term migraine treatment trial. CNS Drugs 2003, 17, 839–851. [Google Scholar] [CrossRef]
- Obaidi, M.; Offman, E.; Messina, J.; Carothers, J.; Djupesland, P.G.; Mahmoud, R.A. Improved pharmacokinetics of sumatriptan with Breath Powered™ nasal delivery of sumatriptan powder. Headache 2013, 53, 1323–1333. [Google Scholar] [CrossRef] [Green Version]
- McGinley, J.S.; Buse, D.C.; Shulman, K.J.; Wirth, R.J.; Hugentobler, E.; Lipton, R.B. Evaluating Mean Level and Within-Person Consistency in Migraine Pain Intensity and Migraine-Related Disability for AVP-825 vs Oral Sumatriptan: Results from the COMPASS Study, A Randomized Trial. Headache 2019, 59, 1002–1013. [Google Scholar] [CrossRef]
- Maggio, E.T. Intravail: Highly effective intranasal delivery of peptide and protein drugs. Expert Opin. Drug Deliv. 2006, 3, 529–539. [Google Scholar] [CrossRef]
- Pillion, D.J.; Hosmer, S.; Meezan, E. Dodecylmaltoside-mediated nasal and ocular absorption of lyspro-insulin: Independence of surfactant action from multimer dissociation. Pharm. Res. 1998, 15, 1637–1639. [Google Scholar] [CrossRef]
- Albrecht, D.; Iwashima, M.; Dillon, D.; Harris, S.; Levy, J. A Phase 1, Randomized, Open-Label, Safety, Tolerability, and Comparative Bioavailability Study of Intranasal Dihydroergotamine Powder (STS101), Intramuscular Dihydroergotamine Mesylate, and Intranasal DHE Mesylate Spray in Healthy Adult Subjects. Headache 2020, 60, 701–712. [Google Scholar] [CrossRef] [Green Version]
- Satsuma Pharmaceuticals Announces Topline Results from EMERGE Phase 3 Trial of STS101 for the Acute Treatment of Migraine. Available online: https://www.globenewswire.com/news-release/2020/09/10/2091685/0/en/Satsuma-Pharmaceuticals-Announces-Topline-Results-from-EMERGE-Phase-3-Trial-of-STS101-for-the-Acute-Treatment-of-Migraine.html (accessed on 13 November 2020).
- Aurora, S.; Jeleva, M.; Hocevar-Trnka, J.; Hoekman, J.; Shrewsbury, S. A Long-Term, Open-label Study of Safety and Tolerability of Precision Olfactory Delivery of DHE in Acute Migraine (STOP 301): Clinical Results. In Proceedings of the Migraine Trust Virtual Symposium (Online), 3–9 October 2020; Available online: https://virtual.mtis2020.org/ (accessed on 2 June 2021).
- Lipton, R.B.; Munjal, S.; Buse, D.C.; Alam, A.; Fanning, K.M.; Reed, M.L.; Schwedt, T.J.; Dodick, D.W. Unmet Acute Treatment Needs From the 2017 Migraine in America Symptoms and Treatment Study. Headache 2019, 59, 1310–1323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shrewsbury, S.B.; Ray, S. Impact and Burden of Episodic, Acute Migraine (I-BEAM): A Patient Experience Study. In Proceedings of the 19th Congress of the International Headache Society, Dublin, Ireland, 5–8 September 2019. IHC-PO-299. [Google Scholar]
- Gallagher, R.M.; Kunkel, R. Migraine Medication Attributes Important for Patient Compliance: Concerns about Side Effects May Delay Treatment. Headache J. Head Face Pain 2003, 43, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Lipton, R.B.; Stewart, W.F. Acute Migraine Therapy: Do Doctors Understand What Patients With Migraine Want From Therapy? Headache J. Head Face Pain 1999, 39. [Google Scholar] [CrossRef]
- Shrewsbury, S.B.; Aurora, S.; Hoekman, J.; Jeleva, M. Patient Acceptability of a Novel Upper Nasal Delivery System for DHE—Using the Precision Olfactory Delivery (POD) Device (INP104). In Proceedings of the Migraine Trust Virtual Symposium 2020 (Online), 3–9 October 2020. MTV20-DP-032. [Google Scholar]
| Product | Initial US Approval Date | Key Product Details | Dosage | BAV | Key Efficacy Details | Key Safety Details |
|---|---|---|---|---|---|---|
| IMITREX® (sumatriptan); GlaxoSmithKline, Research Triangle Park, NC, USA [29,60,61] | 1997 |
| 5, 10, or 20 mg | 17% relative to SC |
|
|
| MIGRANAL® (dihydroergotamine mesylate); Bausch Health Companies Inc. or its affiliates, Bridgewater, NJ, USA [23,51,62,63] | 1997 |
| 2 mg | 32% relative to IV |
|
|
| ZOMIG® (zolmitriptan); Amneal Pharmaceuticals, Bridgewater, NJ, USA [33,64,65,66,67,68] | 2003 |
| 2.5 or 5 mg | 102% relative to oral |
|
|
| ONZETRA® Xsail® (sumatriptan); Currax Pharmaceuticals, Morristown, NJ, USA [69,70,71,72,73] | 2016 |
| 22 mg | 19% relative to SC |
|
|
| TOSYMRA™ (sumatriptan); Upsher-Smith Laboratories, Maple Grove, MN, USA [74,75,76,77,78] | 2019 |
| 10 mg |
|
|
|
| Attributes Desired by Patients | Upper Nasal Space Delivery | Traditional Nasal Delivery |
|---|---|---|
| Speed of onset—headache relief in <30 min [93,94,95] |
|
|
| Provides complete or near complete pain relief [94,95] |
|
|
| Few or minor side effects [94] |
|
|
| Relief from headache-associated symptoms [95] |
|
|
| Ability to carry on with the day [94] |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin, V.; Hoekman, J.; Aurora, S.K.; Shrewsbury, S.B. Nasal Delivery of Acute Medications for Migraine: The Upper Versus Lower Nasal Space. J. Clin. Med. 2021, 10, 2468. https://doi.org/10.3390/jcm10112468
Martin V, Hoekman J, Aurora SK, Shrewsbury SB. Nasal Delivery of Acute Medications for Migraine: The Upper Versus Lower Nasal Space. Journal of Clinical Medicine. 2021; 10(11):2468. https://doi.org/10.3390/jcm10112468
Chicago/Turabian StyleMartin, Vincent, John Hoekman, Sheena K. Aurora, and Stephen B. Shrewsbury. 2021. "Nasal Delivery of Acute Medications for Migraine: The Upper Versus Lower Nasal Space" Journal of Clinical Medicine 10, no. 11: 2468. https://doi.org/10.3390/jcm10112468
APA StyleMartin, V., Hoekman, J., Aurora, S. K., & Shrewsbury, S. B. (2021). Nasal Delivery of Acute Medications for Migraine: The Upper Versus Lower Nasal Space. Journal of Clinical Medicine, 10(11), 2468. https://doi.org/10.3390/jcm10112468





