Monitoring of Changes in Masticatory Muscle Stiffness after Gum Chewing Using Shear Wave Elastography
Abstract
:1. Introduction
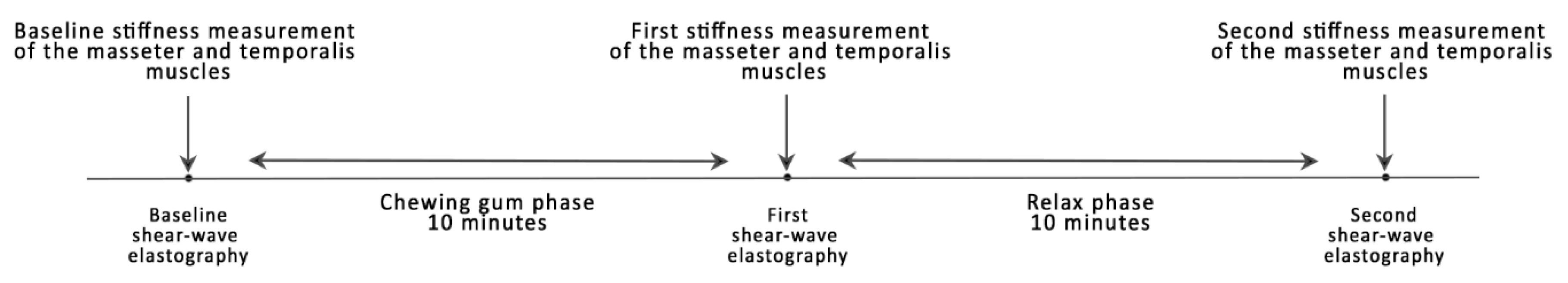
2. Experimental Section
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Basit, H.; Tariq, M.A.; Siccardi, M.A. Anatomy, Head and Neck, Mastication Muscles. Available online: https://www.ncbi.nlm.nih.gov/books/NBK541027/ (accessed on 15 March 2021).
- De Andrade, R.A.; de Cunha, M.D.; da Costa dos Santos Reis, A.M. Morphofunctional analysis of the stomatognathic system in conventional complete dentures users from the Integrated Health Center. Rev. CEFAC 2017, 19, 712–725. [Google Scholar] [CrossRef] [Green Version]
- Alomar, X.; Medrano, J.; Cabratosa, J.; Clavero, J.A.; Lorente, M.; Serra, I.; Monill, J.M.; Salvador, A. Anatomy of the temporomandibular joint. Semin. Ultrasound CT MRI 2007, 28, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, E.; Ohrbach, R. Executive summary of the Diagnostic Criteria for Temporomandibular Disorders for clinical and research applications. J. Am. Dent. Assoc. 2016, 147, 438–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peck, C.C.; Goulet, J.P.; Lobbezoo, F.; Schiffman, E.L.; Alstergren, P.; Anderson, G.C.; de Leeuw, R.; Jensen, R.; Michelotti, A.; Ohrbach, R.; et al. Expanding the taxonomy of the diagnostic criteria for temporomandibular disorders. J. Oral Rehabil. 2014, 41, 2–23. [Google Scholar] [CrossRef] [Green Version]
- Janal, M.N.; Raphael, K.G.; Nayak, S.; Klausner, J. Prevalence of myofascial temporomandibular disorder in US community women. J. Oral Rehabil. 2008, 35, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Al-Jundi, M.A.; John, M.T.; Setz, J.M.; Szentpetery, A.; Kuss, O. Meta-analysis of treatment need for temporomandibular disorders in adult nonpatients. J. Orofac. Pain 2008, 22, 97–107. [Google Scholar] [PubMed]
- Santosh, V.; Hinduja, S.; Manoj, R.; Waghmare, M. Overlaid temporomandibular joint disorders and otology symptoms—A diagnostic approach and management considerations for otolaryngologists and dentists. East. J. Med. Sci. 2020, 5, 25–29. [Google Scholar] [CrossRef]
- Ciancaglini, R.; Testa, M.; Radaelli, G. Association of neck pain with symptoms of temporomandibular dysfunction in the general adult population. Scand. J. Rehabil. Med. 1999, 31, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Cuccia, A.; Caradonna, C. The relationship between the stomatognathic system and body posture. Clinics 2009, 64, 61–66. [Google Scholar] [CrossRef] [Green Version]
- Motta, L.J.; Guedes, C.C.; De Santis, T.O.; Fernandes, K.P.; Mesquita-Ferrari, R.A.; Bussadori, S.K. Association between parafunctional habits and signs and symptoms of temporomandibular dysfunction among adolescents. Oral Health Prev. Dent. 2013, 11, 3–7. [Google Scholar]
- Woźniak, K.; Lipski, M.; Lichota, D.; Szyszka-Sommerfeld, L. Muscle Fatigue in the Temporal and Masseter Muscles in Patients with Temporomandibular Dysfunction. BioMed Res. Int. 2015, 2015, 269734. [Google Scholar] [CrossRef] [Green Version]
- Sarwono, A.P.; Himawan, L.S.; Tant, I. Differences in Pain Threshold Values of Masseter and Temporalis Muscles before and after Mastication. Pesqui. Bras. Odontopediatria Clin. Integr. 2019, 19. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Lee, K.M.; Auh, Q.-S. MRI-Based Assessment of Masticatory Muscle Changes in TMD Patients after Whiplash Injury. J. Clin. Med. 2021, 10, 1404. [Google Scholar] [CrossRef]
- Schiffman, E.; Ohrbach, R.; Truelove, E.; Look, J.; Anderson, G.; Goulet, J.-P.; List, T.; Svensson, P.; Gonzalez, Y.; Lobbezoo, F.; et al. Diagnostic criteria for temporomandibular disorders (DC/TMD) for clinical and research applications: Recommendations of the International RDC/TMD Consortium Network and Orofacial Pain Special Interest Group. J. Oral Facial. Pain Headache 2014, 28, 6–27. [Google Scholar] [CrossRef]
- Komino, M.; Shiga, H. Changes in mandibular movement during chewing of different hardness foods. Odontology 2017, 105, 418–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, G.-H.; Lee, Y. Analysis of Masticatory Muscle Activity Based on Presence of Temporomandibular Joint Disorders. Med. Sci. Monit. 2020, 26, e921337-1–e921337-7. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, A.F.; Albesher, N.; Aljohani, M.; Alsinanni, M.; Turkistani, O.; Salam, M. Association of oral parafunctional habits with anxiety and the Big-Five Personality Traits in the Saudi adult population. Saudi Dent. J. 2021, 33, 90–98. [Google Scholar] [CrossRef]
- Karabicak, G.O.; Hazar Kanik, Z. Temporomandibular disorder prevalence and its association with oral parafunctions, neck pain, and neck function in healthcare students: A cross-sectional study. Cranio 2020, 1–7. [Google Scholar] [CrossRef]
- Owczarek, J.E.; Lion, K.M.; Radwan-Oczko, M. Manifestation of stress and anxiety in the stomatognathic system of undergraduate dentistry students. J. Int. Med. Res. 2020, 48, 300060519889487. [Google Scholar] [CrossRef]
- Ali, K.; Fatima, A.; Ilyas, F.; Khan, M.; Abbassi, Z. Impact of Anxiety and Depression on Temporomandibular Joint Disorders among Sample of Dental Undergraduates of Karachi. J. Pak. Dent. Assoc. 2016, 25, 143–149. [Google Scholar]
- Douglas, C.R.; Avoglio, J.L.V.; de Oliveira, H. Stomatognathic adaptive motor syndrome is the correct diagnosis for temporomandibular disorders. Med. Hypotheses 2010, 74, 710–718. [Google Scholar] [CrossRef]
- Gavish, A.; Winocur, E.; Astandzelov-Nachmias, T.; Gazit, E. Effect of controlled masticatory exercise on pain and muscle performance in myofascial pain patients: A pilot study. Cranio 2006, 24, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Lee, J.-Y.; Lee, E.-S.; Jung, H.-J.; Ahn, H.-J.; Jung, H.I.; Kim, B.I. Simple oral exercise with chewing gum for improving oral function in older adults. Aging Clin. Exp. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Olchowy, A.; Wieckiewicz, M.; Winocur, E.; Dominiak, M.; Dekkers, I.; Łasecki, M.; Olchowy, C. Great potential of ultrasound elastography for the assessment of the masseter muscle in patients with temporomandibular disorders. A systematic review. Dentomaxillofac. Radiol. 2020, 49, 20200024. [Google Scholar] [CrossRef]
- Olchowy, C.; Więckiewicz, M.; Sconfienza, L.M.; Łasecki, M.; Seweryn, P.; Smardz, J.; Hnitecka, S.; Dominiak, M.; Olchowy, A. Potential of Using Shear Wave Elastography in the Clinical Evaluation and Monitoring of Changes in Masseter Muscle Stiffness. Pain Res. Manag. 2020, 2020, 4184268. [Google Scholar] [CrossRef] [PubMed]
- Ariji, Y.; Nakayama, M.; Nishiyama, W.; Ogi, N.; Sakuma, S.; Katsumata, A.; Kurita, K.; Ariji, E. Can sonographic features be efficacy predictors of robotic massage treatment for masseter and temporal muscle in patients with temporomandibular disorder with myofascial pain? Cranio 2016, 34, 13–19. [Google Scholar] [CrossRef] [PubMed]
| Baseline Measurement, Mean (SD), KPa | First Measurement, Mean (SD), KPa | Second Measurement, Mean (SD), KPa | |
|---|---|---|---|
| Left masseter muscle | 10.99 (2.04) | 12.31 (1.38) | 11.29 (2.01) |
| Right masseter muscle | 11.01 (2.21) | 12.32 (1.65) | 11.30 (1.73) |
| Left temporalis muscle | 10.23 (1.23) | 10.92 (1.68) | 10.65 (1.68) |
| Right temporalis muscle | 10.14 (1.32) | 10.73 (1.70) | 10.62 (1.71) |
| Baseline Measurement, Median (IQR), KPa | First Measurement, Median (IQR), KPa | Second Measurement, Median (IQR), KPa | |
|---|---|---|---|
| Masseter muscle | 11.35 (9.7–12.65) | 12.5 (11.1–13.25) | 11.75 (9.95–12.6) |
| Temporalis muscle | 10.1 (9.1–10.95) | 10.3 (10.2–10.52) | 10.2 (9.65–11.9) |
| Difference in medians | 1.25 | 2.2 | 1.55 |
| p-value | 0.0001 | 0.0001 | 0.0033 |
| Median (IQR) | Baseline | 1st Measurement | 2nd Measurement | |||
|---|---|---|---|---|---|---|
| Masseter Muscle (p < 0.0001) | ||||||
| Baseline 11.35 (9.7–12.65) | p < 0.05 | p < 0.05 | ||||
| 1st measurement 12.5 (11.1–13.25) | p < 0.05 | |||||
| 2nd measurement 11.75 (9.95–12.6) | ||||||
| Temporalis Muscle (p < 0.0001) | ||||||
| Baseline 10.1 (9.1–10.95) | p < 0.05 | p < 0.05 | ||||
| 1st measurement 10.3 (9.95–12.2) | p < 0.05 | |||||
| 2nd measurement 10.2 (9.65–11.9) | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olchowy, C.; Grzech-Leśniak, K.; Hadzik, J.; Olchowy, A.; Łasecki, M. Monitoring of Changes in Masticatory Muscle Stiffness after Gum Chewing Using Shear Wave Elastography. J. Clin. Med. 2021, 10, 2480. https://doi.org/10.3390/jcm10112480
Olchowy C, Grzech-Leśniak K, Hadzik J, Olchowy A, Łasecki M. Monitoring of Changes in Masticatory Muscle Stiffness after Gum Chewing Using Shear Wave Elastography. Journal of Clinical Medicine. 2021; 10(11):2480. https://doi.org/10.3390/jcm10112480
Chicago/Turabian StyleOlchowy, Cyprian, Kinga Grzech-Leśniak, Jakub Hadzik, Anna Olchowy, and Mateusz Łasecki. 2021. "Monitoring of Changes in Masticatory Muscle Stiffness after Gum Chewing Using Shear Wave Elastography" Journal of Clinical Medicine 10, no. 11: 2480. https://doi.org/10.3390/jcm10112480
APA StyleOlchowy, C., Grzech-Leśniak, K., Hadzik, J., Olchowy, A., & Łasecki, M. (2021). Monitoring of Changes in Masticatory Muscle Stiffness after Gum Chewing Using Shear Wave Elastography. Journal of Clinical Medicine, 10(11), 2480. https://doi.org/10.3390/jcm10112480







