Efficacy and Safety of Hydroxychloroquine for Hospitalized COVID-19 Patients: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources and Searches
2.2. Study Selection
2.3. Outcomes
2.4. Data Extraction
2.5. Risk of Bias Assessment
2.6. Statistical Analysis
3. Results
3.1. Selection of Studies
3.2. Characteristics of Included Studies
3.3. Risk of Bias of Included RCTs
3.4. Effects of Hydroxichloroquine on Outcomes
3.5. Subgroup Analyses
3.6. Quality of Evidence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Worldometer. COVID-19 Coronavirus Pandemic. Available online: https://www.worldometers.info/coronavirus/ (accessed on 22 February 2021).
- National Institutes of Health. COVID-19 Treatment Guidelines Panel Coronavirus Disease 2019 (COVID-19) Treatment Guidelines; National Institutes of Health: Bethesda, MD, USA, 2020. Available online: https://www.covid19treatmentguidelines.nih.gov/ (accessed on 22 February 2021).
- Satarker, S.; Ahuja, T.; Banerjee, M.; Dogra, S.; Agarwal, T.; Nampoothiri, M. Hydroxychloroquine in COVID-19: Potential Mechanism of Action Against SARS-CoV-2. Curr. Pharmacol. Rep. 2020, 6, 203–211. [Google Scholar] [CrossRef]
- Colson, P.; Rolain, J.-M.; Lagier, J.-C.; Brouqui, P.; Raoult, D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int. J. Antimicrob. Agents 2020, 55, 105932. [Google Scholar] [CrossRef]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Ye, F.; Zhang, M.; Cui, C.; Huang, B.; Niu, P.; Liu, X.; Zhao, L.; Dong, E.; Song, C.; et al. In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Clin. Infect. Dis. 2020, 71, 732–739. [Google Scholar] [CrossRef] [Green Version]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Doyno, C.; Sobieraj, D.M.; Baker, W.L. Toxicity of chloroquine and hydroxychloroquine following therapeutic use or overdose. Clin. Toxicol. 2021, 59, 12–23. [Google Scholar] [CrossRef]
- Hernandez, A.V.; Roman, Y.M.; Pasupuleti, V.; Barboza, J.J.; White, C.M. Hydroxychloroquine or chloroquine for treatment or prophylaxis of COVID-19: A living systematic review. Ann. Intern. Med. 2020, 173, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Hartung, J.; Knapp, G. A refined method for the meta-analysis of controlled clinical trials with binary outcome. Stat. Med. 2001, 20, 3875–3889. [Google Scholar] [CrossRef]
- Veroniki, A.A.; Jackson, D.; Viechtbauer, W.; Bender, R.; Bowden, J.; Knapp, G.; Kuss, O.; Higgins, J.P.T.; Langan, D.; Salanti, G. Methods to Estimate the between-Study Variance and Its Uncertainty in Meta-Analysis. Res. Synth. Methods. 2015, 7, 55–79. [Google Scholar] [CrossRef] [Green Version]
- Balshem, H.; Helfand, M.; Schünemann, H.J.; Oxman, A.D.; Kunz, R.; Brozek, J.; Vist, G.E.; Falck-Ytter, Y.; Meerpohl, J.; Norris, S.L. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011, 64, 401–406. [Google Scholar] [CrossRef] [PubMed]
- McMaster University. GRADEpro GDT: GRADEpro Guideline Development Tool; McMaster University: Hamilton, ON, Canada, 2020; Available online: gradepro.org (accessed on 19 November 2020).
- Chen, J.; Liu, D.; Liu, L.; Liu, P.; Xu, Q.; Xia, L.; Qian, Z. A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease-19 (COVID-19). J. Zhejiang Univ. Med. Sci. 2020, 49, 215–219. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, J.; Zhang, Z.; Jiang, S.; Han, S.; Yan, D.; Zhuang, R.; Hu, B.; Zhang, Z. Efficacy of hydroxychloroquine in patients with COVID-19: Results of a randomized clinical trial. Medrxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Tang, W.; Cao, Z.; Han, M.; Wang, Z.; Chen, J.; Sun, W.; Wu, Y.; Xiao, W.; Liu, S.; Chen, E.; et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: Open label, randomised controlled trial. BMJ 2020, 369, m1849. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, Z.Y.; Fu, J.G.; Feng, Z.P.; Zhang, S.Z.; Han, Q.Y.; Zhang, X.-B.; Xiao, X.; Chen, H.-H.; Liu, L.-L.; et al. Efficacy and safety of chloroquine or hydroxychloroquine in moderate type of COVID-19: A prospective open-label randomized controlled study. Medrxiv 2020. [Google Scholar] [CrossRef]
- Cavalcanti, A.B.; Zampieri, F.G.; Rosa, R.G.; Azevedo, L.C.; Veiga, V.C.; Avezum, A.; Damiani, L.P.; Marcadenti, A.; Kawano-Dourado, L.; Lisboa, T.; et al. Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19. N. Engl. J. Med. 2020, 383, 2041–2052. [Google Scholar] [CrossRef]
- The RECOVERY Collaborative Group. Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2020, 383, 2030–2040. [Google Scholar] [CrossRef]
- Chen, C.P.; Lin, Y.C.; Chen, T.C.; Tseng, T.Y.; Wong, H.L.; Kuo, C.Y.; Lin, W.-P.; Huang, S.-R.; Wang, W.-Y.; Liao, J.-H.; et al. A multicenter, randomized, open-label, controlled trial to evaluate the efficacy and tolerability of hydroxychloroquine and a retrospective study in adult patients with mild to moderate coronavirus disease 2019 (COVID-19). PLoS ONE 2020, 15, e0242763. [Google Scholar]
- Abd-Elsalam, S.; Esmail, E.S.; Khalaf, M.; Abdo, E.F.; Medhat, M.A.; El Ghafar, M.S.A.; Ahmed, O.A.; Soliman, S.; Serangawy, G.N.; Alboraie, M. Hydroxychloroquine in the Treatment of COVID-19: A Multicenter Randomized Controlled Study. Am. J. Trop. Med. Hyg. 2020, 103, 1635–1639. [Google Scholar] [CrossRef]
- Self, W.H.; Semler, M.W.; Leither, L.M.; Casey, J.D.; Angus, D.C.; Brower, R.G.; Chang, S.Y.; Collins, S.P.; Eppensteiner, J.C.; Filbin, M.R.; et al. Effect of Hydroxychloroquine on Clinical Status at 14 Days in Hospitalized Patients With COVID-19: A Randomized Clinical Trial. JAMA 2020, 324, 2165–2176. [Google Scholar] [CrossRef]
- Dubée, V.; Roy, P.-M.; Vielle, B.; Parot-Schinkel, E.; Blanchet, O.; Darsonval, A.; Lefeuvre, C.; Abbara, C.; Boucher, S.; Devaud, E.; et al. Hydroxychloroquine in mild-to-moderate coronavirus disease 2019: A placebo-controlled double blind trial. Clin. Microbiol. Infect. 2021. [Google Scholar] [CrossRef]
- Kamran, S.; Mirza, Z.; Naseem, B.; Saeed, F.; Azam, R.; Ullah, N.; Ahmad, W.; Saleem, S. Clearing the fog: Is HCQ effective in reducing COVID-19 progression: A randomized controlled trial. Medrxiv 2020. [Google Scholar] [CrossRef]
- WHO Solidarity Trial Consortium. Repurposed Antiviral Drugs for Covid-19—Interim WHO Solidarity Trial Results. N. Engl. J. Med. 2021, 384, 497–511. [Google Scholar] [CrossRef]
- Ulrich, R.J.; Troxel, A.B.; Carmody, E.; Eapen, J.; Bäcker, M.; DeHovitz, J.A.; Prasad, P.J.; Li, Y.; Delgado, C.; Jrada, M.; et al. Treating COVID-19 With Hydroxychloroquine (TEACH): A Multicenter, Double-Blind Randomized Controlled Trial in Hospitalized Patients. In Open Forum Infectious; Oxford University Press: New York, NY, USA, 2020; Volume 7. [Google Scholar]
- Hernandez, A.V.; Roman, Y.M.; Pasupuleti, V.; Barboza, J.J.; White, C.M. Update Alert 3: Hydroxychloroquine or Chloroquine for the Treatment or Prophylaxis of COVID-19. Ann. Intern. Med. 2020, 173, w156–w157. [Google Scholar] [CrossRef]
- Ghazy, R.M.; Almaghraby, A.; Shaaban, R.; Kamal, A.; Beshir, H.; Moursi, A.; Ramadan, A.; Taha, S.H.N. A systematic review and meta-analysis on chloroquine and hydroxychloroquine as monotherapy or combined with azithromycin in COVID-19 treatment. Sci. Rep. 2020, 10, 1–18. [Google Scholar] [CrossRef]
- Kashour, Z.; Riaz, M.; Garbati, M.A.; AlDosary, O.; Tlayjeh, H.; Gerberi, D.; Murad, M.H.; Sohail, M.R.; Kashour, T.; Tleyjeh, I.M. Efficacy of chloroquine or hydroxychloroquine in COVID-19 patients: A systematic review and meta-analysis. J. Antimicrob. Chemother. 2021, 76, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Fiolet, T.; Guihur, A.; Rebeaud, M.E.; Mulot, M.; Peiffer-Smadja, N.; Mahamat-Saleh, Y. Effect of hydroxychloroquine with or without azithromycin on the mortality of coronavirus disease 2019 (COVID-19) patients: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2021, 27, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Axfors, C.; Schmitt, A.M.; Janiaud, P.; van’t Hooft, J.; Abd-Elsalam, S.; Abdo, E.F.; Abella, B.S.; Akram, J.; Amaravadi, R.K.; Angus, D.C.; et al. Mortality outcomes with hydroxychloroquine and chloroquine in COVID-19 from an international collaborative meta-analysis of randomized trials. Nat. Commun. 2021, 12, 1–13. [Google Scholar]
- Hoffmann, M.; Mösbauer, K.; Hofmann-Winkler, H.; Kaul, A.; Kleine-Weber, H.; Krüger, N.; Gassen, N.C.; Müller, M.A.; Drosten, C.; Pöhlmann, S. Chloroquine does not inhibit infection of human lung cells with SARS-CoV-2. Nature 2020, 585, 588–590. [Google Scholar] [CrossRef] [PubMed]
- Rother, N.; Yanginlar, C.; Lindeboom, R.G.; Bekkering, S.; van Leent, M.M.; Buijsers, B.; Jonkman, I.; Graaf, M.; Baltissen, M.; Lamers, L.A.; et al. Hydroxychloroquine Inhibits the Trained Innate Immune Response to Interferons. Cell Rep. Med. 2020, 1, 100146. [Google Scholar] [CrossRef] [PubMed]
- Savarino, A.; Boelaert, J.R.; Cassone, A.; Majori, G.; Cauda, R. Effects of chloroquine on viral infections: An old drug against today’s diseases? Lancet Infect. Dis. 2003, 3, 722–727. [Google Scholar] [CrossRef]
- Touret, F.; de Lamballerie, X. Of chloroquine and COVID-19. Antivir. Res. 2020, 177, 104762. [Google Scholar] [CrossRef] [PubMed]





| Author, Year [Ref]/ Registration | Objective | Sample, Country, Population | Overall Key Patient Characteristics | Intervention | Comparison | Outcomes | Follow Up Time |
|---|---|---|---|---|---|---|---|
| Chen, J. et al., 2020 [16], NCT04261517 | To provide a database for exploring the next step in the effectiveness and safety of HCQ sulfate for COVID-19. | 30 (I: 15, C: 15), China, Confirmed COVID-19 patients who were hospitalized in Shanghai Public Health Clinical Center from 6 to 25 February 2020; Inclusion criteria: Age ≥18 years, COVID-19 was diagnosed according to the “diagnosis and treatment plan”.RT-PCR positive for COVID-19: NR% | Mean (SD) age: 49 (4) years Males: 70% Median (IQR) days from symptom onset to enrolment: NR No coexisting disease: 63% | Conventional treatment plus oral HCQ sulfate 400 mg qd for 5 days (total dose: 2000 mg). | Only conventional treatment, including bed rest, oxygen inhalation and symptomatic supportive treatment. | Primary: Virologic clearance of a throat swab, sputum or lower respiratory tract secretion on day 7 or death of the patient within 2 weeks. Secondary: Serious adverse drug event or a change in the subject’s condition within 2 weeks (heavy and critical). | 14 days |
| Chen, Z. et al., 2020 [17], ChiCTR2000029559 | To evaluate the efficacy of HCQ in the treatment of patients with COVID-19. | 62 (I: 31, C: 31), China, Patients with confirmed COVID-19 (diagnosis and classification of COVID-19 based on the criteria of the China National Health Commission). Inclusion criteria: Age ≥18 years; RT-PCR positive for SARS-CoV-2; Chest CT with pneumonia; SaO2/SpO2 ratio >93% or PaO2/FiO2 ratio >300 mmHg under the condition in the hospital room (mild illness).RT-PCR positive for COVID-19: 100% | Mean (SD) age: 45 (15) years Males: 47% Median (IQR) days from symptom onset to enrolment: NR No coexisting disease: NR | Oral HCQ sulfate tablets (Shanghai Pharma) 400 mg/d (200 mg bid) between days 1 and 5 plus standard treatment (total dose: 2000 mg). | Standard treatment only (O2 therapy, antiviral agents, antibacterial agents and immunoglobulin, with or without corticosteroids). | Primary: TTCR, changes in clinical characteristics. Secondary: radiological changes (chest CT) from day 0 to day 6. | 5 days |
| Tang et al., 2020 [18], ChiCTR2000029868 | To assess the efficacy and safety of HCQ plus standard-of-care compared with standard-of-care alone in adult patients with COVID-19. | 150 (I: 75, C: 75), China, Patients (age ≥18 years) with ongoing SARS-CoV-2 infection confirmed in upper or lower respiratory tract specimens with RT-PCR and hospitalized in 16 hospitals in China. RT-PCR positive for COVID-19: 100% | Mean (SD) age: 46 (15) years Males: 55% Mean (SD) days from disease onset to randomization: 17 (11) No coexisting disease: 70% | Oral HCQ in a loading dose of 1200 mg qd for 3 days followed by a maintenance dose of 800 mg qd for remaining days (total duration: 2 weeks mild/moderate (99% patients) plus standard of care (total dose: 12,400 mg). | SOC (aligned with updated national guidelines for COVID-19 in China). No specific details of standard of care. | Primary: negative conversion of SARS-CoV-2 in respiratory specimens by 28 days; clinical improvement in severe COVID-19 by 28 days. Secondary: negative conversion of SARS-CoV-2 at 4, 7, 10, 14 and 21days; adverse events; alleviation of clinical symptoms, laboratory parameters, all-cause death; and chest radiology (all by 28days). | 28 days |
| Chen, L. et al., 2020 [19], ChiCTR2000030054 | To evaluate the clinical utility of CQ and HCQ in treating COVID-19. | 67 (HCQ: 18, C: 12, CQ: 18, Excluded: 19), China, Individuals 18–75 years of age of Wuhan area, with mild or moderate COVID-19 based on the Chinese Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (5th–7th Editions). Patients had positive RT-PCR test for SARS-CoV-2 or lung changes characteristic of COVID-19 on chest CT scan; at admission, all patients had SaO2 >93% at FiO2 21%. RT-PCR positive for COVID-19: 63% | Mean (SD) age: 48 (15) years Males: 47% Mean (SD) days from disease onset to randomization: NR No coexisting disease: 57% | Standard treatment plus oral HCQ sulfate at 200 mg bid for 10 days (total dose: 4000 mg). | Standard treatment only (No details provided). | Primary: TTCR. Secondary: Time to SARS-CoV-2 RNA negativity; length of hospital stay; changes on chest CT scan; days of supplemental oxygenation; adverse events; clinical status; all-cause mortality; vital signs; laboratory testing. | 28 days |
| Cavalcanti et al., 2020 [20], NCT04322123 | To assess whether HCQ, ± AZ, would be effective in improving clinical status at 15 days after hospital admission due to mild-to-moderate COVID-19. | 665 (HCQ: 221, HCQ + AZ: 217, C: 227), Brazil, Patients ≥18 years of age who had been hospitalized with suspected or confirmed Covid-19 with 14 or fewer days since symptom onset.RT-PCR positive for COVID-19: 504 (75.8%) of all randomized (HCQ: 159, HCQ + AZ: 172, C: 173) | Mean (SD) age: 50 (15) years. Males: 58%. Median (IQR) days from symptom onset to randomization: 7 (5–9) No coexisting disease: 10%; O2 supplementation at baseline: 42% | SOC plus HCQ at 400 mg bid for 7 days (total dose: 5600 mg), or standard care plus HCQ at 400 mg bid plus AZ at 500 mg qd for 7 days (total dose: HCQ 5600 mg and AZ 3500 mg). | SOC (at the discretion of the treating physicians). Glucocorticoids, other immunomodulators, antibiotic agents and antiviral agents were allowed. | Primary: Clinical status using 7-point ordinal scale at 15 days. Secondary: Clinical status using 6-point ordinal scale; an indication for intubation; the receipt of supplemental oxygen administered; duration of hospital stay; in-hospital death; thromboembolic complications; acute kidney injury; the number of days alive and free from respiratory support. | 15 days |
| Horby et al., RECOVERY, 2020 [21], NCT04381936 | To evaluate the effects of HCQ versus usual care at 28 days in patients hospitalized with COVID-19. | 4716 (HCQ: 1561, C: 3155), UK, Hospitalized patients with clinically suspected or laboratory confirmed SARS-CoV-2 infection and no medical history that might put patients at substantial risk if they were to participate in the trials. There was an age limit (i.e., only adults), but this was removed on May 9, 2020.RT-PCR positive for COVID-19: 90% | Mean (SD) age: 65 (15) years Males: 62% Median (IQR) days since symptom onset to randomization: 9 (5–14) No coexisting disease: 43% | Usual SOC plus HCQ sulfate tablets at 200 mg (containing 155-mg base equivalent) in a loading dose of 4 tablets at baseline and at 6 h, and followed by 2 tablets starting at 12 h after the initial dose and then bid for the next 9 days or until discharge, whichever occurred earlier (total dose: 9200 mg) | Usual SOC (unspecified). | Primary: All-cause mortality at 28 days. Secondary: Time until discharge from the hospital, a composite of the initiation of invasive MV (extracorporeal membrane oxygenation, death among patients who were not receiving invasive MV at the time of randomization). | 28 days |
| Chen, C.-P. et al., 2020 [22], No registration | To evaluate HCQ efficacy and tolerability in adult patients with mild to moderate COVID-19. | 33 (HCQ: 21, SOC: 12), Taiwan, Patients positive for SARS-CoV-2 infection RT-PCR of 11 public hospitals in northern, central and southern Taiwan affiliated with the Ministry of Health and Welfare. RT-PCR positive for COVID-19: 100% | Mean (SD) age: 33 (11) years Males: 63% Mean (SD) days from disease onset to randomization: NR No coexisting disease: NR | SOC plus HCQ 400 mg bid on day 1 and 200 mg BID for 6 days (total dose: 3200 mg). | SOC: (1) ceftriaxone 2 g qd for 7 days ± AZ 500 mg on day 1 and 250 mg on days 2–5; or (2) levofloxacin 750 mg qd for 5 d; or (3) levofloxacin 500 mg qd; or (4) moxifloxacin 400 mg qd for 7–14 days for subjects allergic to ceftriaxone or AZ. | Primary: Time to negative RT-PCR assessments at 14 days. Secondary: Proportion of negative viral RT-PCR on hospital day 14, resolution of clinical symptoms (time to clinical recovery), proportion of discharges by day 14, mortality rate. | 14 days |
| Abd-Elsalam et al., 2020 [23], NCT04353336 | To evaluate the safety and efficacy of HCQ added to the standard of care versus the standard of care alone in patients with COVID-19. | 194 (HCQ + SOC: 97, SOC: 97), Egypt, Mild, moderate and severe (per WHO guidelines of March 2020) patients admitted to three tertiary centers with confirmed COVID-19 (i.e., SARS-CoV-2 infection) between March and June 2020.RT-PCR positive for COVID-19: 100% | Mean (SD) age: 40 (19) years Males: 59% Mean (SD) days from disease onset to randomization: NR No coexisting disease: 84% | SOC plus HCQ 400 mg BID in day 1 followed by 200 mg tablets bid for 15 days (total dose: 6800 mg). | SOC for 15d (paracetamol, O2 fluids), empiric antibiotic (cephalosporins), oseltamivir if needed (75 mg bid for 5 days), invasive MV with hydrocortisone for severe cases (PaO2 <60 mmHg, O2 saturation <90% despite O2 or NIV, progressive hypercapnia, respiratory acidosis progressive or refractory septic shock. | Primary: Recovery within 28 days, need for mechanical ventilation or death. Secondary: Duration to negative PCR, duration to clinical improvement, duration to hospital discharge. | 28 days |
| Self et al., ORCHID, 2020 [24], NCT04332991 | To test the hypothesis that, compared with placebo, HCQ improves clinical outcomes for adults hospitalized with COVID-19. | 479 (HCQ: 242, Placebo: 237), USA, Adults (aged ≥18 years) who were hospitalized for less than 48 h with laboratory-confirmed SARS-CoV-2 infection and symptoms of respiratory illness for less than 10 days.RT-PCR positive for COVID-19: 100% | Mean (SD) age: 57 (18) years Males: 56% Median (IQR) days of symptoms to randomization: 5 (3–7) No coexisting disease: NR | 400 mg HCQ sulfate pills bid for the first 2 doses followed by 200 mg bid for the subsequent 8 doses, for a total of 10 doses over 5 days (total dose: 2400 mg). | Matching placebo in the same dosing frequency. | Primary: Clinical status 14 days after randomization assessed with a 7-category ordinal scale (COVID Outcomes Scale) by the WHO. Secondary: Scores on the COVID Outcomes Scale, all-cause all-location mortality, time to recovery, composite of death or receipt of ECMO, support-free days. | 28 days |
| Dubee et al., HYCOVID, 2020 [25], NCT04325893 | To evaluate the efficacy and safety of HCQ in adult patients with mild-to-moderate COVID-19 at risk of worsening. | 250 (HCQ: 125, Placebo: 125), France/Monaco, Patients with COVID-19 confirmed by positive SARS-CoV-2 RT-PCR or positive CT scan and had ≥1 of the following risk factors for worsening: (i) age ≥75 years; (ii) age 60–74 years and presence of ≥1 of the following comorbidities: obesity (BMI ≥30 kg/m²), arterial hypertension requiring treatment, diabetes mellitus requiring treatment; (iii) need for O2 to reach a peripheral capillary SpO2 >94% or a PaO2/FiO2 ≤300 mmHg.RT-PCR positive for COVID-19: 99% | Mean (SD) age: 75 (21) years Males: 48% Median (IQR) days from onset to inclusion: 5 (3–9) No coexisting disease: NR | SOC plus oral HCQ (200 mg tablets, orally) at a dose of 2 tablets bid for day 1 followed by 1 tablet bid for8 days (total dose: 4000 mg). | Matching placebo at a dose of 2 tablets bid in day 1 followed by 1 tablet bid for 8 days plus SOC as needed. | Primary: Composite of death, the need for invasive MV within 14 days after randomization. Secondary: Mortality and clinical evolution at day 14 and 28, viral shedding at day 5 and 10. | 28 days |
| Kamran et al., 2020 [26], NCT04491994 | To analyze the efficacy of HCQ in addition to standard of care compared with standard of care alone in reducing disease progression in mild COVID-19. | 500 (HCQ + SOC: 349, SOC: 151), Pakistan, Hospitalized patients aged 18–80 years from both genders with Mild confirmed COVID-19 (positive RT-PCR of oropharyngeal and nasopharyngeal swabs). Mild disease was defined per WHO criteria.RT-PCR positive for COVID-19: 100% | Mean (SD) age: 34 (11) years Males: 93% Mean (SD) days from disease onset to randomization: NR No coexisting disease: NR | SOC plus oral HCQ 400 mg bid for day 1 followed by 200 mg bid for next 5 days (total dose: 2800 mg). | SOC (daily oral vitamin C, oral zinc, oral vitamin D, paracetamol and intravenous fluids). | Primary: Disease progression within 5 days of start of treatment (i.e., development of fever >101 F for >72 h, shortness of breath by minimal exertion (10- Step walk test), derangement of basic lab parameters (ALC <1000 or raised CRP) or appearance of infiltrates on CXR). Secondary: Viral clearance, PCR negativity on day 7 and 14 after admission was recorded. | 14 days |
| Pan et al., SOLIDARITY, 2020 [27], NCT04315948 | To help determine whether any of four repurposed antivirals could at least moderately affect in hospital mortality. | 11266 (HCQ: 954, no drug (SOC):4088, other drugs: 6288), 30 countries worldwide, Patients 18 years of age or older, were hospitalized with a diagnosis of Covid-19, were not known to have received any trial drug, were not expected to be transferred elsewhere within 72 h and, in the physician’s view, had no contraindication to any trial drug.RT-PCR positive for COVID-19: 100% | Age <70 years: 79%. Mean (SD): NR Males: 62% Mean (SD) days from disease onset to randomization: NR No coexisting disease: 44% | Oral HCQ sulfate 200 mg tablets (containing 155-mg base equivalent) at a dose of 4 tablets at hour 0, 4 tablets at hour 6. Starting at hour 12, 2 tablets bid for 10 days (total dose: 9600 mg). | SOC. No details of standard of care. Other interventions (remdesivir, lopinavir, interferon). | Primary: In-hospital mortality (i.e., death during the original hospitalization; follow-up ceased at discharge), regardless of whether death occurred before or after day 28. Secondary: Initiation of MV, hospitalization duration. | 28 days |
| Ulrich et al., TEACH, 2020 [28], NCT04369742 | To evaluate the efficacy and safety of HCQ in hospitalized patients with COVID-19. We hypothesized that HCQ is superior to placebo in preventing severe outcomes among hospitalized COVID-19 patients. | 128 (HCQ: 67, Placebo: 61), USA, Hospitalized patients with a positive RT-PCR for SARS-CoV-2 within 72 h of enrollment, and at least one COVID-19 symptom (e.g., fever, cough, dyspnea, nausea, diarrhea, myalgia, anosmia, dysgeusia) and the subject’s (or legally authorized representative’s), written informed consent.RT-PCR positive for COVID-19: 100%. | Mean (SD) age: 66 (16) years Males: 59% Median (IQR) days since symptom onset: 7 (10) No coexisting disease: 13% | Oral HCQ 400 mg (2 tablets) bid for day 1 and 200 mg (1 tablet) bid for days 2–5 (total dose: 2400 mg). | Placebo: calcium citrate 200-mg tablets, same schedule as HCQ. | Primary: Proportion of subjects meeting a severe COVID-19 progression composite end point (death, ICU admission, VM, ECMO and/or vasopressor use) at day 14. The primary safety outcome was serious adverse events (SAEs), grade 3 or 4 adverse events and/or discontinuation of therapy at day 30. Secondary: changes in an 8-point ordinal COVID-19 clinical severity score, the primary composite outcome and mortality at day 30, hospital LOS, fever-free days, oxygen-free days, laboratory outcomes. | 30 days |
| Outcomes | Anticipated Absolute Effects * (95% CI) | Relative Effect (95% CI) | № of Participants (Studies) | Certainty of the Evidence (GRADE) | |
|---|---|---|---|---|---|
| Risk with Control | Risk with Hydroxychloroquine | ||||
| All-cause mortality follow-up: mean 14 days | 6 per 100 | 7 per 100 | RR 1.07 | 3274 | ⨁⨁⨁◯ |
| (6 to 8) | (0.92 to 1.25) | (7 RCTs) | MODERATE a | ||
| All-cause mortality follow-up: mean 30 days | 20 per 100 | 22 per 100 | RR 1.08 | 7647 | ⨁⨁⨁◯ |
| (20 to 23) | (1.00 to 1.16) | (7 RCTs) | MODERATE b | ||
| Need for mechanical ventilation follow up: mean 14 days | 2 per 100 | 3 per 100 | RR 1.98 | 1419 | ⨁◯◯◯ |
| (0 to 25) | (0.24 to 16.22) | (3 RCTs) | VERY LOW c–e | ||
| Need for mechanical ventilation follow up: mean 30 days | 5 per 100 | 4 per 100 | RR 0.93 | 1048 | ⨁⨁⨁◯ |
| (3 to 7) | (0.61 to 1.41) | (4 RCTs) | MODERATE f | ||
| Need for high-flow nasal cannula or non-invasive ventilation follow up: mean 14 days | 2 per 100 | 2 per 100 | RR 1.06 | 1111 | ⨁◯◯◯ |
| (0 to 25) | (0.11 to 10.69) | (3 RCTs) | VERY LOW g–i | ||
| Need for ICU admission follow up: range 14 days to 30 days | 10 per 100 | 14 per 100 | RR 1.36 | 322 | ⨁◯◯◯ |
| (4 to 42) | (0.44 to 4.19) | (2 RCTs) | VERY LOW j–l | ||
| Need for supplementary oxygen follow up: mean 14 days | 5 per 100 | 6 per 100 | RR 1.26 | 1111 | ⨁⨁◯◯ |
| (3 to 14) | (0.58 to 2.73) | (3 RCTs) | LOW g,m | ||
| Clinical recovery assessed with: Alleviation of clinical symptoms, three consecutive negative PCR tests, discharge from hospital alive OR better ordinal scale at follow up (5 to 7 in 7-point scale or 0 to 2 in 8-point scale) follow up: mean 30 days | 53 per 100 | 67 per 100 | RR 1.26 | 441 | ⨁◯◯◯ |
| (46 to 97) | (0.87 to 1.83) | (2 RCTs) | VERY LOW n,o | ||
| Clinical worsening assessed with: Death or invasive mechanical ventilation follow up: mean 30 days | 26 per 100 | 30 per 100 | RR 1.14 | 4170 | ⨁⨁⨁⨁ |
| (29 to 31) | (1.11 to 1.18) | (2 RCTs) | HIGH | ||
| Discharge from hospital follow up: range 14 days to 30 days | 64 per 100 | 62 per 100 | RR 0.97 | 5569 | ⨁⨁◯◯ |
| (53 to 73) | (0.83 to 1.13) | (4 RCTs) | LOW p,q | ||
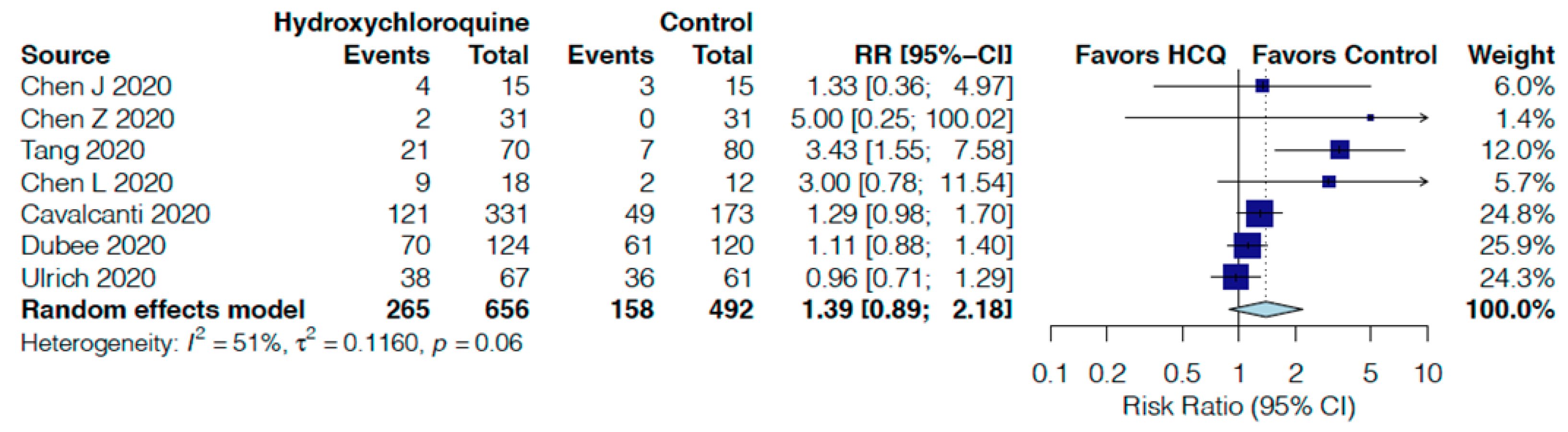
| Adverse events follow up: range 5 days to 30 days | 32 per 100 | 45 per 100 | RR 1.39 | 1148 | ⨁◯◯◯ |
| (29 to 70) | (0.89 to 2.18) | (7 RCTs) | VERY LOW r,s | ||
| Serious adverse events follow up: range 5 days to 30 days | 5 per 100 | 7 per 100 | RR 1.24 | 3786 | ⨁⨁⨁◯ |
| (6 to 8) | (1.05 to 1.46) | (9 RCTs) | MODERATE t | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernandez, A.V.; Phan, M.T.; Rocco, J.; Pasupuleti, V.; Barboza, J.J.; Piscoya, A.; Roman, Y.M.; White, C.M. Efficacy and Safety of Hydroxychloroquine for Hospitalized COVID-19 Patients: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 2503. https://doi.org/10.3390/jcm10112503
Hernandez AV, Phan MT, Rocco J, Pasupuleti V, Barboza JJ, Piscoya A, Roman YM, White CM. Efficacy and Safety of Hydroxychloroquine for Hospitalized COVID-19 Patients: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2021; 10(11):2503. https://doi.org/10.3390/jcm10112503
Chicago/Turabian StyleHernandez, Adrian V., Mi T. Phan, Jonathon Rocco, Vinay Pasupuleti, Joshuan J. Barboza, Alejandro Piscoya, Yuani M. Roman, and Charles M. White. 2021. "Efficacy and Safety of Hydroxychloroquine for Hospitalized COVID-19 Patients: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 10, no. 11: 2503. https://doi.org/10.3390/jcm10112503
APA StyleHernandez, A. V., Phan, M. T., Rocco, J., Pasupuleti, V., Barboza, J. J., Piscoya, A., Roman, Y. M., & White, C. M. (2021). Efficacy and Safety of Hydroxychloroquine for Hospitalized COVID-19 Patients: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 10(11), 2503. https://doi.org/10.3390/jcm10112503









