The Effect of the PCSK9 Inhibitor Evolocumab on Aldosterone Secretion among High Cardiovascular Risk Patients: A Pilot Study
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Procedure
2.2. ACTH Stimulation Test
2.3. Assays
2.4. Twenty-Four Hour Ambulatory Blood Pressure Monitoring
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. Changes in Lipid Profile
3.3. Changes in Steroidogenesis
3.4. Changes in 24-Hour ABPM
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miller, W.L. Steroidogenic Enzymes. Endocr. Dev. 2008, 13, 1–18. [Google Scholar] [CrossRef]
- Hooper, A.J.; van Bockxmeer, F.M.; Burnett, J.R. Monogenic hypocholesterolaemic lipid disorders and apolipoprotein B metabolism. Crit. Rev. Clin. Lab. Sci. 2005, 42, 515–545. [Google Scholar] [CrossRef] [PubMed]
- Arem, R.; Ghusn, H.; Ellerhorst, J.; Comstock, J.P. Effect of decreased plasma low-density lipoprotein levels on adrenal and testicular function in man. Clin. Biochem. 1997, 30, 419–424. [Google Scholar] [CrossRef]
- Krysiak, R.; Okopien, B. Chronic adrenal failure and hypergonadotropic hypogonadism in a patient with abetalipoproteinemia. Eur. Rev. Med. Pharmacol. Sci. 2012, 16 (Suppl. 4), 95–97. [Google Scholar]
- Everett, B.M.; Smith, R.J.; Hiatt, W.R. Reducing LDL with PCSK9 Inhibitors—The Clinical Benefit of Lipid Drugs. N. Engl. J. Med. 2015, 373, 1588–1591. [Google Scholar] [CrossRef]
- Raal, F.J.; Stein, E.A.; Dufour, R.; Turner, T.; Civeira, F.; Burgess, L.; Langslet, G.; Scott, R.; Olsson, A.G.; Sullivan, D.; et al. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): A randomised, double-blind, placebo-controlled trial. Lancet 2015, 385, 331–340. [Google Scholar] [CrossRef]
- Macchi, C.; Ferri, N.; Sirtori, C.R.; Corsini, A.; Banach, M.; Ruscica, M. PCSK9: A view beyond the canonical cholesterol lowering impact. Am. J. Pathol. 2021. [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation 2019, 139, e1082–e1143. [Google Scholar]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; de Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Ivanes, F.; Susen, S.; Mouquet, F.; Pigny, P.; Cuilleret, F.; Sautière, K.; Collet, J.-P.; Beygui, F.; Hennache, B.; Ennezat, P.V.; et al. Aldosterone, mortality, and acute ischaemic events in coronary artery disease patients outside the setting of acute myocardial infarction or heart failure. Eur. Heart J. 2012, 33, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Masana, L.; Girona, J.; Ibarretxe, D.; Rodríguez-Calvo, R.; Rosales, R.; Vallvé, J.-C.; Rodríguez-Borjabad, C.; Guardiola, M.; Rodríguez, M.; Guaita-Esteruelas, S.; et al. Clinical and pathophysiological evidence supporting the safety of extremely low LDL levels—The zero-LDL hypothesis. J. Clin. Lipidol. 2018, 12, 292–299.e3. [Google Scholar] [CrossRef]
- Greco, M.F.; Sirtori, C.R.; Corsini, A.; Ezhov, M.; Sampietro, T.; Ruscica, M. Lipoprotein(a) Lowering—From Lipoprotein Apheresis to Antisense Oligonucleotide Approach. J. Clin. Med. 2020, 9, 2103. [Google Scholar] [CrossRef]
- Ferrario, C.M.; Mullick, A.E. Renin angiotensin aldosterone inhibition in the treatment of cardiovascular disease. Pharmacol. Res. 2017, 125, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, J.R. Lipid management for the prevention of cardiovascular disease. Curr. Pharm. Des. 2011, 17, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Santini, S.A.; Carrozza, C.; Lulli, P.; Zuppi, C.; Tonolo, G.C.; Musumeci, S. Atorvastatin Treatment Does Not Affect Gonadal and Adrenal Hormones in Type 2 Diabetes Patients with Mild to Moderate Hypercholesterolemia. J. Atheroscler. Thromb. 2003, 10, 160–164. [Google Scholar] [CrossRef]
- Dobs, A.S.; Schrott, H.; Davidson, M.H.; Bays, H.; Stein, E.A.; Kush, D.; Wu, M.; Mitchel, Y.; Illingworth, R.D. Effects of high-dose simvastatin on adrenal and gonadal steroidogenesis in men with hypercholesterolemia. Metabolism 2000, 49, 1234–1238. [Google Scholar] [CrossRef]
- Dobs, A.S.; Miller, S.; Neri, G.; Weiss, S.; Tate, A.C.; Shapiro, D.R.; For the Simvastatin, T.A.M. and Pravastatin Male Gonadal Function Study Group. Effects of simvastatin and pravastatin on gonadal function in male hypercholesterolemic patients. Metabolism 2000, 49, 115–121. [Google Scholar] [CrossRef]
- Azzarito, C.; Boiardi, L.; Zini, M.; Agosti, A.; Dotti, C.; Biagi, R.; Portioli, I. Long-term therapy with high-dose simvastatin does not affect adrenocortical and gonadal hormones in hypercholesterolemic patients. Metabolism 1992, 41, 148–153. [Google Scholar] [CrossRef]
- Schooling, C.M.; Yeung, S.L.A.; Freeman, G.; Cowling, B.J. The effect of statins on testosterone in men and women, a systematic review and meta-analysis of randomized controlled trials. BMC Med. 2013, 11, 57. [Google Scholar] [CrossRef]
- Baudrand, R.; Pojoga, L.H.; Vaidya, A.; Garza, A.E.; Vöhringer, P.A.; Jeunemaitre, X.; Hopkins, P.N.; Yao, T.M.; Williams, J.; Adler, G.K.; et al. Statin Use and Adrenal Aldosterone Production in Hypertensive and Diabetic Subjects. Circulation 2015, 132, 1825–1833. [Google Scholar] [CrossRef]
- Toba, H.; Mitani, T.; Takahashi, T.; Imai, N.; Serizawa, R.; Wang, J.; Kobara, M.; Nakata, T. Inhibition of the renal renin-angiotensin system and renoprotection by pitavastatin in type 1 diabetes. Clin. Exp. Pharmacol. Physiol. 2010, 37, 1064–1070. [Google Scholar] [CrossRef]
- Barter, P.J.; Caulfield, M.; Eriksson, M.; Grundy, S.M.; Kastelein, J.J.; Komajda, M.; Lopez-Sendon, J.; Mosca, L.; Tardif, J.C.; Waters, D.D.; et al. Effects of torsetrapib in patients at high risk for coronary events. N. Engl. J. Med. 2007, 357, 2109–2122. [Google Scholar] [CrossRef] [PubMed]
- Blom, D.J.; Djedjos, C.S.; Monsalvo, M.L.; Bridges, I.; Wasserman, S.M.; Scott, R.; Roth, E. Effects of evolocumab on vitamin E and steroid hormone levels results from the 52-week, phase 3, double-blind, randomized, placebo-controlled DESCARTES Study. Circ. Res. 2015, 117, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.G.; Bessac, L.; Berdan, L.G.; Bhatt, D.L.; Bittner, V.; Diaz, R.; Goodman, S.G.; Hanotin, C.; Harrington, R.A.; Jukema, J.W.; et al. Effect of alirocumab, a monoclonal antibody to PCSK9, on long-term cardiovascular outcomes following acute coronary syndromes: Rationale and design of the ODYSSEY Outcomes trial. Am. Heart J. 2014, 168, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Bonaca, M.P.; Nault, P.; Giugliano, R.P.; Keech, A.C.; Pineda, A.L.; Kanevsky, E.; Kuder, J.; Murphy, S.A.; Jukema, J.W.; Lewis, B.S.; et al. Low-Density Lipoprotein Cholesterol Lowering With Evolocumab and Outcomes in Patients With Peripheral Artery Disease: Insights from the FOURIER Trial (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk). Circulation 2018, 137, 338–350. [Google Scholar] [CrossRef] [PubMed]
| Variable | Value |
|---|---|
| Median age, y (Q1–Q3) | 63.8 (59.5–69.6) |
| Male, n (%) | 7 (47) |
| Body mass index, median (Q1–Q3) | 29.4 (27.7–33.9) |
| Weight, kg, median (Q1–Q3) | 86.3 (69.5–97.2) |
| Hyperlipidemia, n (%) | 15 (100) |
| Diabetes mellitus, n (%) | 6 (40) |
| Hypertension, n (%) | 9 (60) |
| Past myocardial infarction, n (%) | 9 (60) |
| Smoking, n (%) | 1 (7) |
| Family history of ischemic heart disease, n (%) | 9 (60) |
| Parameter | Baseline Median (Q1–Q3) | End of Study Median (Q1–Q3) | Difference Median (Q1–Q3) | p Value |
|---|---|---|---|---|
| FBG, mg/dL | 90 (80–107) | 87 (79–98) | −3 (−14–10) | 0.176 |
| Potassium, mmol/L | 4.4 (4.2–4.6) | 4.4 (4.2–4.7) | 0 (−0.6–0.3) | 0.889 |
| Total cholesterol, mg/dL | 219 (156–247) | 136 (118–162) | −55 (−99–−41) | 0.001 |
| HDL cholesterol, mg/dL | 46 (37–64) | 47 (38–59) | 1 (−2–3) | 0.706 |
| LDL cholesterol, mg/dL | 127 (93–153) | 63 (43–73) | −65 (−92–−41) | 0.001 |
| Triglycerides, mg/dL | 146 (97–190) | 114 (101–181) | 1 (−35–7) | 0.496 |
| Lipoprotein (a), mg/dL | 66 (17–92) | 52 (11–83) | −11 (−120–−5) | 0.002 |
| Apo A1, mg/dL | 144 (123–172) | 149 (135–158) | 4 (−4–17) | 0.505 |
| Apo B100, mg/dL | 117 (74–150) | 65 (51–81) | −50 (−65–−13) | 0.002 |
| Parameter | Baseline | End of Study | Difference | p Value |
|---|---|---|---|---|
| ACTH, pg/mL | ||||
| Baseline | 17.6 (6.7–23.4) | 18.0 (11.1–24.1) | 0.1 (−4.1–5.9) | 0.638 |
| Cortisol, mcg/dL | ||||
| Baseline | 13 (10.3–15.8) | 12.3 (8.9–19.3) | 0.1 (−2.5–1.7) | 0.975 |
| 30 min | 30 (25.2–32.4) | 28.1 (23.9–37.2 | 0.9 (−2.6–4.3) | 0.552 |
| 60 min | 33.9 (29.9–38) | 31.1 (28.4–41.1) | −0.1 (−2.4–3.1) | 0.701 |
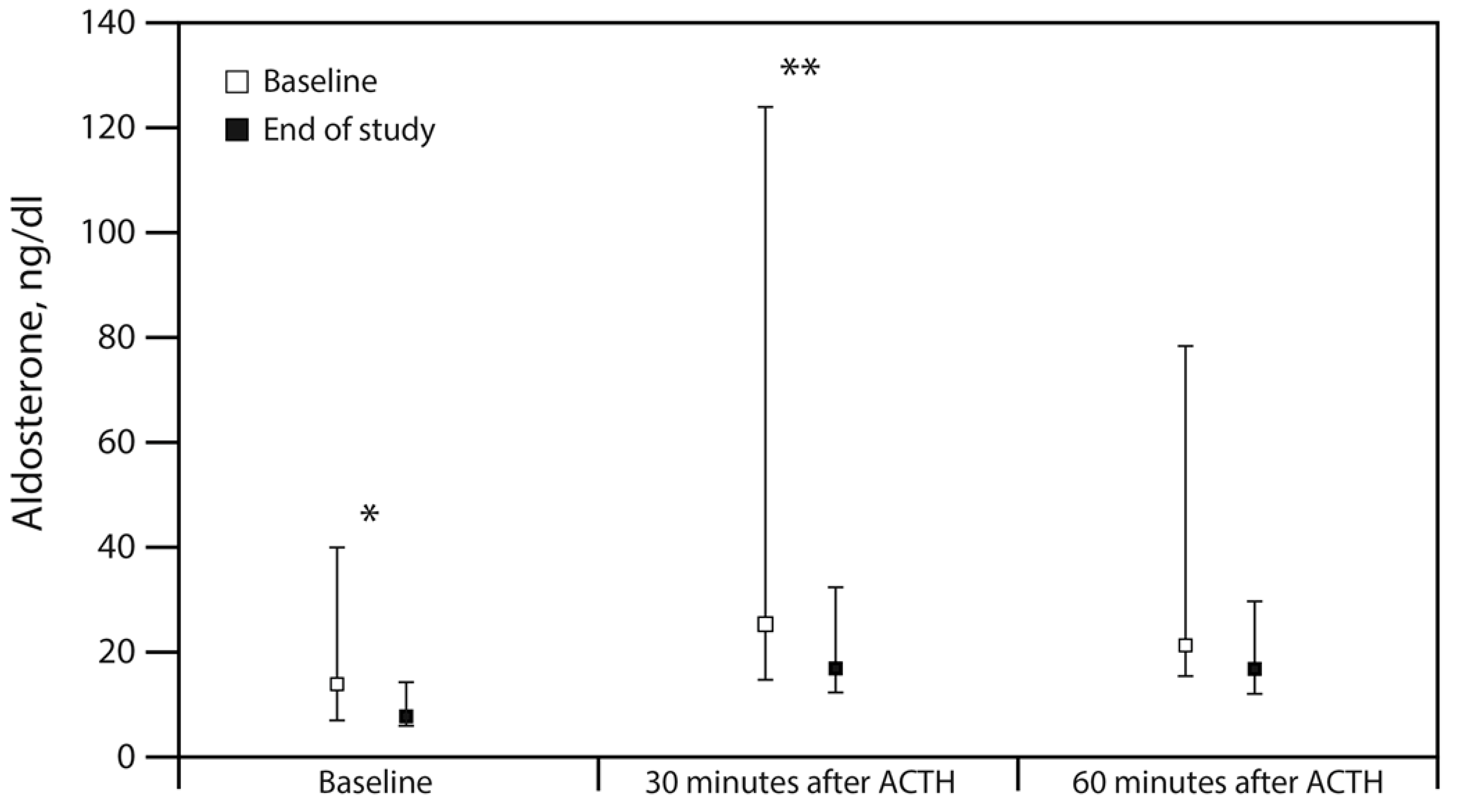
| Aldosterone, ng/dL | ||||
| Baseline | 13.9 (6.7–40.4) | 7.9 (5.6–14.5) | −2.9 (−37.6–-0.3) | 0.036 |
| 30 min | 25.2 (14.6–124.3) | 16.9 (12.0–32.6) | −3.4 (−116.3–−1.1) | 0.008 |
| 60 min | 21.3 (15.2–78.6) | 16.8 (12.2–29.8) | 3.9 (−36.5–1.1) | 0.064 |
| PRA, ng/mL/h | ||||
| Baseline | 1.5 (0.8–3.6) | 1.5 (0.8–3.6) | −0.2 (−1.0–0.6) | 0.65 |
| 30 min | 1.2 (0.3–3.8) | 1.2 (0.3–3.8) | 0.3 (−0.2–1.1) | 0.328 |
| 60 min | 0.8 (0.6–3) | 0.8 (0.6–3) | −0.2 (−0.7–0.8) | 0.972 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izkhakov, E.; Shacham, Y.; Serebro, M.; Yaish, I.; Marcus, Y.; Shefer, G.; Tordjman, K.; Greenman, Y.; Stern, N.; Ziv-Baran, T. The Effect of the PCSK9 Inhibitor Evolocumab on Aldosterone Secretion among High Cardiovascular Risk Patients: A Pilot Study. J. Clin. Med. 2021, 10, 2504. https://doi.org/10.3390/jcm10112504
Izkhakov E, Shacham Y, Serebro M, Yaish I, Marcus Y, Shefer G, Tordjman K, Greenman Y, Stern N, Ziv-Baran T. The Effect of the PCSK9 Inhibitor Evolocumab on Aldosterone Secretion among High Cardiovascular Risk Patients: A Pilot Study. Journal of Clinical Medicine. 2021; 10(11):2504. https://doi.org/10.3390/jcm10112504
Chicago/Turabian StyleIzkhakov, Elena, Yacov Shacham, Merav Serebro, Iris Yaish, Yonit Marcus, Gabi Shefer, Karen Tordjman, Yona Greenman, Naftali Stern, and Tomer Ziv-Baran. 2021. "The Effect of the PCSK9 Inhibitor Evolocumab on Aldosterone Secretion among High Cardiovascular Risk Patients: A Pilot Study" Journal of Clinical Medicine 10, no. 11: 2504. https://doi.org/10.3390/jcm10112504
APA StyleIzkhakov, E., Shacham, Y., Serebro, M., Yaish, I., Marcus, Y., Shefer, G., Tordjman, K., Greenman, Y., Stern, N., & Ziv-Baran, T. (2021). The Effect of the PCSK9 Inhibitor Evolocumab on Aldosterone Secretion among High Cardiovascular Risk Patients: A Pilot Study. Journal of Clinical Medicine, 10(11), 2504. https://doi.org/10.3390/jcm10112504






