Effects of Monotherapy with Clopidogrel vs. Aspirin on Vascular Function and Hemostatic Measurements in Patients with Coronary Artery Disease: The Prospective, Crossover I-LOVE-MONO Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
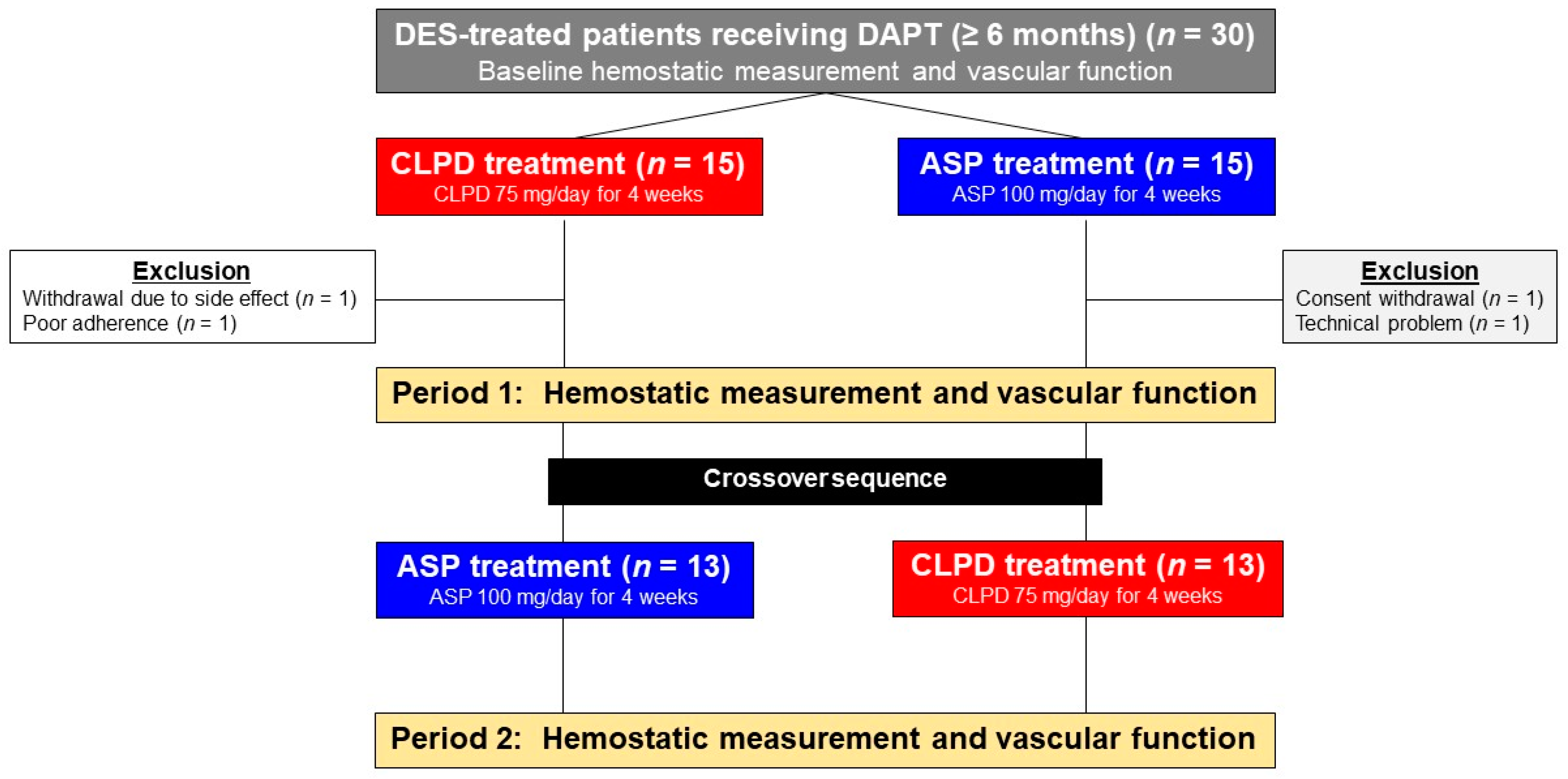
2.2. Study Design
2.3. Hemostatic Measurements
2.3.1. VerifyNow Assay
2.3.2. Thromboelastography (TEG)
2.4. Vascular Function
2.4.1. Reactive Hyperemia-Peripheral Arterial Tonometry (RH-PAT)
2.4.2. Arterial Stiffness Indices
2.5. Endpoints
2.6. Statistical Analysis
3. Results
3.1. Study Population
3.2. Primary Endpoint
3.3. Secondary Endpoint
3.4. Determinants for Normal Endothelial Function (RHI ≥ 2.1)
4. Discussion
4.1. Monotherapy with P2Y12 Inhibitor vs. Aspirin in ASCVD Patients
4.2. Effect of P2Y12 Receptor Inhibitor on Endothelial Function
4.3. Relationship between TEG Measurements and Platelet/Endothelial Function
4.4. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Levine, G.N.; Bates, E.R.; Bittl, J.A.; Brindis, R.G.; Fihn, S.D.; Fleisher, L.A.; Granger, C.B.; Lange, R.A.; Mack, M.J.; Mauri, L.; et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2016, 68, 1082–1115. [Google Scholar] [CrossRef]
- Antithrombotic Trialists’ (ATT) Collaboration; Baigent, C.; Blackwell, L.; Collins, R.; Emberson, J.; Godwin, J.; Peto, R.; Buring, J.; Hennekens, C.; Kearney, P.; et al. Aspirin in the primary and secondary prevention of vascular disease: Collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009, 373, 1849–1860. [Google Scholar]
- Chiarito, M.; Sanz-Sánchez, J.; Cannata, F.; Cao, D.; Sturla, M.; Panico, C.; Godino, C.; Regazzoli, D.; Reimers, B.; De Caterina, R.; et al. Monotherapy with a P2Y(12) inhibitor or aspirin for secondary prevention in patients with established atherosclerosis: A systematic review and meta-analysis. Lancet 2020, 395, 1487–1495. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Angiolillo, D.J.; Rollini, F.; Storey, R.; Bhatt, D.L.; James, S.; Schneider, D.J.; Sibbing, D.; So, D.Y.; Trenk, D.; Alexopoulos, D.; et al. International Expert Consensus on Switching Platelet P2Y(12) Receptor-Inhibiting Therapies. Circulation 2017, 136, 1955–1975. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Domei, T.; Morimoto, T.; Natsuaki, M.; Shiomi, H.; Toyota, T.; Ohya, M.; Suwa, S.; Takagi, K.; Nanasato, M.; et al. Effect of 1-Month Dual Antiplatelet Therapy Followed by Clopidogrel vs 12-Month Dual Antiplatelet Therapy on Cardiovascular and Bleeding Events in Patients Receiving PCI: The STOPDAPT-2 Randomized Clinical Trial. JAMA 2019, 321, 2414–2427. [Google Scholar] [CrossRef]
- Hahn, J.Y.; Song, Y.B.; Oh, J.H.; Chun, W.J.; Park, Y.H.; Jang, W.J.; Im, E.S.; Jeong, J.O.; Cho, B.R.; Oh, S.K.; et al. Effect of P2Y12 Inhibitor Monotherapy vs Dual Antiplatelet Therapy on Cardiovascular Events in Patients Undergoing Percutaneous Coronary Intervention: The SMART-CHOICE Randomized Clinical Trial. JAMA 2019, 321, 2428–2437. [Google Scholar] [CrossRef] [PubMed]
- Park, T.K.; Song, Y.B.; Ahn, J.; Carriere, K.C.; Hahn, J.Y.; Yang, J.H.; Choi, S.H.; Choi, J.H.; Lee, S.H.; Gwon, H.C. Clopidogrel Versus Aspirin as an Antiplatelet Monotherapy After 12-Month Dual-Antiplatelet Therapy in the Era of Drug-Eluting Stents. Circ. Cardiovasc. Interv. 2016, 9, e002816. [Google Scholar] [CrossRef]
- Koo, B.-K.; Kang, J.; Park, K.W.; Rhee, T.-M.; Yang, H.-M.; Won, K.-B.; Rha, S.-W.; Bae, J.-W.; Lee, N.H.; Hur, S.-H.; et al. Aspirin versus clopidogrel for chronic maintenance monotherapy after percutaneous coronary intervention (HOST-EXAM): An investigator-initiated, prospective, randomised, open-label, multicentre trial. Lancet 2021. [Google Scholar] [CrossRef]
- A-CLOSE. A Randomized Comparison of CLOpidogrel Monotherapy Versus Extended Dual-antiplatelet Therapy beyond 12 Months after Implantation of Drug-Eluting StEnts in High-Risk Lesions or Patients Trial. Available online: https://clinicaltrialsgov/ct2/show/NCT03947229 (accessed on 28 May 2021).
- Xu, Y.; Arora, R.C.; Hiebert, B.M.; Lerner, B.; Szwajcer, A.; Macdonald, K.; Rigatto, C.; Komenda, P.; Sood, M.; Tangri, N. Non-invasive endothelial function testing and the risk of adverse outcomes: A systematic review and meta-analysis. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Adamski, P.; Kozinski, M.; Ostrowska, M.; Fabiszak, T.; Navarese, E.P.; Paciorek, P.; Grześk, G.; Kubica, J. Overview of pleiotropic effects of platelet P2Y12 receptor inhibitors. Thromb. Haemost. 2014, 112, 224–242. [Google Scholar] [PubMed]
- Willoughby, S.R.; Luu, L.J.; Cameron, J.D.; Nelson, A.J.; Schultz, C.D.; Worthley, S.G.; Worthley, M.I. Clopidogrel improves microvascular endothelial function in subjects with stable coronary artery disease. Heart Lung Circ. 2014, 23, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Patti, G.; Grieco, D.; Dicuonzo, G.; Pasceri, V.; Nusca, A.; Di Sciascio, G. High versus standard clopidogrel maintenance dose after percutaneous coronary intervention and effects on platelet inhibition, endothelial function, and inflammation results of the ARMYDA-150 mg (antiplatelet therapy for reduction of myocardial damage during angioplasty) randomized study. J. Am. Coll. Cardiol. 2011, 57, 771–778. [Google Scholar]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.H.; Bliden, K.P.; Antonino, M.J.; Park, K.S.; Tantry, U.S.; Gurbel, P.A. Usefulness of the VerifyNow P2Y12 assay to evaluate the antiplatelet effects of ticagrelor and clopidogrel therapies. Am. Heart J. 2012, 164, 35–42. [Google Scholar] [CrossRef]
- Jeong, Y.H.; Bliden, K.P.; Shuldiner, A.R.; Tantry, U.S.; Gurbel, P.A. Thrombin-induced platelet-fibrin clot strength: Relation to high on-clopidogrel platelet reactivity, genotype, and post-percutaneous coronary intervention outcomes. Thromb. Haemost. 2014, 111, 713–724. [Google Scholar] [CrossRef]
- Tanaka, A.; Tomiyama, H.; Maruhashi, T.; Matsuzawa, Y.; Miyoshi, T.; Kabutoya, T.; Kario, K.; Sugiyama, S.; Munakata, M.; Ito, H.; et al. Physiological Diagnostic Criteria for Vascular Failure. Hypertension 2018, 72, 1060–1071. [Google Scholar] [CrossRef]
- Park, H.-W.; Kim, H.-R.; Kang, M.G.; Kim, K.; Koh, J.-S.; Park, J.R.; Hwang, S.-J.; Jeong, Y.-H.; Ahn, J.H.; Park, Y.; et al. Predictive value of the combination of brachial-ankle pulse wave velocity and ankle-brachial index for cardiovascular outcomes in patients with acute myocardial infarction. Coron. Artery Dis. 2020, 31, 157–165. [Google Scholar] [CrossRef]
- Bonaca, M.P.; Bhatt, D.L.; Cohen, M.; Steg, P.G.; Storey, R.; Jensen, E.C.; Magnani, G.; Bansilal, S.; Fish, M.P.; KyungAh Steering Committee and Investigators PEGASUS-TIMI 54; et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N. Engl. J. Med. 2015, 372, 1791–1800. [Google Scholar] [CrossRef]
- Cesaro, A.; Taglialatela, V.; Gragnano, F.; Moscarella, E.; Fimiani, F.; Conte, M.; Barletta, V.; Monda, E.; Limongelli, G.; Severino, S.; et al. Low-Dose Ticagrelor in Patients With High Ischemic Risk and Previous Myocardial Infarction: A Multicenter Prospective Real-World Observational Study. J. Cardiovasc. Pharmacol. 2020, 76, 173–180. [Google Scholar] [CrossRef]
- Kim, H.K.; Tantry, U.S.; Smith, S.C., Jr.; Jeong, M.H.; Park, S.-J.; Kim, M.H.; Lim, D.-S.; Shin, E.-S.; Park, D.-W.; Huo, Y.; et al. The East Asian Paradox: An Updated Position Statement on the Challenges to the Current Antithrombotic Strategy in Patients with Cardiovascular Disease. Thromb. Haemost. 2021, 121, 422–432. [Google Scholar]
- Jeong, Y.H.; Smith, S.C., Jr.; Gurbel, P.A. Long-term use of ticagrelor in patients with prior myocardial infarction. N. Engl. J. Med. 2015, 373, 1273–1274. [Google Scholar]
- CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 1996, 348, 1329–1339. [Google Scholar] [CrossRef]
- Kim, H.K.; Ahn, Y.; Chang, K.; Jeong, Y.H.; Hahn, J.Y.; Choo, E.H.; Kim, M.C.; Kim, H.S.; Kim, W.; Cho, M.C.; et al. 2020 Korean Society of Myocardial Infarction Expert Consensus Document on Pharmacotherapy for Acute Myocardial Infarction. Korean Circ. J. 2020, 50, 845–866. [Google Scholar] [CrossRef]
- Schäfer, A.; Fraccarollo, D.; Pförtsch, S.; Loch, E.; Neuser, J.; Vogt, C.; Bauersachs, J. Clopidogrel improves endothelial function and NO bioavailability by sensitizing adenylyl cyclase in rats with congestive heart failure. Basic Res. Cardiol. 2011, 106, 485–494. [Google Scholar] [CrossRef]
- Bundhoo, S.S.; Anderson, R.A.; Sagan, E.; Hassan, N.; Pinder, A.G.; Rogers, S.C.; Morris, K.; James, P.E. Direct formation of thienopyridine-derived nitrosothiols--just add nitrite! Eur. J. Pharmacol. 2011, 670, 534–540. [Google Scholar] [CrossRef]
- Heitzer, T.; Rudolph, V.; Schwedhelm, E.; Karstens, M.; Sydow, K.; Ortak, M.; Tschentscher, P.; Meinertz, T.; Böger, R.; Baldus, S. Clopidogrel improves systemic endothelial nitric oxide bioavailability in patients with coronary artery disease: Evidence for antioxidant and antiinflammatory effects. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1648–1652. [Google Scholar] [CrossRef]
- Takajo, Y.; Ikeda, H.; Haramaki, N.; Murohara, T.; Imaizumi, T. Augmented oxidative stress of platelets in chronic smokers. Mechanisms of impaired platelet-derived nitric oxide bioactivity and augmented platelet aggregability. J. Am. Coll. Cardiol. 2001, 38, 1320–1327. [Google Scholar] [CrossRef]
- Fujisue, K.; Sugiyama, S.; Ono, T.; Matsuzawa, Y.; Akiyama, E.; Sugamura, K.; Matsubara, J.; Kurokawa, H.; Kaikita, K.; Iwashita, S.; et al. Effects of endothelial dysfunction on residual platelet aggregability after dual antiplatelet therapy with aspirin and clopidogrel in patients with stable coronary artery disease. Cir. Cardiovasc. Interv. 2013, 6, 452–459. [Google Scholar] [CrossRef]
- Jeong, H.S.; Hong, S.J.; Cho, S.A.; Kim, J.H.; Cho, J.Y.; Lee, S.H.; Joo, H.J.; Park, J.H.; Yu, C.W.; Lim, D.S. Comparison of Ticagrelor Versus Prasugrel for Inflammation, Vascular Function, and Circulating Endothelial Progenitor Cells in Diabetic Patients With Non-ST-Segment Elevation Acute Coronary Syndrome Requiring Coronary Stenting: A Prospective, Randomized, Crossover Trial. JACC Cardiovasc. Interv. 2017, 10, 1646–1658. [Google Scholar]
- Ariotti, S.; Ortega-Paz, L.; van Leeuwen, M.; Brugaletta, S.; Leonardi, S.; Akkerhuis, K.M.; Rimoldi, S.F.; Janssens, G.; Gianni, U.; van den Berge, J.C.; et al. Effects of Ticagrelor, Prasugrel, or Clopidogrel on Endothelial Function and Other Vascular Biomarkers: A Randomized Crossover Study. JACC Cardiovasc. Interv. 2018, 11, 1576–1586. [Google Scholar] [CrossRef]
- Schnorbus, B.; Daiber, A.; Jurk, K.; Warnke, S.; Koenig, J.; Lackner, K.J.; Münzel, T.; Gori, T. Effects of clopidogrel vs. prasugrel vs. ticagrelor on endothelial function, inflammatory parameters, and platelet function in patients with acute coronary syndrome undergoing coronary artery stenting: A randomized, blinded, parallel study. Eur. Heart J. 2020, 41, 3144–3152. [Google Scholar] [CrossRef]
- Gurbel, P.A.; Bliden, K.P.; Guyer, K.; Aggarwal, N.; Tantry, U.S. Delayed thrombin-induced platelet-fibrin clot generation by clopidogrel: A new dose-related effect demonstrated by thrombelastography in patients undergoing coronary artery stenting. Thromb. Res. 2007, 119, 563–570. [Google Scholar] [CrossRef]
- Leon, C.; Ravanat, C.; Freund, M.; Cazenave, J.P.; Gachet, C. Differential involvement of the P2Y1 and P2Y12 receptors in platelet procoagulant activity. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1941–1947. [Google Scholar] [CrossRef]
- Park, Y.; Kim, K.H.; Kang, M.G.; Ahn, J.-H.; Jang, J.Y.; Park, H.W.; Koh, J.-S.; Park, J.-R.; Hwang, S.-J.; Jeong, Y.-H.; et al. Antiplatelet Therapy Combinations and Thrombogenicity in Patients with Non-Valvular Atrial Fibrillation. Korean Circ. J. 2017, 47, 366–376. [Google Scholar] [CrossRef]
- Jeong, Y.-H.; Kevin, B.; Ahn, J.-H.; Chaudhary, R.; Kang, M.G.; Park, H.W.; Koh, J.-S.; Park, Y.; Tantry, U.S.; Gurbel, P.A. Viscoelastic properties of clot formation and their clinical impact in East Asian versus Caucasian patients with stable coronary artery disease: A COMPARE-RACE analysis. J. Thromb. Thrombolysis 2021, 51, 454–465. [Google Scholar] [CrossRef]
- Kattula, S.; Byrnes, J.R.; Wolberg, A.S. Fibrinogen and Fibrin in Hemostasis and Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2017, 37, e13–e21. [Google Scholar] [CrossRef]


| R, minutes | Time from the initiation of the until the point where the clot begins to form |
| K, minutes | Interval from the split point of the test to the point where the fibrin cross-linking provides enough clot resistance to produce a 20-mm amplitude |
| Angle, degree | Angle formed by the slope of a tangent line traced from the R time to the K time reflects the rate at which the clot forms |
| MA, mm | Maximum amplitude of the clot dynamics, reflecting ‘platelet-fibrin clot strength’ |
| LY30, % | Percentage of the clot that has lysed 30 min after the time of MA |
| Variables | (n = 26) |
|---|---|
| Duration of DAPT, months | 12 (11, 17) |
| Age, year old | 60.4 ± 7.6 |
| Male, n (%) | 24 (92.3) |
| BMI, kg/m2 | 25.4 ± 2.5 |
| Index disease entity | |
| Stable angina | 5 (19.2) |
| Unstable angina | 6 (23.1) |
| Non-ST-segment elevation MI | 10 (38.5) |
| ST-segment elevation MI | 5 (19.2) |
| Risk factors or past history, n (%) | |
| Diabetes mellitus | 8 (30.8) |
| Hypertension | 14 (53.8) |
| Dyslipidemia | 15 (57.7) |
| Current smoking | 7 (26.9) |
| Chronic kidney disease | 1 (3.8) |
| Peripheral artery disease | 3 (11.5) |
| Previous stroke | 2 (7.7) |
| Concomitant medication, n (%) | |
| Aspirin | 26 (100) |
| Clopidogrel | 26 (100) |
| Statin | 26 (100) |
| Beta blocker | 20 (76.9) |
| Angiotensin blockade | 22 (84.6) |
| Calcium channel blocker | 6 (23.1) |
| Proton pump inhibitor | 21 (80.8) |
| Laboratory data | |
| WBC count, ×103/mm3 | 6.6 ± 1.9 |
| Hemoglobin, g/dL | 14.6 ± 1.2 |
| Platelet count, ×103/mm3 | 229 ± 34 |
| HbA1C, % | 6.0 ± 0.6 |
| GFR, ml/min/1.73 m2 (MDRD) | 90 ± 18 |
| Total cholesterol, mg/dL | 135 ± 38 |
| LV ejection fraction, % | 60 ± 6 |
| Procedural data | |
| Multivessel disease, n (%) | 15 (57.7) |
| Multivessel stenting, n (%) | 9 (34.6) |
| Drug-eluting stent implantation, n (%) | 26 (100) |
| Target lesion, n (%) | |
| Left anterior descending | 17 (65.4) |
| Left circumflex | 2 (7.7) |
| Right coronary | 7 (26.9) |
| Stent number | 1.7 ± 0.7 |
| DAPT (CLPD+ASP) | Monotherapy (CLPD) | Monotherapy (ASP) | p (DAPT vs. CLPD) | p (DAPT vs. ASP) | p (CLPD vs. ASP) | |
|---|---|---|---|---|---|---|
| EndoPAT | ||||||
| RHI, % | 2.19 ± 0.68 | 2.11 ± 0.77 | 1.87 ± 0.72 | 0.479 | 0.023 | 0.045 |
| LnRHI | 0.74 ± 0.32 | 0.68 ± 0.37 | 0.55 ± 0.35 | 0.331 | 0.002 | 0.015 |
| Arterial stiffness indices | ||||||
| Brachial SBP, mmHg | 132.9 ± 17.3 | 130.0 ± 18.1 | 125.5 ± 19.6 | 0.559 | 0.152 | 0.386 |
| Brachial DBP, mmHg | 76.2 ± 10.3 | 74.2 ± 10.1 | 73.4 ± 11.0 | 0.491 | 0.346 | 0.773 |
| Brachial PP, mmHg | 56.0 ± 10.7 | 55.9 ± 12.0 | 52.1 ± 12.7 | 0.961 | 0.239 | 0.282 |
| Central SBP, mmHg | 138.7 ± 19.7 | 134.7 ± 20.7 | 130.4 ± 21.6 | 0.474 | 0.152 | 0.466 |
| Central DBP, mmHg | 76.2 ± 10.3 | 74.2 ± 10.1 | 73.4 ± 11.0 | 0.491 | 0.346 | 0.773 |
| Central PP, mmHg | 62.6 ± 14.0 | 60.5 ± 14.1 | 57.0 ± 13.9 | 0.597 | 0.159 | 0.377 |
| AI, % | 83.5 ± 12.2 | 81.6 ± 12.4 | 81.6 ± 11.6 | 0.583 | 0.570 | 1.000 |
| AI@75, % | 77.0 ± 11.7 | 75.4 ± 11.6 | 75.8 ± 11.1 | 0.602 | 0.690 | 0.893 |
| PWV (mean), m/sec | 15.9 ± 2.8 | 15.5 ± 2.8 | 15.2 ± 2.6 | 0.620 | 0.327 | 0.642 |
| Ankle-brachial index (mean) | 1.14 ± 0.07 | 1.12 ± 0.07 | 1.12 ± 0.08 | 0.565 | 0.344 | 0.662 |
| VerifyNow P2Y12 assay | ||||||
| PRU | 138 ± 77 | 130 ± 64 | 214 ± 50 | 0.178 | <0.001 | <0.001 |
| BASE | 218 ± 39 | 208 ± 35 | 206 ± 36 | 0.102 | 0.133 | 0.639 |
| Thromboelastography | ||||||
| R, minutes | 5.8 ± 1.3 | 5.5 ± 1.2 | 5.1 ± 1.1 | 0.379 | 0.017 | 0.037 |
| K, minutes | 1.9 ± 0.8 | 1.9 ± 0.7 | 1.6 ± 0.5 | 0.727 | 0.124 | 0.157 |
| Angle, degree | 61.4 ± 8.5 | 62.9 ± 9.0 | 65.2 ± 8.1 | 0.441 | 0.145 | 0.346 |
| MAthrombin, mm | 60.3 ± 5.9 | 59.9 ± 5.2 | 61.0 ± 5.2 | 0.748 | 0.603 | 0.388 |
| LY30, % | 1.2 ± 1.7 | 1.1 ± 1.7 | 1.4 ± 2.4 | 0.684 | 0.722 | 0.490 |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| Arterial stiffness indices | ||||||
| Central SBP ≥ 121 mmHg | 4.687 | 1.240–17.715 | 0.016 | 3.780 | 0.605–23.634 | 0.155 |
| Central PP ≥ 63 mmHg | 3.606 | 1.367–9.513 | 0.010 | 0.939 | 0.225–3.924 | 0.931 |
| PWV ≥ 14.5 m/sec | 5.538 | 1.676–18.298 | 0.005 | 3.834 | 0.887–16.581 | 0.072 |
| VerifyNow test | ||||||
| PRU ≤ 132 | 3.555 | 1.307–9.621 | 0.013 | 4.028 | 1.087–14.925 | 0.037 |
| Thromboelastography | ||||||
| Angle ≤ 68 degree | 9.414 | 2.010–44.087 | 0.004 | 7.420 | 1.445–38.099 | 0.016 |
| LY30 ≥ 1.5% | 3.417 | 1.208–9.662 | 0.021 | 2.790 | 0.755–10.312 | 0.124 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.-W.; Kang, M.-G.; Ahn, J.-H.; Bae, J.-S.; Tantry, U.S.; Gurbel, P.A.; Jeong, Y.-H. Effects of Monotherapy with Clopidogrel vs. Aspirin on Vascular Function and Hemostatic Measurements in Patients with Coronary Artery Disease: The Prospective, Crossover I-LOVE-MONO Trial. J. Clin. Med. 2021, 10, 2720. https://doi.org/10.3390/jcm10122720
Park H-W, Kang M-G, Ahn J-H, Bae J-S, Tantry US, Gurbel PA, Jeong Y-H. Effects of Monotherapy with Clopidogrel vs. Aspirin on Vascular Function and Hemostatic Measurements in Patients with Coronary Artery Disease: The Prospective, Crossover I-LOVE-MONO Trial. Journal of Clinical Medicine. 2021; 10(12):2720. https://doi.org/10.3390/jcm10122720
Chicago/Turabian StylePark, Hyun-Woong, Min-Gyu Kang, Jong-Hwa Ahn, Jae-Seok Bae, Udaya S. Tantry, Paul A. Gurbel, and Young-Hoon Jeong. 2021. "Effects of Monotherapy with Clopidogrel vs. Aspirin on Vascular Function and Hemostatic Measurements in Patients with Coronary Artery Disease: The Prospective, Crossover I-LOVE-MONO Trial" Journal of Clinical Medicine 10, no. 12: 2720. https://doi.org/10.3390/jcm10122720
APA StylePark, H.-W., Kang, M.-G., Ahn, J.-H., Bae, J.-S., Tantry, U. S., Gurbel, P. A., & Jeong, Y.-H. (2021). Effects of Monotherapy with Clopidogrel vs. Aspirin on Vascular Function and Hemostatic Measurements in Patients with Coronary Artery Disease: The Prospective, Crossover I-LOVE-MONO Trial. Journal of Clinical Medicine, 10(12), 2720. https://doi.org/10.3390/jcm10122720






