Arrhythmogenic Cardiomyopathy—Current Treatment and Future Options
Abstract
:1. Introduction
1.1. Definition and Classification
1.2. Genetic Background
1.3. Role of Inflammation in Arrhythmogenic Cardiomyopathy
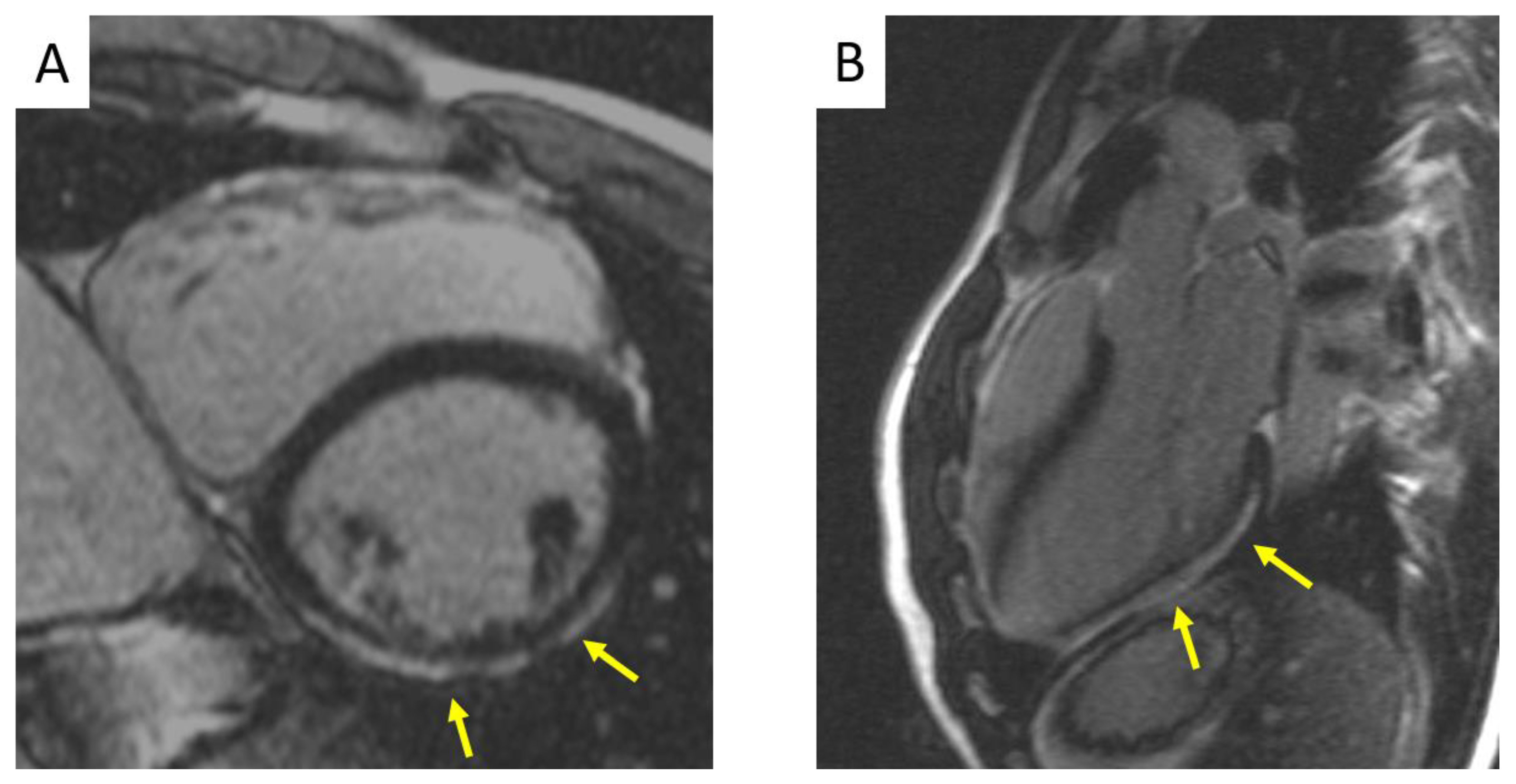
1.4. Diagnosis
2. Management
2.1. Prevention of Sudden Cardiac Death
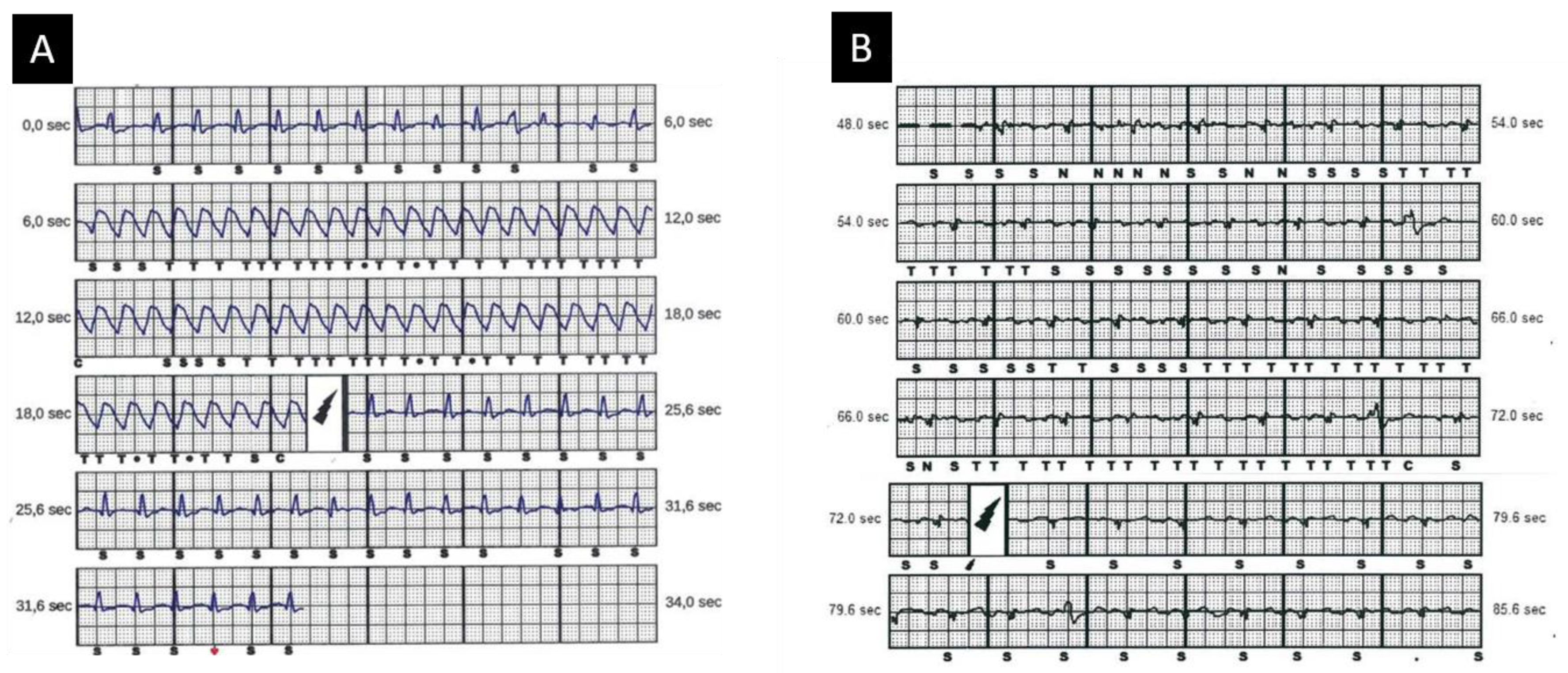
2.1.1. Risk Stratification
2.1.2. New Risk Predictors
2.1.3. Indications for ICD Implantation
2.1.4. Transvenous Versus Subcutaneous ICD
2.2. Improvement of Symptoms and Quality of Life
2.2.1. Traditional Pharmacologic Therapy
2.2.2. New Pharmacological Options
Heart Failure Drugs
Anti-Inflammatory Drugs
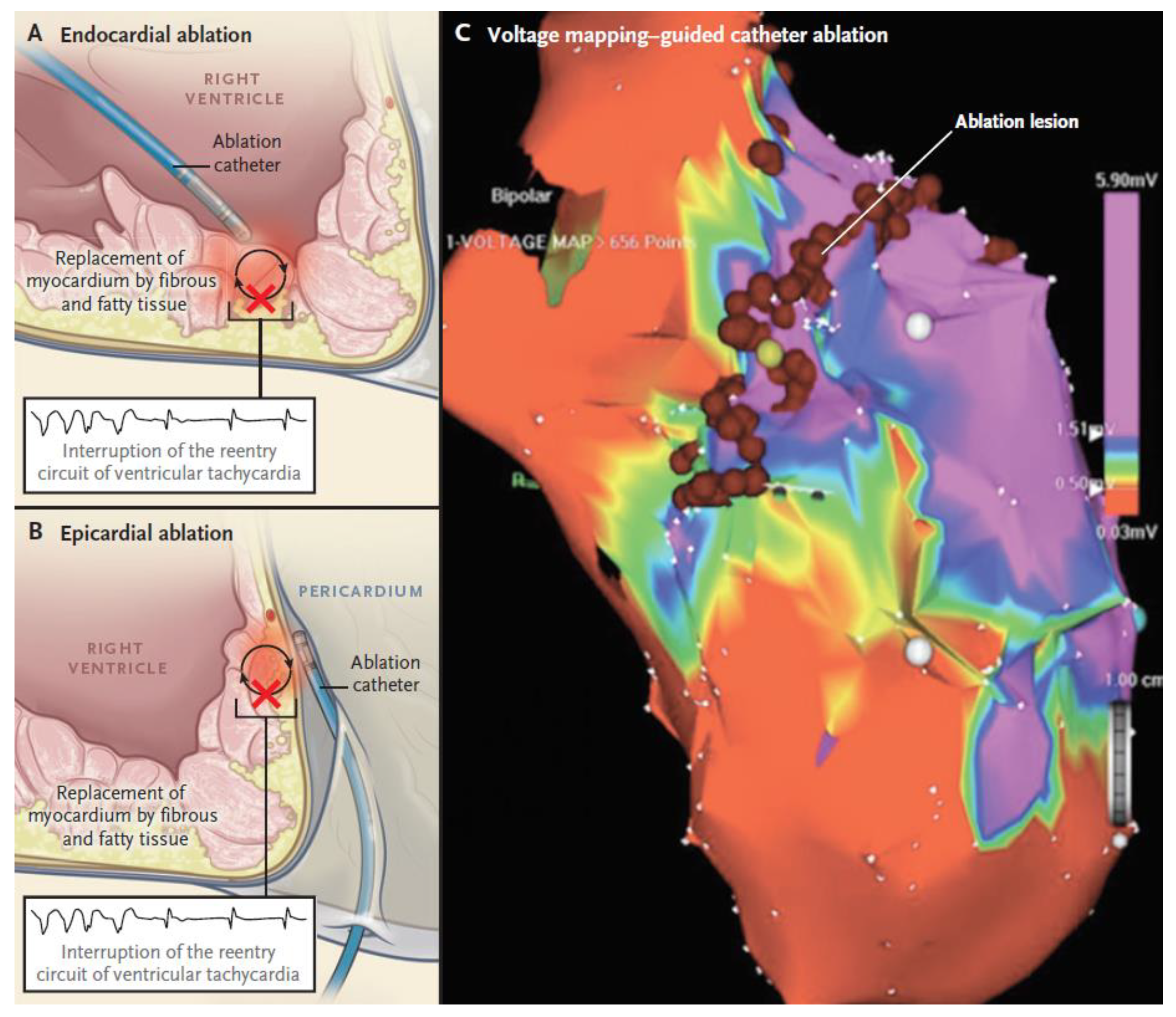
2.2.3. Non-Pharmacologic Therapy by Catheter Ablation
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Corrado, D.; Basso, C.; Judge, D. Arrhythmogenic Cardiomyopathy. Circ. Res. 2017, 121, 784–802. [Google Scholar] [CrossRef] [Green Version]
- Basso, C.; Thiene, G.; Corrado, D.; Angelini, A.; Nava, A.; Valente, M. Arrhythmogenic Right Ventricular Cardiomyopathy: Dysplasia, dystrophy, or myocarditis? Circulation 1996, 94, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Pilichou, K.; Remme, C.A.; Basso, C.; Campian, M.E.; Rizzo, S.; Barnett, P.; Scicluna, B.; Bauce, B.; Hoff, M.J.B.V.D.; De Bakker, J.M.T.; et al. Myocyte necrosis underlies progressive myocardial dystrophy in mouse dsg2-related arrhythmogenic right ventricular cardiomyopathy. J. Exp. Med. 2009, 206, 1787–1802. [Google Scholar] [CrossRef] [Green Version]
- Thiene, G.; Basso, C. Arrhythmogenic right ventricular cardiomyopathy: An update. Cardiovasc. Pathol. 2001, 10, 109–117. [Google Scholar] [CrossRef]
- Sen-Chowdhry, S.; Syrris, P.; Prasad, S.K.; Hughes, S.E.; Merrifield, R.; Ward, D.; Pennell, D.; McKenna, W.J. Left-Dominant Arrhythmogenic Cardiomyopathy. J. Am. Coll. Cardiol. 2008, 52, 2175–2187. [Google Scholar] [CrossRef] [Green Version]
- Te Riele, A.S.J.M.; James, C.A.; Philips, B.; Rastegar, N.; Bhonsale, A.; Groeneweg, J.A.; Murray, B.; Tichnell, C.; Judge, D.; Van Der Heijden, J.F.; et al. Mutation-Positive Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy: The Triangle of Dysplasia Displaced. J. Cardiovasc. Electrophysiol. 2013, 24, 1311–1320. [Google Scholar] [CrossRef] [PubMed]
- Corrado, D.; Van Tintelen, P.J.; McKenna, W.J.; Hauer, R.N.W.; Anastastakis, A.; Asimaki, A.; Basso, C.; Bauce, B.; Brunckhorst, C.; Bucciarelli-Ducci, C.; et al. International Experts. Arrhythmogenic right ventricular cardiomyopathy: Evaluation of the current diagnostic criteria and differential diagnosis. Eur. Heart J. 2020, 41, 1414–1429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKoy, G.; Protonotarios, N.; Crosby, A.; Tsatsopoulou, A.; Anastasakis, A.; Coonar, A.; Norman, M.; Baboonian, C.; Jeffery, S.; McKenna, W.J. Identification of a deletion in plakoglobin in arrhythmogenic right ventricular cardiomyopathy with palmoplantar keratoderma and woolly hair (Naxos disease). Lancet 2000, 355, 2119–2124. [Google Scholar] [CrossRef]
- Rampazzo, A.; Nava, A.; Malacrida, S.; Beffagna, G.; Bauce, B.; Rossi, V.; Zimbello, R.; Simionati, B.; Basso, C.; Thiene, G.; et al. Mutation in Human Desmoplakin Domain Binding to Plakoglobin Causes a Dominant Form of Arrhythmogenic Right Ventricular Cardiomyopathy. Am. J. Hum. Genet. 2002, 71, 1200–1206. [Google Scholar] [CrossRef] [Green Version]
- Gerull, B.; Heuser, A.; Wichter, T.; Paul, M.; Basson, C.T.; A McDermott, D.; Lerman, B.B.; Markowitz, S.M.; Ellinor, P.T.; Macrae, C.A.; et al. Mutations in the desmosomal protein plakophilin-2 are common in arrhythmogenic right ventricular cardiomyopathy. Nat. Genet. 2004, 36, 1162–1164. [Google Scholar] [CrossRef]
- Pilichou, K.; Nava, A.; Basso, C.; Beffagna, G.; Bauce, B.; Lorenzon, A.; Frigo, G.; Vettori, A.; Valente, M.; Towbin, J.; et al. Mutations in Desmoglein-2 Gene Are Associated with Arrhythmogenic Right Ventricular Cardiomyopathy. Circulation 2006, 113, 1171–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syrris, P.; Ward, D.; Evans, A.; Asimaki, A.; Gandjbakhch, E.; Sen-Chowdhry, S.; McKenna, W.J. Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy Associated with Mutations in the Desmosomal Gene Desmocollin-2. Am. J. Hum. Genet. 2006, 79, 978–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Hengel, J.; Calore, M.; Bauce, B.; Dazzo, E.; Mazzotti, E.; De Bortoli, M.; Lorenzon, A.; Mura, I.E.L.; Beffagna, G.; Rigato, I.; et al. Mutations in the area composita protein αT-catenin are associated with arrhythmogenic right ventricular cardiomyopathy. Eur. Hear. J. 2013, 34, 201–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayosi, B.M.; Fish, M.; Shaboodien, G.; Mastantuono, E.; Kraus, S.; Wieland, T.; Kotta, M.-C.; Chin, A.; Laing, N.; Ntusi, N.B.; et al. Identification of Cadherin 2 (CDH2) Mutations in Arrhythmogenic Right Ventricular Cardiomyopathy. Circ. Cardiovasc. Genet. 2017, 10. [Google Scholar] [CrossRef] [Green Version]
- Gras, E.G.; Lombardi, R.; Giocondo, M.J.; Willerson, J.T.; Schneider, M.D.; Khoury, D.S.; Marian, A.J. Suppression of canonical Wnt/ -catenin signaling by nuclear plakoglobin recapitulates phenotype of arrhythmogenic right ventricular cardiomyopathy. J. Clin. Investig. 2006, 116, 2012–2021. [Google Scholar] [CrossRef] [Green Version]
- Mattesi, G.; Zorzi, A.; Corrado, D.; Cipriani, A. Natural History of Arrhythmogenic Cardiomyopathy. J. Clin. Med. 2020, 9, 878. [Google Scholar] [CrossRef] [Green Version]
- Corrado, D.; Zorzi, A.; Cerrone, M.; Rigato, I.; Mongillo, M.; Bauce, B.; Delmar, M. Relationship Between Arrhythmogenic Right Ventricular Cardiomyopathy and Brugada Syndrome. Circ. Arrhythmia Electrophysiol. 2016, 9, e003631. [Google Scholar] [CrossRef] [Green Version]
- Merner, N.D.; Hodgkinson, K.A.; Haywood, A.F.; Connors, S.; French, V.M.; Drenckhahn, J.-D.; Kupprion, C.; Ramadanova, K.; Thierfelder, L.; McKenna, W.; et al. Arrhythmogenic Right Ventricular Cardiomyopathy Type 5 Is a Fully Penetrant, Lethal Arrhythmic Disorder Caused by a Missense Mutation in the TMEM43 Gene. Am. J. Hum. Genet. 2008, 82, 809–821. [Google Scholar] [CrossRef] [Green Version]
- Quarta, G.; Syrris, P.; Ashworth, M.; Jenkins, S.; Alapi, K.Z.; Morgan, J.; Muir, A.; Pantazis, A.; McKenna, W.J.; Elliott, P.M. Mutations in the Lamin A/C gene mimic arrhythmogenic right ventricular cardiomyopathy. Eur. Hear. J. 2011, 33, 1128–1136. [Google Scholar] [CrossRef] [Green Version]
- van Tintelen, J.P.; Van Gelder, I.C.; Asimaki, A.; Suurmeijer, A.J.; Wiesfeld, A.C.; Jongbloed, J.D.; Wijngaard, A.V.D.; Kuks, J.B.; van Spaendonck-Zwarts, K.Y.; Notermans, N.; et al. Severe cardiac phenotype with right ventricular predominance in a large cohort of patients with a single missense mutation in the DES gene. Hear. Rhythm. 2009, 6, 1574–1583. [Google Scholar] [CrossRef]
- Taylor, M.; Graw, S.; Sinagra, G.; Barnes, C.; Slavov, D.; Brun, F.; Pinamonti, B.; Salcedo, E.E.; Sauer, W.; Pyxaras, S.; et al. Genetic Variation in Titin in Arrhythmogenic Right Ventricular Cardiomyopathy–Overlap Syndromes. Circulation 2011, 124, 876–885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Der Zwaag, P.A.; Van Rijsingen, I.A.; Asimaki, A.; Jongbloed, J.D.; Van Veldhuisen, D.J.; Wiesfeld, A.C.; Cox, M.G.; Van Lochem, L.T.; De Boer, R.A.; Hofstra, R.M.; et al. Phospholamban R14del mutation in patients diagnosed with dilated cardiomyopathy or arrhythmogenic right ventricular cardiomyopathy: Evidence supporting the concept of arrhythmogenic cardiomyopathy. Eur. J. Hear. Fail. 2012, 14, 1199–1207. [Google Scholar] [CrossRef]
- Beffagna, G.; Occhi, G.; Nava, A.; Vitiello, L.; Ditadi, A.; Basso, C.; Bauce, B.; Carraro, G.; Thiene, G.; Towbin, J.A. Regulatory mutations in transforming growth factor-?3 gene cause arrhythmogenic right ventricular cardiomyopathy type 1. Cardiovasc. Res. 2005, 65, 366–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sen-Chowdhry, S.; Syrris, P.; Ward, D.; Asimaki, A.; Sevdalis, E.; McKenna, W.J. Clinical and Genetic Characterization of Families with Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy Provides Novel Insights Into Patterns of Disease Expression. Circulation 2007, 115, 1710–1720. [Google Scholar] [CrossRef] [Green Version]
- Bhonsale, A.; Groeneweg, J.A.; James, C.A.; Dooijes, D.; Tichnell, C.; Jongbloed, J.D.H.; Murray, B.; Riele, A.S.J.M.T.; Berg, M.P.V.D.; Bikker, H.; et al. Impact of genotype on clinical course in arrhythmogenic right ventricular dysplasia/cardiomyopathy-associated mutation carriers. Eur. Hear. J. 2015, 36, 847–855. [Google Scholar] [CrossRef]
- Ortiz-Genga, M.F.; Cuenca, S.; Ferro, M.D.; Zorio, E.; Aranda, R.S.; Climent, V.; Padrón-Barthe, L.; Duro-Aguado, I.; Jiménez-Jáimez, J.; Hidalgo-Olivares, V.M.; et al. Truncating FLNC Mutations Are Associated With High-Risk Dilated and Arrhythmogenic Cardiomyopathies. J. Am. Coll. Cardiol. 2016, 68, 2440–2451. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, F.; Zorio, E.; Jimenez-Jaimez, J.; Salguero-Bodes, R.; Zwart, R.; Gonzalez-Lopez, E.; Molina, P.; Jiménez, F.J.B.; Delgado, J.F.; Braza-Boïls, A.; et al. Clinical characteristics and determinants of the phenotype in TMEM43 arrhythmogenic right ventricular cardiomyopathy type 5. Hear. Rhythm. 2020, 17, 945–954. [Google Scholar] [CrossRef]
- Begay, R.; Graw, S.L.; Sinagra, G.; Asimaki, A.; Rowland, T.J.; Slavov, D.B.; Gowan, K.; Jones, K.L.; Brun, F.; Merlo, M.; et al. Filamin C Truncation Mutations Are Associated with Arrhythmogenic Dilated Cardiomyopathy and Changes in the Cell–Cell Adhesion Structures. JACC Clin. Electrophysiol. 2018, 4, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Augusto, J.B.; Eiros, R.; Nakou, E.; Moura-Ferreira, S.; Treibel, T.; Captur, G.; Akhtar, M.M.; Protonotarios, A.; Gossios, T.D.; Savvatis, K.; et al. Dilated cardiomyopathy and arrhythmogenic left ventricular cardiomyopathy: A comprehensive genotype-imaging phenotype study. Eur. Hear. J. Cardiovasc. Imaging 2019, 21, 326–336. [Google Scholar] [CrossRef]
- Segura-Rodríguez, D.; Jiménez, F.J.B.; Carriel, V.; López-Fernández, S.; González-Molina, M.; Ramírez, J.M.O.; Fernández-Navarro, L.; García-Roa, M.D.; Cabrerizo, E.M.; Durand-Herrera, D.; et al. Myocardial fibrosis in arrhythmogenic cardiomyopathy: A genotype–phenotype correlation study. Eur. Hear. J. Cardiovasc. Imaging 2019, 21, 378–386. [Google Scholar] [CrossRef]
- Mattesi, G.; Cipriani, A.; Bauce, B.; Rigato, I.; Zorzi, A.; Corrado, D. Arrhythmogenic Left Ventricular Cardiomyopathy: Genotype-Phenotype Correlations and New Diagnostic Criteria. J. Clin. Med. 2021, 10, 2212. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, F.; Angelini, A.; Thiene, G.; Basso, C.; Nava, A.; Valente, M. No detection of enteroviral genome in the myocardium of patients with arrhythmogenic right ventricular cardiomyopathy. J. Clin. Pathol. 2000, 53, 382–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiene, G.; Corrado, D.; Nava, A.; Rossi, L.; Poletti, A.; Boffa, G.M.; Daliento, L.; Pennelli, N. Right ventricular cardiomyopathy: Is there evidence of an inflammatory aetiology? Eur. Hear. J. 1991, 12, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Bariani, R.; Cipriani, A.; Rizzo, S.; Celeghin, R.; Marinas, M.B.; Giorgi, B.; De Gaspari, M.; Rigato, I.; Leoni, L.; Zorzi, A.; et al. ‘Hot phase’ clinical presentation in arrhythmogenic cardiomyopathy. Europace 2021, 23, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.; Fatah, M.; Akdis, D.; A Spears, D.; Koopmann, T.T.; Mittal, K.; A Rafiq, M.; Cattanach, B.M.; Zhao, Q.; Healey, J.S.; et al. An autoantibody identifies arrhythmogenic right ventricular cardiomyopathy and participates in its pathogenesis. Eur. Hear. J. 2018, 39, 3932–3944. [Google Scholar] [CrossRef] [Green Version]
- Caforio, A.L.; Re, F.; Avella, A.; Marcolongo, R.; Baratta, P.; Seguso, M.; Gallo, N.; Plebani, M.; Izquierdo-Bajo, A.; Cheng, C.-Y.; et al. Evidence From Family Studies for Autoimmunity in Arrhythmogenic Right Ventricular Cardiomyopathy. Circulation 2020, 141, 1238–1248. [Google Scholar] [CrossRef] [PubMed]
- McKenna, W.J.; Thiene, G.; Nava, A.; Fontaliran, F.; Blomstrom-Lundqvist, C.; Fontaine, G.; Camerini, F. Diagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Task Force of the Working Group Myocardial and Pericardial Disease of the European Society of Cardiology and of the Scientific Council on Cardiomyopathies of the International Society and Federation of Cardiology. Br. Heart. J. 1994, 71, 215–218. [Google Scholar] [CrossRef] [Green Version]
- Marcus, F.I.; McKenna, W.J.; Sherrill, D.; Basso, C.; Bauce, B.; Bluemke, D.; Calkins, H.; Corrado, D.; Cox, M.G.; Daubert, J.P.; et al. Diagnosis of Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia. Circulation 2010, 121, 1533–1541. [Google Scholar] [CrossRef]
- Towbin, J.A.; McKenna, W.J.; Abrams, D.J.; Ackerman, M.J.; Calkins, H.; Darrieux, F.C.; Daubert, J.P.; De Chillou, C.; DePasquale, E.C.; Desai, M.Y.; et al. 2019 HRS expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy. Hear. Rhythm. 2019, 16, e301–e372. [Google Scholar] [CrossRef] [Green Version]
- Corrado, D.; Perazzolo Marra, M.; Zorzi, A.; Beffagna, G.; Cipriani, A.; Lazzari, M.; Migliore, F.; Pilichou, K.; Rampazzo, A.; Rigato, I.; et al. Diagnosis of arrhythmogenic cardiomyopathy: The Padua criteria. Int. J. Cardiol. 2020, 319, 106–114. [Google Scholar] [CrossRef]
- Pontone, G.; Di Bella, G.; Castelletti, S.; Maestrini, V.; Festa, P.; Ait-Ali, L.; Masci, P.G.; Monti, L.; Di Giovine, G.; De Lazzari, M.; et al. Clinical recommendations of cardiac magnetic resonance, Part II. J. Cardiovasc. Med. 2017, 18, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Corrado, D.; Wichter, T.; Link, M.S.; Hauer, R.N.; Marchlinski, F.E.; Anastasakis, A.; Bauce, B.; Basso, C.; Brunckhorst, C.; Tsatsopoulou, A.; et al. Treatment of Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia. Circulation 2015, 132, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Rigato, I.; Bauce, B.; Rampazzo, A.; Zorzi, A.; Pilichou, K.; Mazzotti, E.; Migliore, F.; Marra, M.P.; Lorenzon, A.; De Bortoli, M.; et al. Compound and Digenic Heterozygosity Predicts Lifetime Arrhythmic Outcome and Sudden Cardiac Death in Desmosomal Gene–Related Arrhythmogenic Right Ventricular Cardiomyopathy. Circ. Cardiovasc. Genet. 2013, 6, 533–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corrado, D.; Link, M.S.; Calkins, H. Arrhythmogenic Right Ventricular Cardiomyopathy. New Engl. J. Med. 2017, 376, 61–72. [Google Scholar] [CrossRef]
- Cadrin-Tourigny, J.; Bosman, L.P.; Nozza, A.; Wang, W.; Tadros, R.; Bhonsale, A.; Bourfiss, M.; Fortier, A.; Lie, Ø.H.; Saguner, A.M.; et al. A new prediction model for ventricular arrhythmias in arrhythmogenic right ventricular cardiomyopathy. Eur. Hear. J. 2019, 40, 1850–1858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cadrin-Tourigny, J.; Bosman, L.P.; Wang, W.; Tadros, R.; Bhonsale, A.; Bourfiss, M.; Lie, Ø.H.; Saguner, A.M.; Svensson, A.; Andorin, A.; et al. Sudden Cardiac Death Prediction in Arrhythmogenic Right Ventricular Cardiomyo-pathy: A Multinational Collaboration. Circ. Arrhythm. Electrophysiol. 2021, 14, e008509. [Google Scholar] [CrossRef] [PubMed]
- Van Der Voorn, S.M.; Riele, A.S.J.M.T.; Basso, C.; Calkins, H.; Remme, C.A.; Veen, T.A.B.V. Arrhythmogenic cardiomyopathy: Pathogenesis, pro-arrhythmic remodelling, and novel approaches for risk stratification and therapy. Cardiovasc. Res. 2020, 116, 1571–1584. [Google Scholar] [CrossRef]
- Akdis, D.; Saguner, A.M.; Shah, K.; Wei, C.; Medeiros-Domingo, A.; Von Eckardstein, A.; Lüscher, T.F.; Brunckhorst, C.; Chen, H.V.; Duru, F. Sex hormones affect outcome in arrhythmogenic right ventricular cardiomyopathy/dysplasia: From a stem cell derived cardiomyocyte-based model to clinical biomarkers of disease outcome. Eur. Hear. J. 2017, 38, 1498–1508. [Google Scholar] [CrossRef] [PubMed]
- Coats, C.J.; E Heywood, W.; Mills, K.; Elliott, P.M. Current applications of biomarkers in cardiomyopathies. Expert Rev. Cardiovasc. Ther. 2015, 13, 825–837. [Google Scholar] [CrossRef] [PubMed]
- De Jong, S.; Van Veen, T.A.B.; De Bakker, J.M.T.; Vos, M.A.; van Rijen, H. Biomarkers of Myocardial Fibrosis. J. Cardiovasc. Pharmacol. 2011, 57, 522–535. [Google Scholar] [CrossRef]
- De Jong, S.; Van Veen, T.A.B.; De Bakker, J.M.T.; Van Rijen, H.V.M. Monitoring cardiac fibrosis: A technical challenge. Neth. Hear. J. 2011, 20, 44–48. [Google Scholar] [CrossRef] [Green Version]
- Chalikias, G.K.; Tziakas, D.N. Biomarkers of the extracellular matrix and of collagen fragments. Clin. Chim. Acta 2015, 443, 39–47. [Google Scholar] [CrossRef]
- Spinale, F.G. Matrix Metalloproteinases. Circ. Res. 2002, 90, 520–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sommariva, E.; D’Alessandra, Y.; Farina, F.M.; Casella, M.; Cattaneo, F.; Catto, V.; Chiesa, M.; Stadiotti, I.; Brambilla, S.; Russo, A.D.; et al. MiR-320a as a Potential Novel Circulating Biomarker of Arrhythmogenic CardioMyopathy. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, S.; Dong, T.; Yang, J.; Xie, Y.; Wu, Y.; Kang, K.; Hu, S.; Gou, D.; Wei, Y. Profiling of differentially expressed microRNAs in arrhythmogenic right ventricular cardiomyopathy. Sci. Rep. 2016, 6, 28101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thum, T.; Condorelli, G. Long Noncoding RNAs and MicroRNAs in Cardiovascular Pathophysiology. Circ. Res. 2015, 116, 751–762. [Google Scholar] [CrossRef]
- Bauersachs, J. Regulation of Myocardial Fibrosis by MicroRNAs. J. Cardiovasc. Pharmacol. 2010, 56, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Thum, T.; Gross, C.; Fiedler, J.; Fischer, T.; Kissler, S.; Bussen, M.; Galuppo, P.; Just, S.; Rottbauer, W.; Frantz, S.; et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nat. Cell Biol. 2008, 456, 980–984. [Google Scholar] [CrossRef] [PubMed]
- Broch, K.; Leren, I.S.; Saberniak, J.; Ueland, T.; Edvardsen, T.; Gullestad, L.; Haugaa, K. Soluble ST2 is associated with disease severity in arrhythmogenic right ventricular cardiomyopathy. Biomarkers 2017, 22, 367–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, T.-T.; Cogswell, R.; James, C.A.; Kang, G.; Pullinger, C.R.; Malloy, M.J.; Kane, J.P.; Wojciak, J.; Calkins, H.; Scheinman, M.M.; et al. Plasma BIN1 correlates with heart failure and predicts arrhythmia in patients with arrhythmogenic right ventricular cardiomyopathy. Hear. Rhythm. 2012, 9, 961–967. [Google Scholar] [CrossRef] [Green Version]
- Asimaki, A.; Protonotarios, A.; James, C.A.; Chelko, S.; Tichnell, C.; Murray, B.; Tsatsopoulou, A.; Anastasakis, A.; Riele, A.T.; Kléber, A.G.; et al. Characterizing the Molecular Pathology of Arrhythmogenic Cardiomyopathy in Patient Buccal Mucosa Cells. Circ. Arrhythmia Electrophysiol. 2016, 9, e003688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rolf, S.; Bruns, H.-J.; Wichter, T.; Kirchhof, P.; Ribbing, M.; Wasmer, K.; Paul, M.; Breithardt, G.; Haverkamp, W.; Eckardt, L. The ajmaline challenge in Brugada syndrome: Diagnostic impact, safety, and recommended protocol. Eur. Hear. J. 2003, 24, 1104–1112. [Google Scholar] [CrossRef] [Green Version]
- Peters, S. Arrhythmogenic right ventricular dysplasia-cardiomyopathy and provocable coved-type ST-segment elevation in right precordial leads: Clues from long-term follow-up. Europace 2008, 10, 816–820. [Google Scholar] [CrossRef] [PubMed]
- Oostendorp, T.F.; Van Dessel, P.F.H.M.; Coronel, R.; Belterman, C.; Linnenbank, A.C.; Van Schie, I.H.; Van Oosterom, A.; Oosterhoff, P.; Van Dam, P.M.; De Bakker, J.M.T. Noninvasive detection of epicardial and endocardial activity of the heart. Neth. Hear. J. 2011, 19, 488–491. [Google Scholar] [CrossRef] [Green Version]
- Teske, A.J.; Cox, M.G.; Riele, A.T.; De Boeck, B.W.; Doevendans, P.A.; Hauer, R.N.; Cramer, M.J. Early Detection of Regional Functional Abnormalities in Asymptomatic ARVD/C Gene Carriers. J. Am. Soc. Echocardiogr. 2012, 25, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Haugaa, K.H.; Haland, T.F.; Leren, I.S.; Saberniak, J.; Edvardsen, T. Arrhythmogenic right ventricular cardiomyopathy, clinical manifestations, and diagnosis. Europace 2015, 18, 965–972. [Google Scholar] [CrossRef]
- Knops, R.E.; Nordkamp, L.R.O.; Delnoy, P.-P.H.; Boersma, L.V.; Kuschyk, J.; El-Chami, M.F.; Bonnemeier, H.; Behr, E.R.; Brouwer, T.F.; Kääb, S.; et al. Subcutaneous or Transvenous Defibrillator Therapy. N. Engl. J. Med. 2020, 383, 526–536. [Google Scholar] [CrossRef]
- Migliore, F.; Viani, S.; Bongiorni, M.G.; Zorzi, A.; Silvetti, M.S.; Francia, P.; D’Onofrio, A.; De Franceschi, P.; Sala, S.; Donzelli, S.; et al. Subcutaneous implantable cardioverter defibrillator in patients with arrhythmogenic right ventricular cardiomyopathy: Results from an Italian multicenter registry. Int. J. Cardiol. 2019, 280, 74–79. [Google Scholar] [CrossRef] [Green Version]
- Link, M.S.; Laidlaw, D.; Polonsky, B.; Zareba, W.; McNitt, S.; Gear, K.; Marcus, F.; Estes, N.A.M. Ventricular Arrhythmias in the North American Multidisciplinary Study of ARVC. J. Am. Coll. Cardiol. 2014, 64, 119–125. [Google Scholar] [CrossRef] [Green Version]
- Migliore, F.; Bertaglia, E.; Zorzi, A.; Corrado, D. Subcutaneous Implantable Cardioverter-Defibrillator and Arrhythmogenic Right Ventricular Cardiomyopathy. JACC Clin. Electrophysiol. 2017, 3, 785–786. [Google Scholar] [CrossRef]
- Migliore, F.; Mattesi, G.; De Franceschi, P.; Allocca, G.; Crosato, M.; Calzolari, V.; Fantinel, M.; Ortis, B.; Facchin, D.; Daleffe, E.; et al. Multicentre experience with the second-generation subcutaneous implantable cardioverter defibrillator and the intermuscular two-incision implantation technique. J. Cardiovasc. Electrophysiol. 2019, 30, 854–864. [Google Scholar] [CrossRef]
- Theuns, D.A.; Brouwer, T.F.; Jones, P.W.; Allavatam, V.; Donnelley, S.; Auricchio, A.; Knops, R.E.; Burke, M.C. Prospective blinded evaluation of a novel sensing methodology designed to reduce inappropriate shocks by the subcutaneous implantable cardioverter-defibrillator. Hear. Rhythm. 2018, 15, 1515–1522. [Google Scholar] [CrossRef] [PubMed]
- Priori, S.; Blomström-Lundqvist, C.; Mazzanti, A.; Blom, N.; Borggrefe, M.; Camm, J.; Elliott, P.M.; Fitzsimons, D.; Hatala, R.; Hindricks, G.; et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur. Hear. J. 2015, 36, 2793–2867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tedford, R.J.; James, C.; Judge, D.; Tichnell, C.; Murray, B.; Bhonsale, A.; Philips, B.; Abraham, T.; Dalal, D.; Halushka, M.; et al. Cardiac Transplantation in Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy. J. Am. Coll. Cardiol. 2012, 59, 289–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2013, 62, e147–e239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016, 18, 891–975. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, U.M.; Gong, J.; Jhund, P.; Shen, L.; Køber, L.; Desai, A.S.; Lefkowitz, M.P.; Packer, M.; Rouleau, J.L.; Solomon, S.D.; et al. Effect of sacubitril/valsartan on recurrent events in the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF). Eur. J. Hear. Fail. 2018, 20, 760–768. [Google Scholar] [CrossRef]
- Asimaki, A.; Kapoor, S.; Plovie, E.; Arndt, A.K.; Adams, E.; Liu, Z.; James, C.A.; Judge, D.; Calkins, H.; Churko, J.; et al. Identification of a New Modulator of the Intercalated Disc in a Zebrafish Model of Arrhythmogenic Cardiomyopathy. Sci. Transl. Med. 2014, 6, 240ra74. [Google Scholar] [CrossRef] [Green Version]
- Chelko, S.; Asimaki, A.; Andersen, P.; Bedja, D.; Amat-Alarcon, N.; DeMazumder, D.; Jasti, R.; Macrae, C.A.; Leber, R.; Kleber, A.G.; et al. Central role for GSK3β in the pathogenesis of arrhythmogenic cardiomyopathy. JCI Insight 2016, 1, 1. [Google Scholar] [CrossRef] [Green Version]
- Chelko, S.; Asimaki, A.; Lowenthal, J.; Bueno-Beti, C.; Bedja, D.; Scalco, A.; Amat-Alarcon, N.; Andersen, P.; Judge, D.P.; Tung, L.; et al. Therapeutic Modulation of the Immune Response in Arrhythmogenic Cardiomyopathy. Circulation 2019, 140, 1491–1505. [Google Scholar] [CrossRef]
- Zhang, X.; Meng, F.; Song, J.; Zhang, L.; Wang, J.; Li, D.; Li, L.; Dong, P.; Yang, B.; Chen, Y. Pentoxifylline Ameliorates Cardiac Fibrosis, Pathological Hypertrophy, and Cardiac Dysfunction in Angiotensin II-induced Hypertensive Rats. J. Cardiovasc. Pharmacol. 2016, 67, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Szekely, Y.; Arbel, Y. A Review of Interleukin-1 in Heart Disease: Where Do We Stand Today? Cardiol. Ther. 2018, 7, 25–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Muchir, A.; Wu, W.; Choi, J.C.; Iwata, S.; Morrow, J.; Homma, S.; Worman, H.J. Abnormal p38 mitogen-activated protein kinase signaling in dilated cardiomyopathy caused by lamin A/C gene mutation. Hum. Mol. Genet. 2012, 21, 4325–4333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Philips, B.; Madhavan, S.; James, C.; Tichnell, C.; Murray, B.; Dalal, D.; Bhonsale, A.; Nazarian, S.; Judge, D.; Russell, S.D.; et al. Outcomes of Catheter Ablation of Ventricular Tachycardia in Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy. Circ. Arrhythmia Electrophysiol. 2012, 5, 499–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, F.C.; Bazan, V.; Zado, E.S.; Ren, J.-F.; Marchlinski, F. Epicardial Substrate and Outcome with Epicardial Ablation of Ventricular Tachycardia in Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia. Circulation 2009, 120, 366–375. [Google Scholar] [CrossRef] [Green Version]
- Polin, G.M.; Haqqani, H.; Tzou, W.; Hutchinson, M.; Garcia, F.C.; Callans, D.J.; Zado, E.S.; Marchlinski, F.E. Endocardial unipolar voltage mapping to identify epicardial substrate in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Hear. Rhythm. 2011, 8, 76–83. [Google Scholar] [CrossRef]
- Santangeli, P.; Tung, R.; Xue, Y.; Chung, F.-P.; Lin, Y.-J.; Di Biase, L.; Zhan, X.; Lin, C.-Y.; Wei, W.; Mohanty, S.; et al. Outcomes of Catheter Ablation in Arrhythmogenic Right Ventricular Cardiomyopathy Without Background Implantable Cardioverter Defibrillator Therapy. JACC Clin. Electrophysiol. 2019, 5, 55–65. [Google Scholar] [CrossRef]
- Berruezo, A.; Acosta, J.; Fernández-Armenta, J.; Pedrote, A.; Barrera, A.; Arana-Rueda, E.; Bodegas, A.I.; Anguera, I.; Tercedor, L.; Penela, D.; et al. Safety, long-term outcomes and predictors of recurrence after first-line combined endoepicardial ventricular tachycardia substrate ablation in arrhythmogenic cardiomyopathy. Impact of arrhythmic substrate distribution pattern. A prospective multicentre study. Europace 2016, 19, 607–616. [Google Scholar] [CrossRef]



| Author (Year) | Patients n (Men) | Ablation Technique | Complete Acute Success (%) | Procedure-Related Complications | Follow-up | ||||
|---|---|---|---|---|---|---|---|---|---|
| Electro- Anatomic Map | Irrigated Tip | Epicardial Map/abl (%) | Mean (Months) | VT Recurrences (%) | Deaths or HT | ||||
| Santangeli 2019 | 32 (23) | Yes | Yes | 72% | 100 | 1 (RV laceration) | 46 | 19 | N/A |
| Berruezo 2017 | 41 (36) | Yes | Yes | 100% | 90 | 2 (tamponade, death) | 32 | 26.8 | N/A |
| Mussigbrodt 2017 | 45 (30) | Yes | Yes | 48.9% | 84 | 5 (TIA, tamponade x2, PE x2 1 fatal) | 31 | 44 * | N/A |
| Souissi 2018 | 49 (44) | Yes | Yes | 100% | 71 | 3 (tamponade, femoral AV fistula, intestinal perforation) | 64 | 81 at 5 years 31 at 1 years * | 6 deaths, 2 HT |
| Santangeli 2015 | 62 (45) | Yes | Yes | 63% | 77 | 5 (PE x2, pericardial effusion, RV puncture, CT) | 56 | 29 * | 5 NC, 5HT |
| Philips 2012 | 87 (45) | Yes | Yes | 26.4% | 82 | 2 (death, MI) | 88 | 85 | N/A |
| Berruezo 2012 | 11 (9) | Yes | Yes | 100% | 100 | 1 (tamponade) | 11 | 9 | 0 |
| Garcia 2009 | 13 (10) | Yes | Yes | Yes | 92 | 0 | 18 | 23 | 1 HT |
| Nogami 2008 | 18 (13) | Yes | No | No | 72 | 0 | 61 | 33 | 2 HF, 1 NC |
| Dalal, 2007 | 24 (11) | Yes | No | No | 77 | 1 (death) | 32 | 85 | 2 HT |
| Satomi 2006 | 17 (13) | Yes | No | No | 88 | 0 | 26 | 24 | 0 |
| Verma 2005 | 22 (15) | Yes | Yes | No | 82 | 1 (tamponade) | 37 | 36 | 0 |
| Miljoen 2005 | 11 (8) | Yes | No | No | 73 | 0 | 20 | 45 | 1 NC |
| Marchlinski 2004 | 19 (18) | Yes | Yes | No | 74 | 0 | 27 | 11 | 0 |
| Reithmann 2003 | 5 (3) | Yes | No | No | 80 | 0 | 7 | 20 | 0 |
| Ellison 1998 | 5 (4) | No | No | No | 42 | 0 | 17 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Migliore, F.; Mattesi, G.; Zorzi, A.; Bauce, B.; Rigato, I.; Corrado, D.; Cipriani, A. Arrhythmogenic Cardiomyopathy—Current Treatment and Future Options. J. Clin. Med. 2021, 10, 2750. https://doi.org/10.3390/jcm10132750
Migliore F, Mattesi G, Zorzi A, Bauce B, Rigato I, Corrado D, Cipriani A. Arrhythmogenic Cardiomyopathy—Current Treatment and Future Options. Journal of Clinical Medicine. 2021; 10(13):2750. https://doi.org/10.3390/jcm10132750
Chicago/Turabian StyleMigliore, Federico, Giulia Mattesi, Alessandro Zorzi, Barbara Bauce, Ilaria Rigato, Domenico Corrado, and Alberto Cipriani. 2021. "Arrhythmogenic Cardiomyopathy—Current Treatment and Future Options" Journal of Clinical Medicine 10, no. 13: 2750. https://doi.org/10.3390/jcm10132750
APA StyleMigliore, F., Mattesi, G., Zorzi, A., Bauce, B., Rigato, I., Corrado, D., & Cipriani, A. (2021). Arrhythmogenic Cardiomyopathy—Current Treatment and Future Options. Journal of Clinical Medicine, 10(13), 2750. https://doi.org/10.3390/jcm10132750







