Noncompaction Cardiomyopathy—History and Current Knowledge for Clinical Practice
Abstract
1. Introduction
2. Noncompaction Cardiomyopathy in the Cardiomyopathy Classifications
3. Subtypes of Noncompaction Cardiomyopathy
4. Epidemiology
5. Clinical Features
6. Diagnostic Criteria
6.1. Diagnostic Criteria for Echocardiography
6.2. Diagnostic Criteria for Magnet Resonance Imaging
7. Additional Diagnostic Armamentarium
7.1. The Multimodality Imaging Approach
7.2. Left Ventricular Angiography
7.3. Computer Tomography
7.4. Electrocardiography
7.5. Biomarkers
7.6. Endomyocardial Biopsy
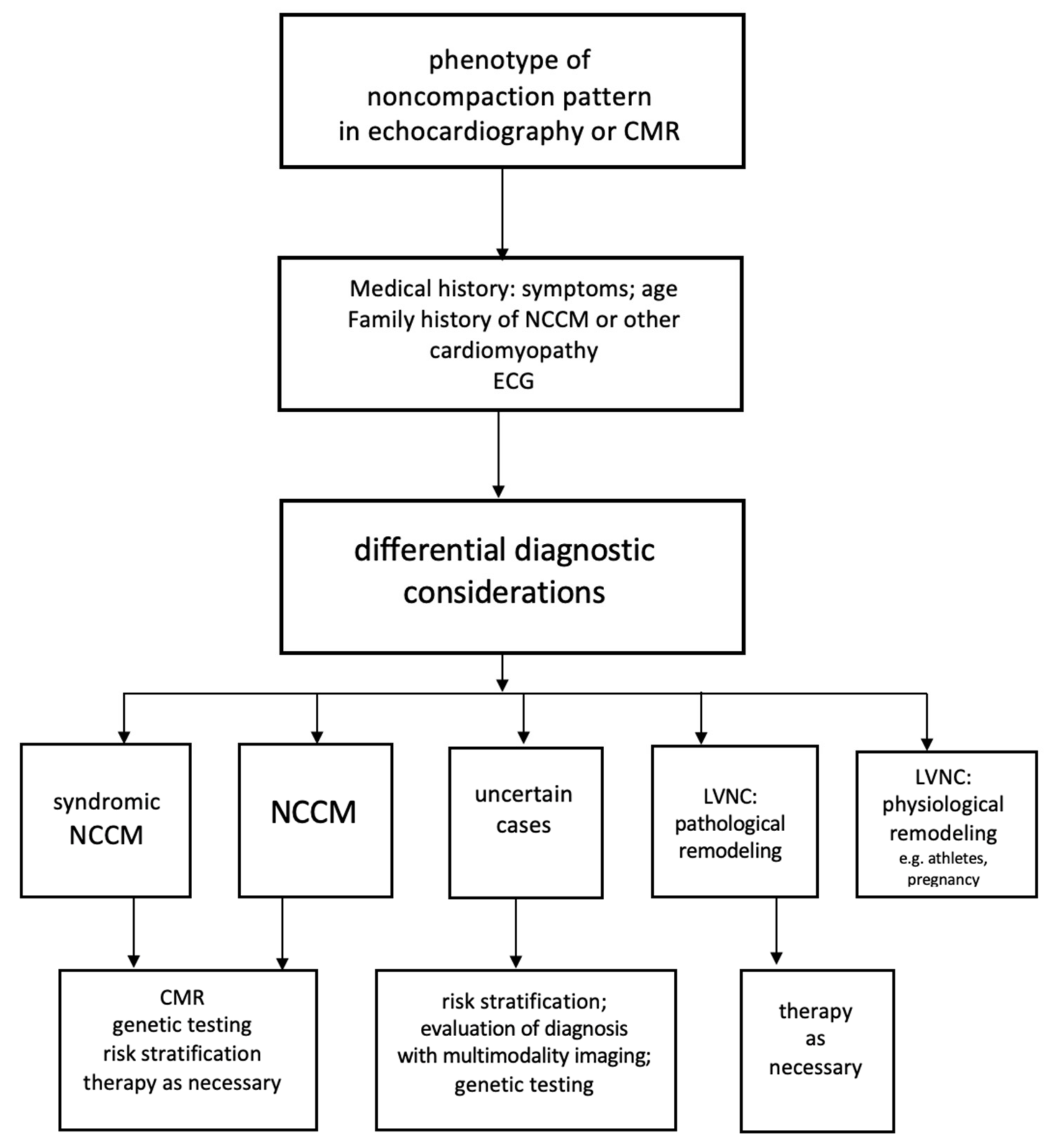
8. Differential Diagnosis
9. Pathogenesis—Embryogenesis and the Pathophysiological Concept
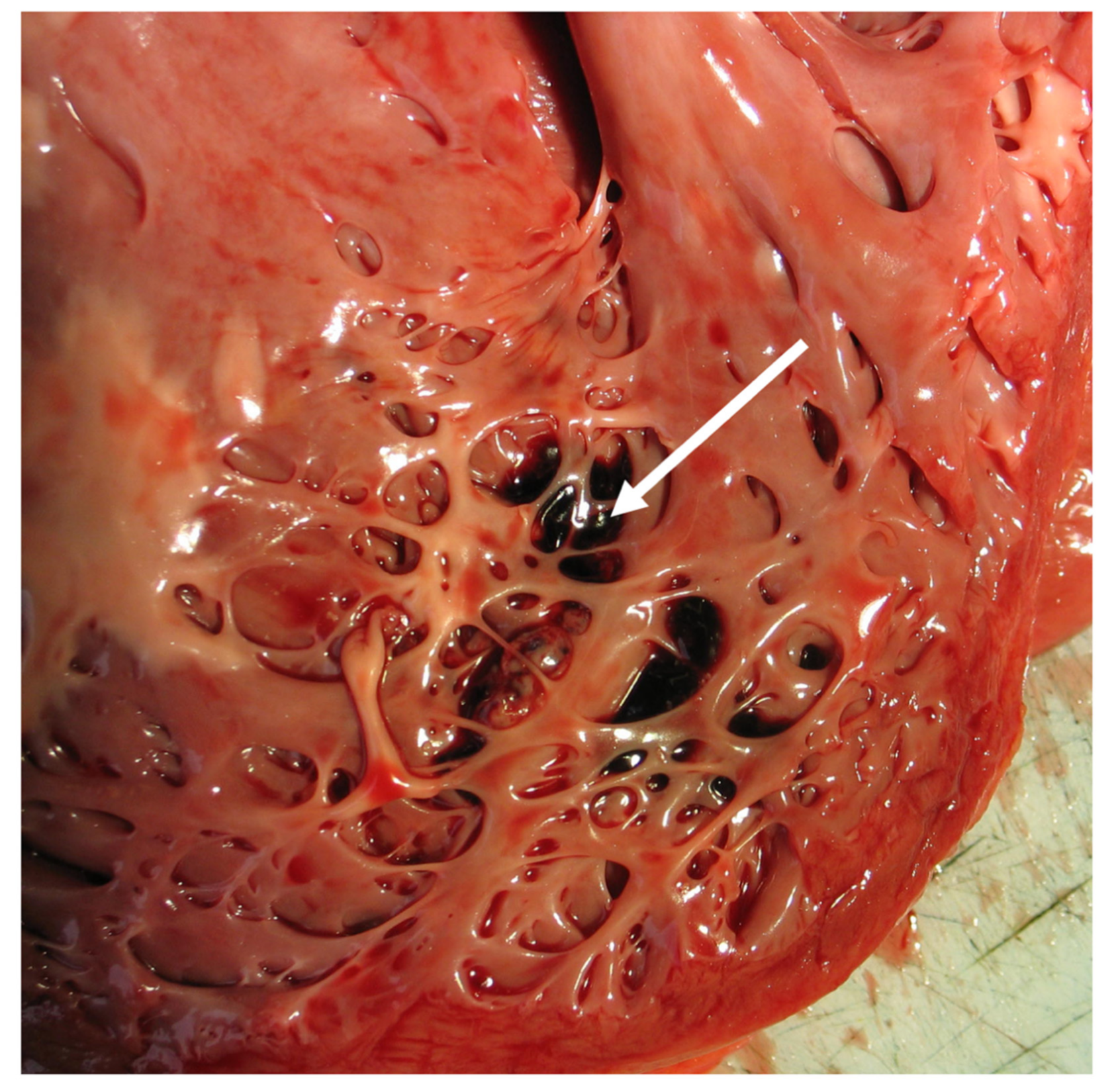
10. Pathology
11. Genetics in Noncompaction Cardiomyopathy
11.1. Basic Aspects
11.2. Genetic Testing in Familial Noncompaction Cardiomyopathy
12. Prognosis
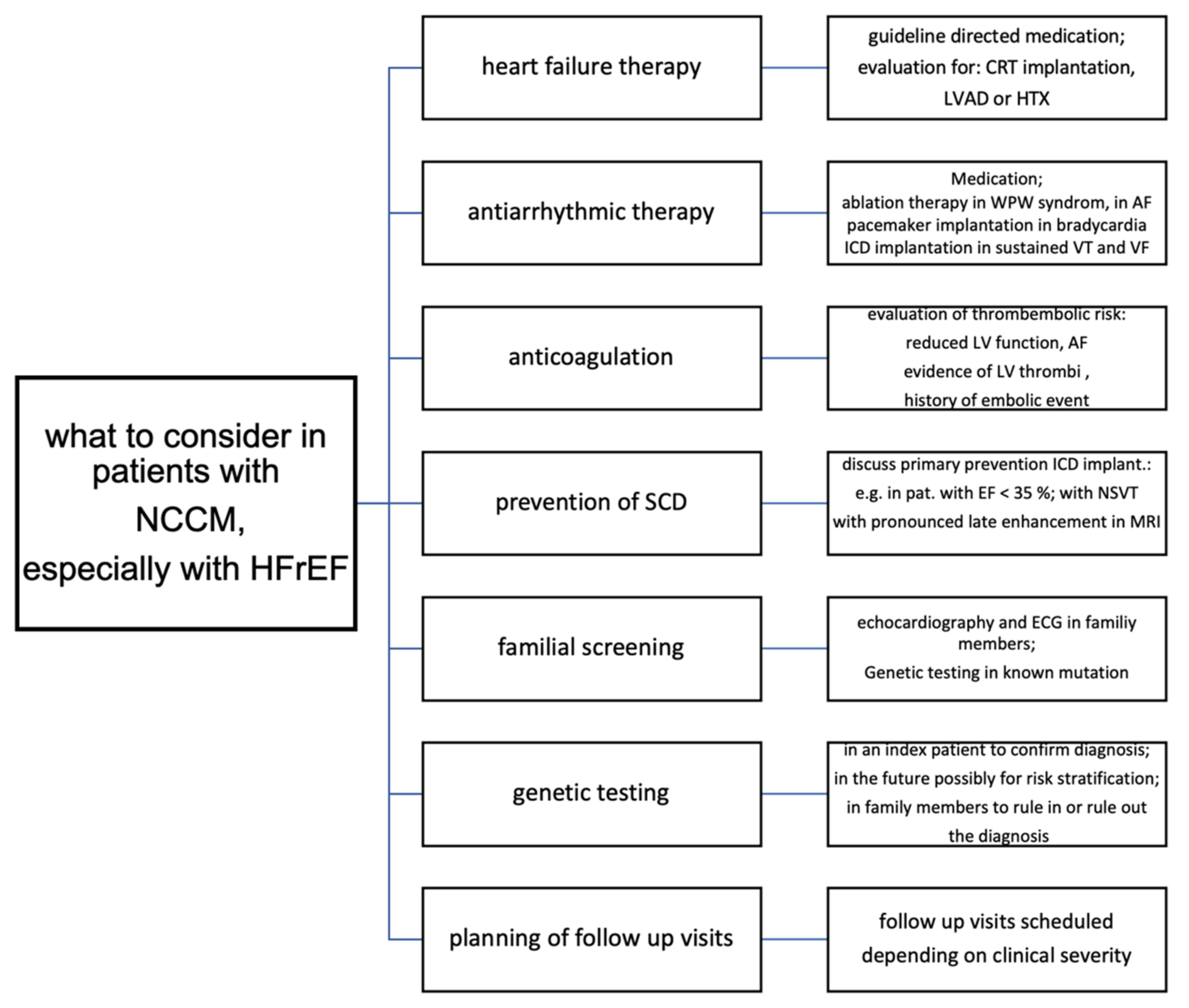
13. Therapy
14. Future Work
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Brandenburg, R.O. Report of the WHO/ISFC task force on the definition and classification of cardiomyopathies. Br. Heart J. 1980, 44, 672–673. [Google Scholar] [CrossRef]
- Grant, R.T. An unusual anomaly of the coronary vessels in the malformed heart of a child. Heart 1926, 1, 273–283. [Google Scholar]
- Bellet, S.; Gouley, B.A. Congenital heart disease with multiple cardiac anomalies: Report of case showing aortic atresia, fibrous scar in myocardium, and embryonal sinusoidal remains. Am. J. Med. Sci. 1932, 183, 458–465. [Google Scholar] [CrossRef]
- Dusek, J.; Ostádal, B.; Duskova, M. Postnatal persistence of spongy myocardium with embryonic blood supply. Arch. Pathol. 1975, 99, 312–317. [Google Scholar]
- Lauer, R.M.; Fink, H.P.; Petry, E.L.; Dunn, M.I.; Diehl, A.M. Angiographic Demonstration of Intramyocardial Sinusoids in Pulmonary-Valve Atresia with Intact Ventricular Septum and Hypoplastic Right Ventricle. N. Engl. J. Med. 1964, 271, 68–72. [Google Scholar] [CrossRef]
- Feldt, R.H.; Rahimtoola, S.H.; Davis, G.D.; Swan, H.; Titus, J.L. Anomalous ventricular myocardial patterns in a child with complex congenital heart disease. Am. J. Cardiol. 1969, 23, 732–734. [Google Scholar] [CrossRef]
- Engberding, R.; Bender, F. Identification of a rare congenital anomaly of the myocardium by two-dimensional echocardiography: Persistence of isolated myocardial sinusoids. Am. J. Cardiol. 1984, 53, 1733–1734. [Google Scholar] [CrossRef]
- Jenni, R.; Goebel, N.; Tartini, R.; Schneider, J.; Arbenz, U.; Oelz, O. Persisting myocardial sinusoids of both ventricles as an isolated anomaly: Echocardiographic, angiographic, and pathologic anatomical findings. Cardiovasc. Interv. Radiol. 1986, 9, 127–131. [Google Scholar] [CrossRef]
- Chin, T.K.; Perloff, J.K.; Williams, R.G.; Jue, K.; Mohrmann, R. Isolated noncompaction of left ventricular myocardium. A study of eight cases. Circulation 1990, 82, 507–513. [Google Scholar] [CrossRef]
- Jensen, B.; van der Wal, A.C.; Moorman, A.F.M.; Christoffels, V.M. Excessive trabecularions in noncompaction do not have the embryonic identity. Int. J. Cardiol. 2017, 227, 325–330. [Google Scholar] [CrossRef]
- Nel, S.; Khandheria, B.K.; Libhaber, E.; Peters, F.; dos Santos, C.F.; Matioda, H.; Grinter, S.; Maharaj, N.; Essop, M.R. Prevalence and significance of isolated left ventricular non-compaction phenotype in normal black Africans using echocardiography. IJC Heart Vasc. 2020, 30, 100585. [Google Scholar] [CrossRef]
- Towbin, J.; Lorts, A.; Jefferies, J.L. Left ventricular non-compaction cardiomyopathy. Lancet 2015, 386, 813–825. [Google Scholar] [CrossRef]
- Zemrak, F.; Ahlman, M.A.; Captur, G.; Mohiddin, S.; Kawel-Boehm, N.; Prince, M.R.; Moon, J.C.; Hundley, W.G.; Lima, J.A.; Bluemke, D.; et al. The Relationship of Left Ventricular Trabeculation to Ventricular Function and Structure Over a 9.5-Year Follow-Up. J. Am. Coll. Cardiol. 2014, 64, 1971–1980. [Google Scholar] [CrossRef] [PubMed]
- Andreini, D.; Pontone, G.; Bogaert, J.; Roghi, A.; Barison, A.; Schwitter, J.; Mushtaq, S.; Vovas, G.; Sormani, P.; Aquaro, G.D.; et al. Long-Term Prognostic Value of Cardiac Magnetic Resonance in Left Ventricle Noncompaction. J. Am. Coll. Cardiol. 2016, 68, 2166–2181. [Google Scholar] [CrossRef]
- Ivanov, A.; Dabiesingh, D.S.; Bhumireddy, G.P.; Mohamed, A.; Asfour, A.; Briggs, W.M.; Ho, J.; Khan, S.A.; Grossman, A.; Klem, I.; et al. Prevalence and Prognostic Significance of Left Ventricular Noncompaction in Patients Referred for Cardiac Magnetic Resonance Imaging. Circ. Cardiovasc. Imaging 2017, 10, e006174. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, V.R.; Lyle, M.; Miranda, W.R.; Farwati, M.; Isath, A.; Patlolla, S.H.; Hodge, D.O.; Asirvatham, S.J.; Kapa, S.; Deshmukh, A.J.; et al. Long-Term Survival of Patients with Left Ventricular Noncompaction. J. Am. Heart Assoc. 2021, 10, e015563. [Google Scholar] [CrossRef]
- Stöllberger, C.; Finsterer, J.; Blazek, G. Isolated left ventricular abnormal trabeculation is a cardiac manifestation of neuromuscular disorders. Cardiology 2000, 94, 72–76. [Google Scholar] [CrossRef]
- Towbin, J.A.; Jefferies, J.L. Cardiomyopathies Due to Left Ventricular Noncompaction, Mitochondrial and Storage Diseases, and Inborn Errors of Metabolism. Circ. Res. 2017, 121, 838–854. [Google Scholar] [CrossRef]
- Caselli, S.; Jost, C.H.A.; Jenni, R.; Pelliccia, A. Left Ventricular Noncompaction Diagnosis and Management Relevant to Pre-participation Screening of Athletes. Am. J. Cardiol. 2015, 116, 801–808. [Google Scholar] [CrossRef]
- Gati, S.; Papadakis, M.; Papamichael, N.D.; Zaidi, A.; Sheikh, N.; Reed, M.; Sharma, R.; Thilaganathan, B.; Sharma, S. Reversible De Novo Left Ventricular Trabeculations in Pregnant Women. Circulation 2014, 130, 475–483. [Google Scholar] [CrossRef]
- Brigden, W. Uncommon myocardial diseases the non-coronary cardiomyopathies. Lancet 1957, 270, 1179–1184. [Google Scholar] [CrossRef]
- Goodwin, J.F.; Oakley, C.M. The cardiomyopathies. Br. Heart J. 1972, 34, 545–552. [Google Scholar] [CrossRef]
- Richardson, P.; McKenna, W.; Bristow, M.; Maisch, B.; Mautner, B.; O´Connell, J.; Olsen, E.; Thiene, G.; Goodwin, J.; Gyarfas, I.; et al. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of Cardiomyopathies. Circulation 1996, 93, 841–842. [Google Scholar] [PubMed]
- Maron, B.J.; Towbin, J.A.; Thiene, G.; Antzelevitch, C.; Corrado, D.; Arnett, D.; Moss, A.J.; Seidman, C.E.; Young, J.B. Contemporary definitions and classifications of the cardiomyopathies: An American Heart Association Scientific Statement form the Council on Clinical Cardiology, Heart Failure and Transplantation Committee: Quality of Care and Outcomes Research and functional genomics and translational biology interdisciplinary working groups; and the Council on Epidemiology and Prevention. Circulation 2006, 113, 1807–1816. [Google Scholar] [CrossRef]
- Elliott, P.; Andersson, B.; Arbustini, E.; Bilinska, Z.; Cecchi, F.; Charron, P.; Dubourg, O.; Kuhl, U.; Maisch, B.; McKenna, W.J.; et al. Classification of the cardiomyopathies: A position statement from the European Society of Cardiology Working Group on myocardial and pericardial diseases. Eur. Heart J. 2008, 29, 270–276. [Google Scholar] [CrossRef]
- Arbustini, E.; Narula, N.; Dec, G.W.; Reddy, K.S.; Greenberg, B.; Kushwaha, S.; Marwick, T.; Pinney, S.; Bellazzi, R.; Favalli, V.; et al. The MOGE(S) Classification for a Phenotype–Genotype Nomenclature of Cardiomyopathy: Endorsed by the World Heart Federation. Glob. Heart 2013, 8, 355–382. [Google Scholar] [CrossRef] [PubMed]
- Arbustini, E.; Favalli, V.; Narula, N.; Serio, A.; Grasso, M. Left ventricular noncompaction. A distinct genetic cardiomyopathy. J. Am. Coll. Cardiol. 2016, 68, 949–966. [Google Scholar] [CrossRef]
- van Waning, J.I.; Moesker, J.; Heijsman, D.; Boersma, E.; Majoor-Krakauer, D. Systematic Review of Genotype-Phenotype Correlations in Noncompaction Cardiomyopathy. J. Am. Heart Assoc. 2019, 8, e012993. [Google Scholar] [CrossRef]
- Maheshwari, M.; Gokroo, R.K.; Kaushik, S.K. Isolated non-compacted right ventricular myocardium. J. Assoc. Physicians India 2012, 60, 56–57. [Google Scholar]
- Gomathi, S.B.; Makadia, N.; Ajit, S.M. An unusual case of isolated non-compacted right ventricular myocardium. Eur. J. Echocardiogr. 2008, 9, 424–425. [Google Scholar] [CrossRef]
- Nugent, A.W.; Daubeney, P.E.; Chondros, P.; Carlin, J.B.; Cheung, M.; Wilkinson, L.C.; Davis, A.M.; Kahler, S.G.; Chow, C.; Wilkinson, J.L.; et al. The Epidemiology of Childhood Cardiomyopathy in Australia. N. Engl. J. Med. 2003, 348, 1639–1646. [Google Scholar] [CrossRef]
- Shi, W.Y.; Moreno-Betancur, M.; Nugent, A.W.; Cheung, M.; Colan, S.; Turner, C.; Sholler, G.F.; Robertson, T.; Justo, R.; Bullock, A.; et al. Long-Term Outcomes of Childhood Left Ventricular Noncompaction Cardiomyopathy. Circulation 2018, 138, 367–376. [Google Scholar] [CrossRef]
- Jefferies, J.L.; Wilkinson, J.D.; Sleeper, L.A.; Colan, S.D.; Lu, M.; Pahl, E.; Kantor, P.; Everitt, M.D.; Webber, S.A.; Kaufman, B.D.; et al. Cardiomyopathy Phenotypes and Outcomes for Children with Left Ventricular Myocardial Noncompaction: Results From the Pediatric Cardiomyopathy Registry. J. Card. Fail. 2015, 21, 877–884. [Google Scholar] [CrossRef]
- Charron, P.; Elliott, P.M.; Gimeno, J.R.; Caforio, A.L.P.; Kaski, J.P.; Tavazzi, L.; Tendera, M.; Maupain, C.; Laroche, C.; Rubis, P.; et al. The Cardiomyopathy Registry of the EURObservational Research Programme of the European Society of Cardiology: Baseline data and contemporary management of adult patients with cardiomyopathies. Eur. Heart J. 2018, 39, 1784–1793. [Google Scholar] [CrossRef]
- Seyler, C.; Meder, B.; Weis, T.; Schwaneberg, T.; Weitmann, K.; Hoffmann, W.; Katus, H.A.; Dösch, A. TranslatiOnal Registry for CardiomyopatHies (TORCH)—rationale and first results. ESC Heart Fail. 2017, 4, 209–215. [Google Scholar] [CrossRef]
- Arunamata, A.; Punn, R.; Cuneo, B.; Bharati, S.; Silverman, N.H.; Silverman, N.H. Echocardiographic Diagnosis and Prognosis of Fetal Left Ventricular Noncompaction. J. Am. Soc. Echocardiogr. 2012, 25, 112–120. [Google Scholar] [CrossRef]
- Vinograd, C.A.; Srivastava, S.; Panesar, L.E. Fetal Diagnosis of Left-Ventricular Noncompaction Cardiomyopathy in Identical Twins with Discordant Congenital Heart Disease. Pediatr. Cardiol. 2012, 34, 1503–1507. [Google Scholar] [CrossRef]
- Tian, L.; Zhou, Q.; Zhou, J.; Zeng, S.; Cao, D.; Zhang, M. Ventricular non-compaction cardiomyopathy: Prenatal diagnosis and pathology. Prenat. Diagn. 2014, 35, 221–227. [Google Scholar] [CrossRef]
- Sato, Y.; Matsumoto, N.; Matsuo, S.; Yoda, S.; Iida, K.; Kunimasa, T.; Kunimoto, S.; Saito, S. Isolated noncompaction of the ventricular myocardium in a 94-year-old patient: Depiction at echocardiography and magnetic resonance imaging. Int. J. Cardiol. 2007, 119, e32–e34. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.T.; Thaman, R.; Blanes, J.G.; Ward, D.; Sevdalis, E.; Papra, E.; Kiotsekolglou, A.; Tome, M.T.; Pellerin, D.; McKenna, W.J.; et al. Natural history and familial characteristics of isolated left ventricular non-compaction. Eur. Heart J. 2004, 26, 187–192. [Google Scholar] [CrossRef]
- Oechslin, E.N.; Jost, C.H.A.; Rojas, J.R.; Kaufmann, P.; Jenni, R. Long-term follow-up of 34 adults with isolated left ventricular noncompaction: A distinct cardiomyopathy with poor prognosis. J. Am. Coll. Cardiol. 2000, 36, 493–500. [Google Scholar] [CrossRef]
- Sandhu, R.; Finkelhor, R.S.; Gunawardena, D.R.; Bahler, R.C. Prevalence and characteristics of left ventricular noncompaction in a community hospital cohort of patients with systolic dysfunction. Echocardiography 2008, 25, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Ritter, M.; Oechslin, E.; Sütsch, G.; Attenhofer, C.; Schneider, J.; Jenni, R. Isolated Noncompaction of the Myocardium in Adults. Mayo Clin. Proc. 1997, 72, 26–31. [Google Scholar] [CrossRef]
- Ross, S.B.; Jones, K.; Blanch, B.; Puranik, R.; McGeechan, K.; Barratt, A.; Semsarian, C. A systematic review and meta-analysis of the prevalence of left ventricular non-compaction in adults. Eur. Heart J. 2020, 41, 1428–1436. [Google Scholar] [CrossRef]
- Weir-McCall, J.R.; Yeap, P.M.; Papagiorcopulo, C.; Fitzgerald, K.; Gandy, S.J.; Lambert, M.; Belch, J.J.F.; Cavin, I.; Littleford, R.; Macfarlane, J.A.; et al. Left Ventricular Noncompaction Anatomical phenotype or distinct cardiomyopathy? J. Am. Coll. Cardiol. 2016, 68, 2157–2165. [Google Scholar] [CrossRef]
- Engberding, R.; Stöllberger, C.; Schneider, B.; Nothnagel, D.; Fehske, W.; Gerecke, B.J. Heart failure in noncompaction cardiomyopathy—Data from the German noncompaction registry (ALKK). Circulation 2012, 126, A14769. [Google Scholar]
- Gerecke, B.; Stoellberger, C.; Schneider, B.; Fehske, W.; Nothnagel, D.; Engberding, R. Arrhythmias in isolated noncompaction cardiomyopathy—Data form the German Noncompaction Registry (ALKK). Circulation 2011, 124, A11978. [Google Scholar]
- Gerecke, B.; Engberding, R. Isolated noncompaction cardiomyopathy with special emphasis on arrhythmia complications. Herzschr. Elektrophys. 2012, 23, 201–210. [Google Scholar] [CrossRef]
- Howard, T.S.; Valdes, S.O.; Hope, K.; Morris, S.A.; Landstrom, A.P.; Schneider, A.E.; Miyake, C.Y.; Denfield, S.W.; Pignatelli, R.H.; Wang, Y.; et al. Association of Wolff-Parkinson-White with Left Ventricular Noncompaction Cardiomyopathy in Children. J. Card. Fail. 2019, 25, 1004–1008. [Google Scholar] [CrossRef]
- Stöllberger, C.; Blazek, G.; Dobias, C.; Hanafin, A.; Wegner, C.; Finsterer, J. Frequency of Stroke and Embolism in Left Ventricular Hypertrabeculation/Noncompaction. Am. J. Cardiol. 2011, 108, 1021–1023. [Google Scholar] [CrossRef]
- Stöllberger, C.; Blazek, G.; Gessner, M.; Bichler, K.; Wegner, C.; Finsterer, J. Neuromuscular comorbidity, heart failure, and atrial fibrillation as prognostic factors in left ventricular hypertrabeculation/noncompaction. Herz 2015, 40, 906–911. [Google Scholar] [CrossRef]
- Stöllberger, C.; Wegner, C.; Finsterer, J. Left ventricular hypertrabeculation/noncompaction, cardiac phenotype, and neuromuscular disorders. Herz 2018, 44, 659–665. [Google Scholar] [CrossRef]
- Moric-Janiszewska, E.; Markiewicz-Łoskot, G. Genetic Heterogeneity of Left-ventricular Noncompaction Cardiomyopathy. Clin. Cardiol. 2008, 31, 201–204. [Google Scholar] [CrossRef]
- Stähli, B.E.; Gebhard, C.; Biaggi, P.; Klaassen, S.; Buechel, E.V.; Jost, C.H.A.; Jenni, R.; Tanner, F.C.; Greutmann, M. Left ventricular non-compaction: Prevalence in congenital heart disease. Int. J. Cardiol. 2013, 167, 2477–2481. [Google Scholar] [CrossRef] [PubMed]
- Jost, C.H.A.; Connolly, H.M.; O’Leary, P.W.; Warnes, C.A.; Tajik, A.J.; Seward, J.B. Left Heart Lesions in Patients With Ebstein Anomaly. Mayo Clin. Proc. 2005, 80, 361–368. [Google Scholar] [CrossRef]
- Friedberg, M.K.; Ursell, P.C.; Silverman, N.H. Isomerism of the Left Atrial Appendage Associated With Ventricular Noncompaction. Am. J. Cardiol. 2005, 96, 985–990. [Google Scholar] [CrossRef]
- Lilje, C.; Porciani, M.C.; Lilli, A.; Macioce, R.; Cappelli, F.; Demarchi, G.; Pappone, A.; Ricciardi, G.; Padeletti, L. Complications of non-compaction of the left ventricular myocardium in a paediatric population: A prospective study. Eur. Heart J. 2006, 27, 1855–1860. [Google Scholar] [CrossRef] [PubMed]
- Finsterer, J. Barth syndrome: Mechanisms and management. Appl. Clin. Genet. 2019, 12, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Panduranga, P.; Mukhaini, M.K. Left-ventricular non-compaction with coronary artery disease. Int. J. Cardiol. 2011, 150, e37–e39. [Google Scholar] [CrossRef] [PubMed]
- Toufan, M.; Shahvalizadeh, R.; Khalili, M. Myocardial infarction in a patient with left ventricular noncompaction: A case report. Int. J. Gen. Med. 2012, 5, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Yavuzgil, O.; Gurgun, C.; Çinar, C.S.; Yüksel, A. Anterior myocardial infarction in an adult patient with left ventricular hypertrabeculation/noncompaction. Int. J. Cardiol. 2006, 106, 394–395. [Google Scholar] [CrossRef] [PubMed]
- Engberding, R.; Stöllberger, C.; Ong, P.; Yelbuz, T.M.; Gerecke, B.J.; Breithardt, G. Isolated Non-Compaction Cardiomyopathy. Dtsch. Aerzteblatt Online 2010, 107, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Paterick, T.E.; Tajik, A.J. Left ventricular noncompaction—A diagnostically challenging cardiomyopathy. Circ. J. 2012, 76, 1556–1562. [Google Scholar] [CrossRef]
- Senior, R.; Becher, H.; Monaghan, M.; Agati, L.; Zamorano, J.; Vanoverschelde, J.L.; Nihoyannopoulos, P.; Edvardsen, T.; Lancellotti, P.; Delgado, V.; et al. Clinical practice of contrast echocardiography: Recommendation by the European Association of Cardiovascular Imaging (EACVI) 2017. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1205–1205af. [Google Scholar] [CrossRef]
- Bhat, T.; Lafferty, J.; Teli, S.; Rjaili, G.A.; Olkovsky, Y.; Costantino, T. Isolated left ventricular noncompaction cardiomyo-pathy diagnosed by transesophageal echocardiography. Clin. Med. Insights Cardiol. 2011, 5, 23–27. [Google Scholar] [CrossRef]
- Soliman, O.I.; McGhie, J.; ten Cate, F.J.; Paelinck, B.P.; Caliskan, K. Multimodality Imaging, Diagnostic Challenges and Proposed Diagnostic Algorithm for Noncompaction Cardiomyopathy. In Noncompaction Cardiomyopathy; Caliskan, K., Soliman, O.I., ten Cate, F.J., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 17–40. [Google Scholar]
- Engberding, R.; Stöllberger, C.; Gerecke, B.J. Left ventricular noncompaction: Affected regions in respect to LV function—Data from the German Noncompaction Registry (ALKK). Circulation 2012, 126, A14830. [Google Scholar]
- Engberding, R.; Gerecke, B. Noncompaction Cardiomyopathy, a Novel Clinical Entity (Historical Perspective). In Noncompaction Cardiomyopathy; Caliskan, K., Soliman, O.I., ten Cate, F.J., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 1–16. [Google Scholar] [CrossRef]
- Engberding, R.; Yelbuz, T.M.; Breithardt, G. Isolated noncompaction of the left ventricular myocardium—A review of the literature two decades after the initial case description. Clin. Res. Cardiol. 2007, 96, 481–488. [Google Scholar] [CrossRef]
- Paterick, T.E.; Umland, M.M.; Jan, M.F.; Ammar, K.A.; Kramer, C.; Khandheria, B.K.; Seward, J.B.; Tajik, A.J. Left Ventricular Noncompaction: A 25-Year Odyssey. J. Am. Soc. Echocardiogr. 2012, 25, 363–375. [Google Scholar] [CrossRef]
- Frischknecht, B.S.; Attenhofer Jost, C.H.; Oechslin, E.N.; Seifert, B.; Hoigné, P.; Roos, M.; Jenni, R. Validation of noncompaction criteria in dilated cardiomyopathy, and valvular and hypertensive heart disease. J. Am. Soc. Echocardiogr. 2005, 18, 865–872. [Google Scholar] [CrossRef]
- Belanger, A.R.; Miller, M.A.; Donthireddi, U.R.; Najovits, A.J.; Goldman, M.E. New Classification Scheme of Left Ventricular Noncompaction and Correlation with Ventricular Performance. Am. J. Cardiol. 2008, 102, 92–96. [Google Scholar] [CrossRef]
- Gebhard, C.; Stähli, B.E.; Greutmann, M.; Biaggi, P.; Jenni, R.; Tanner, F.C. Reduced Left Ventricular Compacta Thickness: A Novel Echocardiographic Criterion for Non-Compaction Cardiomyopathy. J. Am. Soc. Echocardiogr. 2012, 25, 1050–1057. [Google Scholar] [CrossRef]
- Sabatino, J.; Di Salvo, G.; Krupickova, S.; Fraisse, A.; Prota, C.; Bucciarelli, V.; Josen, M.; Paredes, J.; Sirico, D.; Voges, I.; et al. Left Ventricular Twist Mechanics to Identify Left Ventricular Noncompaction in Childhood. Circ. Cardiovasc. Imaging 2019, 12, e007805. [Google Scholar] [CrossRef]
- Van Dalen, B.M.; Caliskan, K.; Soliman, O.I.; Nemes, A.; Vletter, W.B.; Cate, F.J.T.; Geleijnse, M.L. Left ventricular solid body rotation in non-compaction cardiomyopathy: A potential new objective and quantitative functional diagnostic criterion? Eur. J. Heart Fail. 2008, 10, 1088–1093. [Google Scholar] [CrossRef] [PubMed]
- Caliskan, K.; Soliman, O.; Nemes, A.; Van Domburg, R.T.; Simoons, M.L.; Geleijnse, M.L. No relationship between left ventricular radial wall motion and longitudinal velocity and the extent and severity of noncompaction cardiomyopathy. Cardiovasc. Ultrasound 2012, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Rudolecká, J.; Veiser, T.; Plášek, J.; Homza, M.; Fürstová, J. Ventricular twist in isolated left ventricular noncompaction. Cor. Vasa 2014, 56, e471–e477. [Google Scholar] [CrossRef]
- Jenni, R.; Oechslin, E.; Schneider, J.; Attenhofer Jost, J.; Kaufmann, P.A. Echocardiographic and pathoanatomical characteristics of isolated left ventricular non-compaction: A step towards classification as a distinct cardiomyopathy. Heart 2001, 86, 666–671. [Google Scholar] [CrossRef]
- Arunamata, A.; Stringer, J.; Balasubramanian, S.; Tacy, T.A.; Silverman, N.H.; Punn, R. Cardiac Segmental Strain Analysis in Pediatric Left Ventricular Noncompaction Cardiomyopathy. J. Am. Soc. Echocardiogr. 2019, 32, 763–773. [Google Scholar] [CrossRef]
- Huttin, O.; Venner, C.; Frikha, Z.; Voilliot, D.; Marie, P.-Y.; Aliot, E.; Sadoul, N.; Juillière, Y.; Brembilla-Perrot, B.; Selton-Suty, C. Myocardial deformation pattern in left ventricular non-compaction: Comparison with dilated cardiomyopathy. IJC Heart Vasc. 2014, 5, 9–14. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stöllberger, C.; Gerecke, B.; Finsterer, J.; Engberding, R. Refinement of echocardiographic criteria for left ventricular noncompaction. Int. J. Cardiol. 2013, 165, 463–467. [Google Scholar] [CrossRef]
- Stöllberger, C.; Gerecke, B.; Engberding, R.; Grabner, B.; Wandaller, C.; Finsterer, J.; Gietzelt, M.; Balzereit, A. Interobserver Agreement of the Echocardiographic Diagnosis of LV Hypertrabeculation/Noncompaction. JACC Cardiovasc. Imaging 2015, 8, 1252–1257. [Google Scholar] [CrossRef]
- Joong, A.; Hayes, D.A.; Anderson, B.R.; Zuckerman, W.A.; Carroll, S.J.; Lai, W.W. Comparison of Echocardiographic Diagnostic Criteria of Left Ventricular Noncompaction in a Pediatric Population. Pediatr. Cardiol. 2017, 38, 1493–1504. [Google Scholar] [CrossRef] [PubMed]
- Kohli, S.K.; Pantazis, A.A.; Shah, J.S.; Adeyemi, B.; Jackson, G.; McKenna, W.J.; Sharma, S.; Elliott, P.M. Diagnosis of left-ventricular non-compaction in patients with left-ventricular systolic dysfunction: Time for a reappraisal of diagnostic criteria? Eur. Heart J. 2007, 29, 89–95. [Google Scholar] [CrossRef]
- Petersen, S.E.; Selvanayagam, J.B.; Wiesmann, F.; Robson, M.D.; Francis, J.M.; Anderson, R.H.; Watkins, H.; Neubauer, S. Left Ventricular Non-Compaction. Insights from cardiovascular magnetic resonance imaging. J. Am. Coll. Cardiol. 2005, 46, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Stacey, R.B.; Andersen, M.M.; Clair, M.S.; Hundley, W.G.; Thohan, V. Comparison of Systolic and Diastolic Criteria for Isolated LV Noncompaction in CMR. JACC Cardiovasc. Imaging 2013, 6, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Jacquier, A.; Thuny, F.; Jop, B.; Giorgi, R.; Cohen, F.; Gaubert, J.-Y.; Vidal, V.; Bartoli, J.M.; Habib, G.; Moulin, G. Measurement of trabeculated left ventricular mass using cardiac magnetic resonance imaging in the diagnosis of left ventricular non-compaction. Eur. Heart J. 2010, 31, 1098–1104. [Google Scholar] [CrossRef]
- Captur, G.; Zemrak, F.; Muthurangu, V.; Petersen, S.E.; Chumming, L.; Bassett, P.; Kawel-Boehm, N.; McKenna, W.J.; Elliott, P.M.; Lima, J.A.; et al. Fractal analysis of myocardial trabeculations in 2547 study participants: Multi-ethnic study of atherosclerosis. Radiology 2015, 277, 707–715. [Google Scholar] [CrossRef]
- Grothoff, M.; Pachowsky, M.; Hoffmann, J.; Posch, M.; Klaassen, S.; Lehmkuhl, L.; Gutberlet, M. Value of cardiovascular MR in diagnosing left ventricular non-compaction cardiomyopathy and in discriminating between other cardiomyopathies. Eur. Radiol. 2012, 22, 2699–2709. [Google Scholar] [CrossRef]
- Dreisbach, J.G.; Mathur, S.; Houbois, C.P.; Oechslin, E.; Ross, H.; Hanneman, K.; Wintersperger, B.J. Cardiovascular magnetic resonance based diagnosis of left ventricular non-compaction cardiomyopathy: Impact of cine bSSFP strain analysis. J. Cardiovasc. Magn. Reson. 2020, 22, 1–14. [Google Scholar] [CrossRef]
- Dodd, J.D.; Holmvang, G.; Hoffmann, U.; Ferencik, M.; Abbara, S.; Brady, T.J.; Cury, R.C. Quantification of Left Ventricular Noncompaction and Trabecular Delayed Hyperenhancement with Cardiac MRI: Correlation with Clinical Severity. Am. J. Roentgenol. 2007, 189, 974–980. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, S.M.; Lee, S.-C.; Chang, S.-A.; Jang, S.Y.; Choe, Y.H. Quantification of left ventricular trabeculae using cardiovascular magnetic resonance for the diagnosis of left ventricular non-compaction: Evaluation of trabecular volume and refined semi-quantitative criteria. J. Cardiovasc. Magn. Reson. 2016, 18, 1–13. [Google Scholar] [CrossRef]
- Miller, E.M.; Hinton, R.B.; Czosek, R.; Lorts, A.; Parrott, A.; Shikany, A.R.; Ittenbach, R.F.; Ware, S.M. Genetic Testing in Pediatric Left Ventricular Noncompaction. Circ. Cardiovasc. Genet. 2017, 10, e001735. [Google Scholar] [CrossRef]
- Donal, E.; Delgado, V.; Bucciarelli-Ducci, C.; Galli, E.; Haugaa, K.H.; Charron, P.; Voigt, J.-U.; Cardim, N.; Masci, P.G.; Galderisi, M.; et al. Multimodality imaging in the diagnosis, risk stratification, and management of patients with dilated cardiomyopathies: An expert consensus document from the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 1075–1093. [Google Scholar] [CrossRef]
- Conces, D.J.; Ryan, T.; Tarver, R.D. Noncompaction of ventricular myocardium: CT appearance. Am. J. Roentgenol. 1991, 156, 717–718. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Meléndez-Ramírez, G.; Castillo-Castellon, F.; Espínola-Zavaleta, N.; Meave, A.; Kimura-Hayama, E.T. Left ventricular noncompaction: A proposal of new diagnostic criteria by multidetector computed tomography. J. Cardiovasc. Comput. Tomogr. 2012, 6, 346–354. [Google Scholar] [CrossRef]
- Fuchs, T.A.; Erhart, L.; Ghadri, J.R.; Herzog, B.A.; Giannopoulos, A.; Buechel, R.R.; Stämpfli, S.F.; Gruner, C.; Pazhenkottil, A.P.; Niemann, M.; et al. Diagnostic criteria for left ventricular non-compaction in cardiac computed tomography. PLoS ONE 2020, 15, e0235751. [Google Scholar] [CrossRef]
- Stöllberger, C.; Winkler-Dworak, M.; Blazek, G.; Finsterer, J. Association of Electrocardiographic Abnormalities with Cardiac Findings and Neuromuscular Disorders in Left Ventricular Hypertrabeculation/Non-Compaction. Cardiology 2007, 107, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Rapatz, K.; Finsterer, J.; Voill-Glaninger, A.; Wilfinger-Lutz, N.; Winkler-Dworak, M.; Stöllberger, C. NT-pro-BNP in patients with left ventricular hypertrabeculation/non-compaction. ESC Heart Fail. 2020, 7, 4126–4133. [Google Scholar] [CrossRef] [PubMed]
- Finsterer, J.; Stöllberger, C.; Krugluger, W. Positive troponin-T in noncompaction is associated with neuromuscular disorders and poor outcome. Clin. Res. Cardiol. 2006, 96, 109–113. [Google Scholar] [CrossRef]
- Biagini, E.; Ragni, L.; Ferlito, M.; Pasquale, F.; Lofiego, C.; Leone, O.; Rocchi, G.; Perugini, E.; Zagnoni, S.; Branzi, A.; et al. Different Types of Cardiomyopathy Associated with Isolated Ventricular Noncompaction. Am. J. Cardiol. 2006, 98, 821–824. [Google Scholar] [CrossRef]
- Stollberger, C.; Finsterer, J. Pitfalls in the diagnosis of left ventricular hypertrabeculation/non-compaction. Postgrad. Med. J. 2006, 82, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Schaufelberger, M. Cardiomyopathy and pregnancy. Heart 2019, 105, 1543–1551. [Google Scholar] [CrossRef]
- Reimold, S.C. Reversible left ventricular trabeculations in pregnancy. Is this sufficient to make the diagnosis of left ventricular noncompaction? Circulation 2014, 130, 453–456. [Google Scholar] [CrossRef]
- Rajagopalan, N.; Attili, A.K.; Bodiwala, K.; Bailey, A.L. Features of left ventricular noncompaction in peripartum cardiomyopathy: A case series. Int. J. Cardiol. 2013, 165, e13–e14. [Google Scholar] [CrossRef]
- Luijkx, T.; Cramer, M.J.; Zaidi, A.; Rienks, R.; Senden, P.J.; Sharma, S.; Van Hellemondt, F.J.; Buckens, C.F.; Mali, W.P.; Velthuis, B.K. Ethnic differences in ventricular hypertrabeculation on cardiac MRI in elite football players. Neth. Heart J. 2012, 20, 389–395. [Google Scholar] [CrossRef]
- de la Chica, J.A.; Gómez-Talavera, S.; García-Ruiz, J.M.; García-Lunar, I.; Oliva, B.; Fernández-Alvira, J.M.; López-Melgar, B.; Sánchez-González, J.; de la Pompa, J.L.; Mendiguren, J.M.; et al. Association Between Left Ventricular Noncompaction and Vigorous Physical Activity. J. Am. Coll. Cardiol. 2020, 76, 1723–1733. [Google Scholar] [CrossRef]
- Loria, V.; Colizzi, C.; Vaccarella, M.; Franceschi, F.; Aspromonte, N. Left Ventricular Noncompaction: Cause or Consequence of Myocardial Disease? A Case Report and Literature Review. Cardiology 2019, 143, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Luckie, M.; Irwin, B.; Nair, S.; Greenwood, J.; Khattar, R. Left ventricular non-compaction in identical twins with thalassaemia and cardiac iron overload. Eur. J. Echocardiogr. 2009, 10, 509–512. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Harvey, R.P. Patterning the vertebrate heart. Nat. Rev. Genet. 2002, 3, 544–556. [Google Scholar] [CrossRef]
- Srivastava, D.; Olson, E.N. A genetic blueprint for cardiac development. Nat. Cell Biol. 2000, 407, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Sedmera, D.; Pexieder, T.; Vuillemin, M.; Thompson, R.P.; Anderson, R.H. Developmental patterning of the myocardium. Anat. Rec. 2000, 258, 319–337. [Google Scholar] [CrossRef]
- Agmon, Y.; Connolly, H.M.; Olson, L.J.; Khandheria, B.K.; Seward, J.B. Noncompaction of the Ventricular Myocardium. J. Am. Soc. Echocardiogr. 1999, 12, 859–863. [Google Scholar] [CrossRef]
- Bernanke, D.H.; Velkey, J.M. Development of the coronary blood supply: Changing concepts and current ideas. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2002, 269, 198–208. [Google Scholar] [CrossRef]
- Freedom, R.M.; Yoo, S.-J.; Perrin, D.; Taylor, G.; Petersen, S.; Anderson, R.H. The morphological spectrum of ventricular noncompaction. Cardiol. Young 2005, 15, 345–364. [Google Scholar] [CrossRef] [PubMed]
- Henderson, D.J.; Anderson, R.H. The Development and Structure of the Ventricles in the Human Heart. Pediatr. Cardiol. 2009, 30, 588–596. [Google Scholar] [CrossRef] [PubMed]
- MacGrogan, D.; Münch, J.; De La Pompa, J.L. Notch and interacting signalling pathways in cardiac development, disease, and regeneration. Nat. Rev. Cardiol. 2018, 15, 685–704. [Google Scholar] [CrossRef]
- Luxán, G.; Casanova, J.C.; Martínez-Poveda, B.; Prados, B.; D’Amato, G.; MacGrogan, D.; Gonzalez-Rajal, A.; Dobarro, D.; Torroja, C.; Martinez, F.; et al. Mutations in the NOTCH pathway regulator MIB1 cause left ventricular noncompaction cardiomyopathy. Nat. Med. 2013, 19, 193–201. [Google Scholar] [CrossRef]
- Meyer, D.; Birchmeier, C. Multiple essential functions of neuregulin in development. Nat. Cell Biol. 1995, 378, 386–390. [Google Scholar] [CrossRef]
- Gassmann, M.; Casagranda, F.; Orioli, D.; Simon, H.; Lai, C.; Klein, R.; Lemke, G. Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor. Nat. Cell Biol. 1995, 378, 390–394. [Google Scholar] [CrossRef]
- Choquet, C.; Kelly, R.; Miquerol, L. Defects in Trabecular Development Contribute to Left Ventricular Noncompaction. Pediatr. Cardiol. 2019, 40, 1331–1338. [Google Scholar] [CrossRef]
- Allenby, P.A.; Gould, N.S.; Schwartz, M.F.; Chiemmongkoltip, P. Dysplastic cardiac development presenting as cardiomyopathy. Arch Pathol. Lab Med. 1988, 122, 1255–1258. [Google Scholar]
- Boyd, M.T.; Seward, J.B.; Tajik, A.J.; Edwards, W.D. Frequency and location of prominent left ventricular trabeculations at autopsy in 474 normal human hearts: Implications for evaluation of mural thrombi by two-dimensional echocardiography. J. Am. Coll. Cardiol. 1987, 9, 323–326. [Google Scholar] [CrossRef]
- Stöllberger, C.; Finsterer, J. Left ventricular hypertrabeculation/noncompaction. J. Am. Soc. Echocardiogr. 2004, 17, 91–100. [Google Scholar] [CrossRef]
- Burke, A.; Mont, E.; Kutys, R.; Virmani, R. Left ventricular noncompaction: A pathological study of 14 cases. Hum. Pathol. 2005, 36, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Finsterer, J.; Stöllberger, C. Ultrastructural Findings in Noncompaction Prevail with Neuromuscular Disorders. Cardiology 2013, 126, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Prentice, H. Studies on Left Ventricular Hypertrabeculation/Noncompaction: The Need for In-Depth Ultrastructural Investigations. Cardiology 2013, 126, 255–257. [Google Scholar] [CrossRef] [PubMed]
- Bleyl, S.B.; Mumford, B.R.; Thompson, V.; Carey, J.C.; Pysher, T.J.; Chin, T.K.; Ward, K. Neonatal, Lethal Noncompaction of the Left Ventricular Myocardium Is Allelic with Barth Syndrome. Am. J. Hum. Genet. 1997, 61, 868–872. [Google Scholar] [CrossRef]
- Bozkurt, B.; Colvin, M.; Cook, J.; Cooper, L.T.; Deswal, A.; Fonarow, G.; Francis, G.S.; Lenihan, D.; Lewis, E.F.; McNamara, D.M.; et al. Current Diagnostic and Treatment Strategies for Specific Dilated Cardiomyopathies: A Scientific Statement from the American Heart Association. Circulation 2016, 134, e579–e646. [Google Scholar] [CrossRef] [PubMed]
- Corrado, G.; Checcarelli, N.; Santarone, M.; Stöllberger, C.; Finsterer, J. Left Ventricular Hypertrabeculation/Noncompaction with PMP22 Duplication-Based Charcot-Marie-Tooth Disease Type 1A. Cardiology 2006, 105, 142–145. [Google Scholar] [CrossRef]
- Johnson, M.T.; Zhang, S.; Gilkeson, R.; Ameduri, R.; Siwik, E.; Patel, C.R.; Chebotarev, O.; Kenton, A.B.; Bowles, K.R.; Towbin, J.A.; et al. Intrafamilial variability of noncompaction of the ventricular myocardium. Am. Heart J. 2006, 151, 1012–e7. [Google Scholar] [CrossRef]
- Klaassen, S.; Probst, S.; Oechslin, E.; Gerull, B.; Krings, G.; Schuler, P.; Greutmann, M.; Hurlimann, D.; Yegitbasi, M.; Pons, L.; et al. Mutations in Sarcomere Protein Genes in Left Ventricular Noncompaction. Circulation 2008, 117, 2893–2901. [Google Scholar] [CrossRef]
- Vio, R.; Angelini, A.; Basso, C.; Cipriani, A.; Zorzi, A.; Melacini, P.; Thiene, G.; Rampazzo, A.; Corrado, D.; Calore, C. Hypertrophic Cardiomyopathy and Primary Restrictive Cardiomyopathy: Similarities, Differences and Phenocopies. J. Clin. Med. 2021, 10, 1954. [Google Scholar] [CrossRef]
- van Waning, J.I.; Caliskan, K.; Michels, M.; Schinkel, A.F.; Hirsch, A.; Dalinghaus, M.; Hoedemaekers, Y.M.; Wessels, M.W.; IJpma, A.S.; Hofstra, R.M.; et al. Cardiac Phenotypes, Genetics, and Risks in Familial Noncompaction Cardiomyopathy. J. Am. Coll. Cardiol. 2019, 73, 1601–1611. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; Makita, N.; Xing, Y.; Watanabe, S.; Futatani, T.; Ye, F.; Saito, K.; Ibuki, K.; Watanabe, K.; Hirono, K. SCN5A variants in Japanese patients with left ventricular noncompaction and arrhythmia. Mol. Genet. Metab. 2008, 93, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Kolokotronis, K.; Kühnisch, J.; Klopocki, E.; Dartsch, J.; Rost, S.; Huculak, C.; Mearini, G.; Störk, S.; Carrier, L.; Klaassen, S.; et al. Biallelic mutation in MYH7 and MYBPC3 leads to severe cardiomyopathy with left ventricular noncompaction phenotype. Hum. Mutat. 2019, 40, 1101–1114. [Google Scholar] [CrossRef]
- Ichida, F. Left ventricular noncompaction—risk stratification and genetic consideration. J. Cardiol. 2019, 75, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mazzarotto, F.; Hawley, M.H.; Beltrami, M.; Beekman, L.; de Marvao, A.; McGurk, K.A.; Statton, B.; Boschi, B.; Girolami, F.; Roberts, A.M.; et al. Systematic large-scale assessment of the genetic architecture of left ventricular noncompaction reveals diverse etiologies. Genet. Med. 2021, 23, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.B.; Singer, E.S.; Driscoll, E.; Nowak, N.; Yeates, L.; Puranik, R.; Sy, R.W.; Rajagopalan, S.; Barratt, A.; Ingles, J.; et al. Genetic architecture of left ventricular noncompaction in adults. Hum. Genome Var. 2020, 7, 33. [Google Scholar] [CrossRef]
- Hershberger, R.E.; Givertz, M.M.; Ho, C.Y.; Judge, D.P.; Kantor, P.F.; McBride, K.L.; Morales, A.; Taylor, M.R.; Vatta, M. Genetic evaluation of cardiomyopathy: A clinical practice resource of the American College of Medical Genetics and Genomics (ACMG). Genet. Med. 2018, 20, 899–909. [Google Scholar] [CrossRef]
- Ackerman, M.J.; Priori, S.G.; Willems, S.; Berul, C.; Brugada, R.; Calkins, H.; Camm, J.; Ellinor, P.T.; Gollob, M.; Hamilton, R.; et al. HRS/EHRA Consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies. Europace 2011, 13, 1077–1109. [Google Scholar] [CrossRef] [PubMed]
- Schulze-Bahr, E.; Klaassen, S.; Abdul-Khaliq, H.; Schunkert, H. Gendiagnostik bei kardiovaskulären Erkrankungen. Positionspapier der Deutschen Gesellschaft für Kardiologie (DGK) und der Deutschen Gesellschaft für Pädiatrische Kardiologie (DGPK). Kardiologe 2015, 9, 213–243. [Google Scholar] [CrossRef]
- Musunuru, K.; Hershberger, R.E.; Day, S.M.; Klinedinst, N.J.; Landstrom, A.P.; Parikh, V.N.; Prakash, S.; Semsarian, C.; Sturm, A.C. on behalf of the American Heart Association Council on Genomic and Precision Medicine; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular and Stroke Nursing; and Council on Clinical Cardiology. Genetic Testing for inherited cardiovascular diseases. Circ. Genom. Precis. Med. 2020, 13, e000067. [Google Scholar] [CrossRef]
- Zemrak, F.; Raisi-Estabragh, Z.; Khanji, M.Y.; Mohiddin, S.A.; Bruder, O.; Wagner, A.; Lombardi, M.; Schwitter, J.; Van Rossum, A.C.; Pilz, G.; et al. Left Ventricular Hypertrabeculation Is Not Associated With Cardiovascular Morbity or Mortality: Insights From the Eurocmr Registry. Front. Cardiovasc. Med. 2020, 7, 158. [Google Scholar] [CrossRef]
- Petersen, S.; Neubauer, S. Excessive trabeculations and prognosis. The plot thickens. Circ. Cardiovasc. Imaging 2017, 10, e006908. [Google Scholar] [CrossRef]
- Lofiego, C.; Biagini, E.; Ferlito, M.; Pasquale, F.; Rocchi, G.; Perugini, E.; Leone, O.; Bracchetti, G.; Caliskan, K.; Branzi, A.; et al. Paradoxical Contributions of Non-Compacted and Compacted Segments to Global Left Ventricular Dysfunction in Isolated Left Ventricular Noncompaction. Am. J. Cardiol. 2006, 97, 738–741. [Google Scholar] [CrossRef]
- Gerecke, B.J.; Stoellberger, C.; Gietzelt, M.; Haux, R.; Engberding, R. Risk factors in noncompaction cardiomyopathy—Data form the German Noncompaction Registry (ALKK). Eur. Heart J. 2013, 34 (Suppl. 1), 166. [Google Scholar] [CrossRef][Green Version]
- Pignatelli, R.H.; MacMahon, C.J.-; Dreyer, W.J.; Denfield, S.W.; Price, J.; Belmont, J.W.; Craigen, W.J.; Wu, J.; El Said, H.; Bezold, L.I.; et al. Clinical characterization of left ventricular noncompaction in children. A relatively common form of cardiomyopathy. Circulation 2003, 108, 2672–2678. [Google Scholar] [CrossRef] [PubMed]
- Al-Wakeel-Marquard, N.; Degener, F.; Herbst, C.; Kühnisch, J.; Dartsch, J.; Schmitt, B.; Kuehne, T.; Messroghli, D.; Berger, F.; Klaassen, S. RIKADA Study Reveals Risk Factors in Pediatric Primary Cardiomyopathy. J. Am. Heart Assoc. 2019, 8, e012531. [Google Scholar] [CrossRef] [PubMed]
- Van Waning, J.I.; Caliskan, K.; Hoedemaekers, Y.M.; Van Spaendonck-Zwarts, K.Y.; Baas, A.F.; Boekholdt, S.M.; Van Melle, J.P.; Teske, A.J.; Asselbergs, F.W.; Backx, P.C.M.; et al. Genetics, Clinical Features, and Long-Term Outcome of Noncompaction Cardiomyopathy. J. Am. Coll. Cardiol. 2018, 71, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Sedaghat-Hamedani, F.; Haas, J.; Zhu, F.; Geier, C.; Kayvanpour, E.; Liss, M.; Lai, A.; Frese, K.; Pribe-Wolferts, R.; Amr, A.; et al. Clinical genetics and outcome of left ventricular non-compaction cardiomyopathy. Eur. Heart J. 2017, 38, 3449–3460. [Google Scholar] [CrossRef]
- Aung, N.; Doimo, S.; Ricci, F.; Sanghvi, M.M.; Pedrosa, C.; Woodbridge, S.P.; Al-Balah, A.; Zemrak, F.; Khanji, M.Y.; Naci, H.; et al. Prognostic significance of left ventricular noncompaction. Systematic review and meta-analysis of observational studies. Circ. Cardiovasc. Imaging 2020, 13, e009712. [Google Scholar] [CrossRef] [PubMed]
- Amzulescu, M.S.; Rousseau, M.F.; Ahn, S.A.; Boileau, L.; de Mester de Ravenstein, C.; Vancraeynest, D.; Pasquet, A.; Vanoverschelde, J.L.; Pouleur, A.C.; Gerber, B.L. Prognostic impact of hypertrabeculation and noncompaction phenotype in dilated cardiomyopathy. A CMR study. J. Am. Coll. Cardiol. Imging 2015, 8, 934–946. [Google Scholar] [CrossRef] [PubMed]
- Romano, S.; Judd, R.M.; Kim, R.J.; Kim, H.W.; Klem, I.; Heitner, J.F.; Shah, D.J.; Jue, J.; White, B.E.; Indorkar, R.; et al. Feature-Tracking Global Longitudinal Strain Predicts Death in a Multicenter Population of Patients with Ischemic and Nonischemic Dilated Cardiomyopathy Incremental to Ejection Fraction and Late Gadolinium Enhancement. JACC Cardiovasc. Imaging 2018, 11, 1419–1429. [Google Scholar] [CrossRef]
- Grigoratos, C.; Barison, A.; Ivanov, A.; Andreini, D.; Amzulescu, M.S.; Mazurkiewicz, L.; de Luca, A.; Grzybowski, J.; Masci, P.G.; Marczak, M.; et al. Meta-analysis of the prognostic role of late gadolinium enhancement and global systolic impairment in left ventricular noncompaction. J. Am. Coll. Cardiol. Imag 2019, 12, 2141–2151. [Google Scholar] [CrossRef]
- Ashrith, G.; Gupta, D.; Hanmer, J.; Weiss, R.M. Cardiovascular magnetic resonance characterization of left ventricular non-compaction provides independent prognostic information in patients with incident heart failure or suspected cardiomyopathy. J. Cardiovasc. Magn. Reson. 2014, 16, 64. [Google Scholar] [CrossRef]
- Minamisawa, M.; Koyama, J.; Kozuka, A.; Miura, T.; Ebisawa, S.; Motoki, H.; Okada, A.; Izawa, A.; Ikeda, U.; Information, P.E.K.F.C. Regression of left ventricular hypertrabeculation is associated with improvement in systolic function and favorable prognosis in adult patients with non-ischemic cardiomyopathy. J. Cardiol. 2016, 68, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Bertini, M.; Ziacchi, M.; Biffi, M.; Biagini, E.; Rocchi, G.; Martignani, C.; Ferlito, M.; Pasquale, F.; Cervi, E.; Branzi, A.; et al. Effects of cardiac resynchronisation therapy on dilated cardiomyopathy with isolated ventricular non-compaction. Heart 2010, 97, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Uribarri, A.; Rojas, S.V.; Avsar, M.; Hanke, J.S.; Napp, L.C.; Berliner, D.; Bavendiek, U.; Bauersachs, J.; Bara, C.; Sanchez, P.L.; et al. First series of mechanical circulatory support in non-compaction cardiomyopathy: Is LVAD implantation a safe alternative? Int. J. Cardiol. 2015, 197, 128–132. [Google Scholar] [CrossRef]
- Kovacevic-Preradovic, T.; Jenni, R.; Oechslin, E.; Noll, G.; Seifert, B.; Jost, C.A. Isolated Left Ventricular Noncompaction as a Cause for Heart Failure and Heart Transplantation: A Single Center Experience. Cardiology 2009, 112, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Gerecke, B.; Stöllberger, C.; Gradaus, F.; Andresen, H.; Engberding, R. ICD therapy in noncompaction cardiomyopathy: Data from the German left ventricular noncompaction registry (ALKK). Circulation 2009, 120, A2342. [Google Scholar]
- Sohns, C.; Ouyang, F.; Volkmer, M.; Metzner, A.; Nürnberg, J.H.; Ventura, R.; Gerecke, B.; Jansen, H.; Reinhardt, A.; Kuck, K.-H.; et al. Therapy of ventricular arrhythmias in patients suffering from isolated left ventricular non-compaction cardiomyopathy. Europace 2019, 21, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2020, 1125. [Google Scholar] [CrossRef]
- Epstein, A.E.; DiMarco, J.P.; Ellenbogen, K.A.; Estes, N.A.M.; Freedman, R.A.; Gettes, L.S.; Gillinov, A.M.; Gregoratos, G.; Hammill, S.C.; Hayes, D.L.; et al. ACC/AHA/HRS 2008 Guidelines for device-based therapy of cardiac rhythm abnormalities. J. Am. Coll. Cardiol. 2008, 51, e1–e61. [Google Scholar] [CrossRef]
- Priori, S.G.; Blomström-Lundqvist, C.; Mazzanti, A.; Blom, N.; Borggrefe, M.; Camm, J.; Elliott, P.M.; Fitzsimons, D.; Hatala, R. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur. Heart J. 2015, 36, 2793–2867. [Google Scholar] [CrossRef] [PubMed]
- Al-Khatib, S.; Stevenson, W.G.; Ackerman, M.J.; Bryant, W.J.; Callans, D.J.; Curtis, A.B.; Deal, B.J.; Dickfeld, T.; Field, M.E.; Fonarow, G.C.; et al. 2017 AHA/ACC/HRS Guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardia death. Circulation 2018, 138, e272–e391. [Google Scholar] [CrossRef] [PubMed]
- Takamatsu, M.; Kamohara, K.; Sato, M.; Koga, Y. Effect of Noncompacted Myocardial Resection on Isolated Left Ventricular Noncompaction. Ann. Thorac. Surg. 2020, 110, e387–e389. [Google Scholar] [CrossRef]
- Sharma, S.; Gati, S.; Back, M.; Börjesson, M.; Caselli, S.; Collet, J.P.; Corrado, D.; Drezner, J.A.; Halle, M.; Hansen, D.; et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur. Heart J. 2021, 41, 17–96. [Google Scholar] [CrossRef] [PubMed]

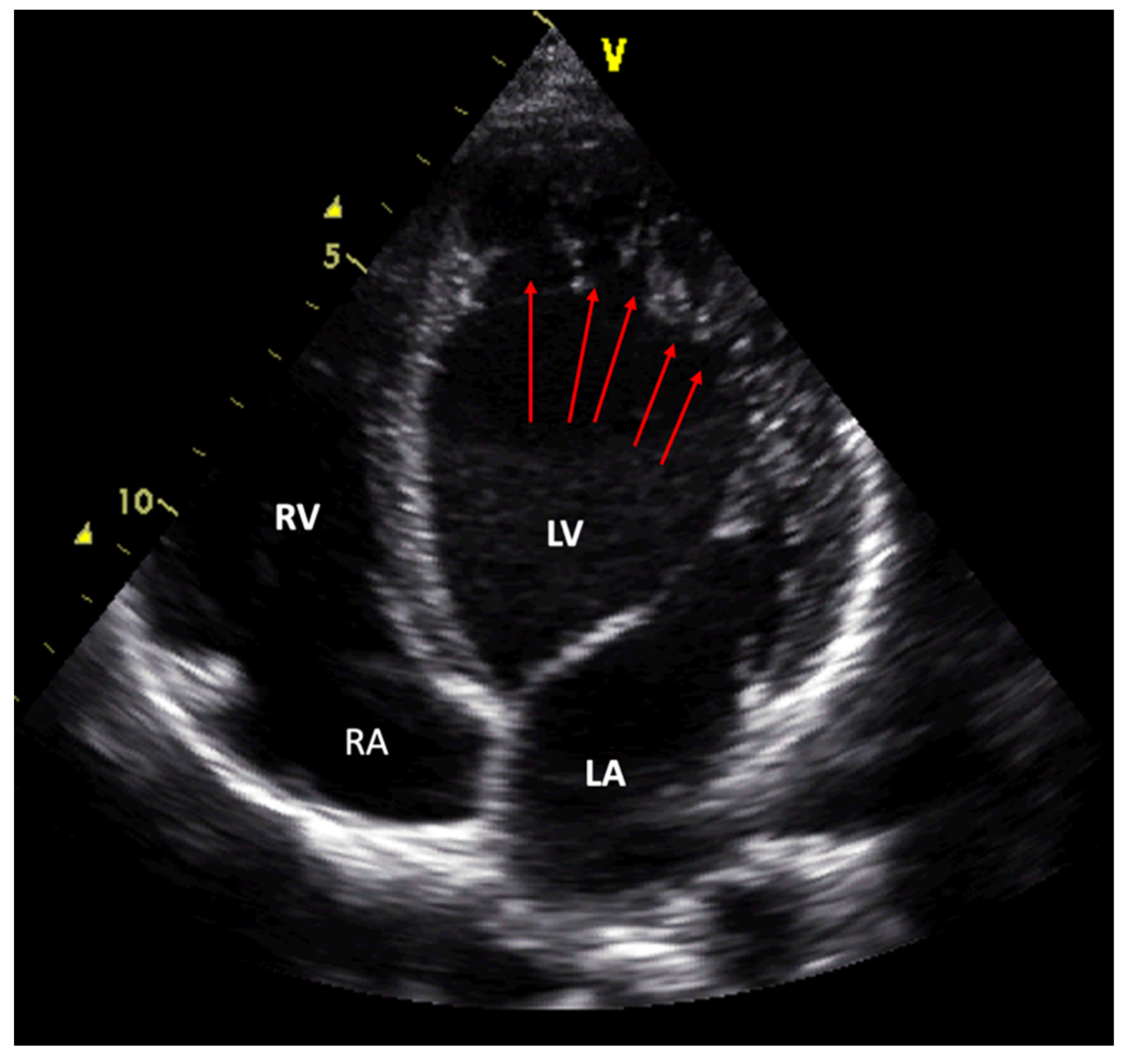
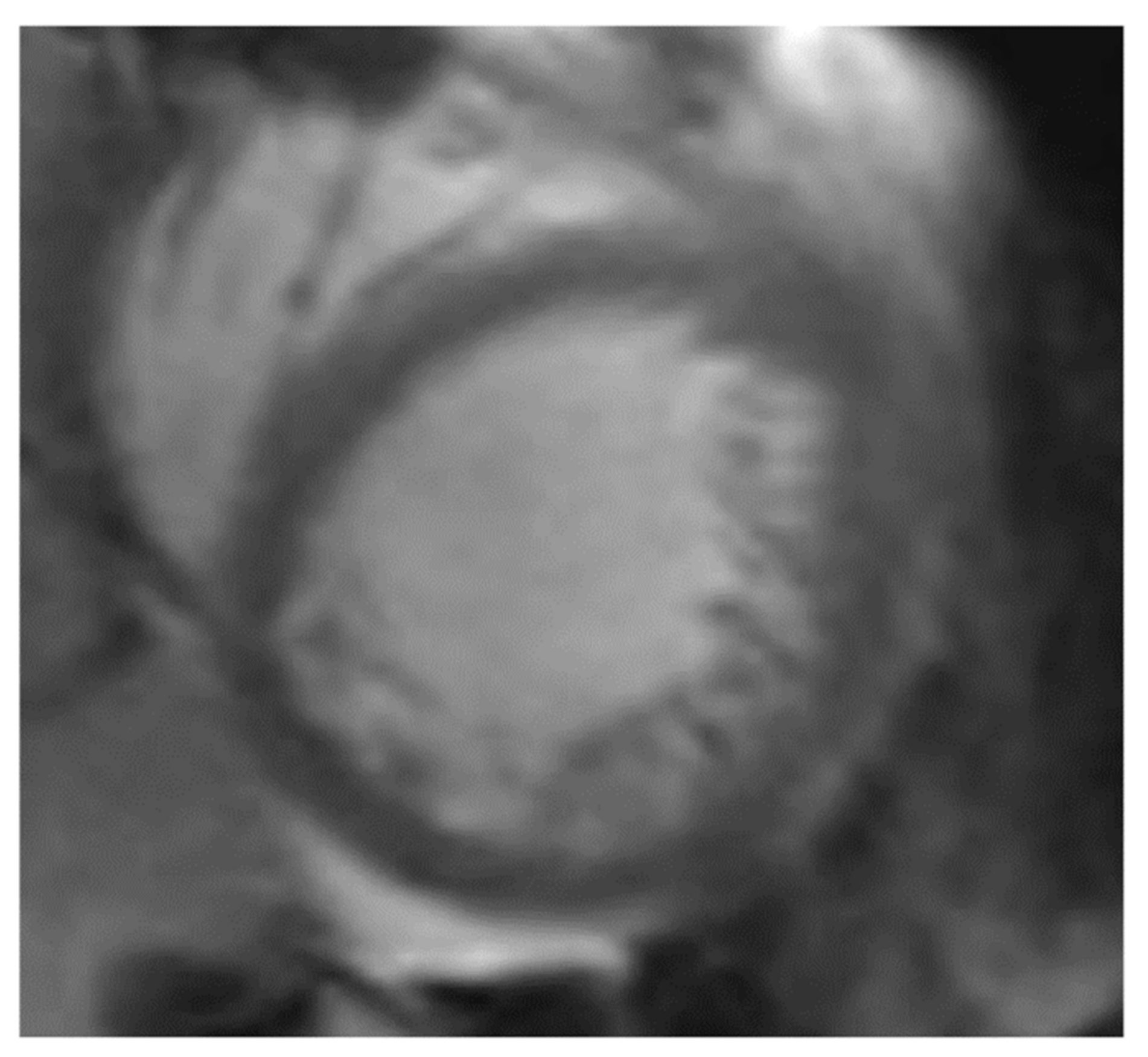
| Term | Abbreviation |
|---|---|
| Spongy myocardium | |
| Fetal myocardium | |
| Honeycomb myocardium | |
| Persistent sinusoids | |
| Isolated noncompaction of the left ventricular myocardium | INVM |
| Left ventricular noncompaction | LVNC |
| Noncompaction cardiomyopathy | NCCM |
| Left ventricular hypertrabeculation | LVHT |
| Hypertrabeculation syndrome | |
| Left ventricular myocardial noncompaction cardiomyopathy | |
| Non-compaction of the left ventricular myocardium |
| (a) Subtypes of NCCM in the Pediatric Population, modified after [12] |
| 1. The isolated or benign form of LVNC, (M LVNC); |
| 2. The arrhythmogenic form of LVNC; |
| 3. The dilated form of LVNC, (M LVNC + D); |
| 4. The hypertrophic form of LVNC, (M LVNC + H); |
| 5. The “mixed” form of LVNC; |
| 6. The restrictive form of LVNC, (M LVNC + R); |
| 7. The biventricular form of LVNC; |
| 8. The right ventricular hypertrabeculation with normal LV form; |
| 9. The congenital heart disease form of LVNC. |
| (b) Subtypes of NCCM, modified after [27] |
| 1. iLVNC. NC morphology in left ventricles with normal systolic and diastolic function, size, and wall thickness; |
| 2. LVNC with LV dilation and dysfunction at onset, such as in the paradigmatic infantile CMP of Barth syndrome; |
| 3. LVNC in hearts fulfilling the diagnostic criteria for DCM, HCM, RCM, or ARVC; |
| 4. LVNC associated with congenital heart disease; |
| 5. Syndromes with LVNC, either sporadic or familial, in which the noncompaction morphology is one of the cardiac traits associated with both monogenic defects and chromosomal anomalies, i.e., complex syndromes with several multiorgan defects; |
| 6. Acquired and potentially reversible LVNC, which has been reported in athletes; it has also been reported in sickle cell anemia, pregnancy, myopathies, and chronic renal failure; |
| 7. Right ventricular noncompaction, concomitant with that of the left ventricle, or present as a unique anatomic area of NC. |
| Type of Arrhythmia | Subtype | Prevalence |
|---|---|---|
| Bradyarrhythmias | Sinus bradycardia | |
| First-degree AV block | ||
| Second-degree AV block | ||
| Mobitz II | ||
| Third-degree AV block | ||
| indication for | ||
| pacemaker implantation | 5% | |
| Supraventricular | Atrial fibrillation | 18% |
| Tachycardias | Atrial flutter | 1.5% |
| Atrial tachycardia | ||
| AV nodal reentrant tachycardia | 1.0% | |
| AV reentrant tachycardia | 1.5% | |
| Ventricular arrhythmias |  | 6% |
1. At least four prominent trabeculations and deep intertrabecular recesses; |
2. Blood flow between the cavity of the left ventricle and the recesses demonstrable by color Doppler echocardiography or by the use of ultrasonographic contrast medium; |
3. The left ventricular wall segments show a typical bilaminar structure, and the noncompact subendocardial layer is at least twice as thick as the compact subepicardial layer in systole; |
4. No other cardiac abnormalities present. |
| Author Year; [ref.] | Appellation | Used Criteria | Cardiac Phase Used for Measurement | Recommended Views | |||||
|---|---|---|---|---|---|---|---|---|---|
| Trabeculations | Intertrabecular Recesses | Two-Layered Myocardial Structure | NC/C Ratio | Coexisting Cardiac Disease | Additional Criteria | ||||
| Chin 1990; [9] | California | Excessive prominent | Deep intertrabecular | X/Y ratio decrease i.e., C/NC+C; no exact cut-off value | Abnormalities excluded | End diastole | Apical view; subcostal view | ||
| Jenni 2001; [8,78] | Zurich | Excessive prominent trabeculations | Deep intertrabecular recesses | Compacted thin epicardial and much thicker noncompacted endocardial | NC/C > 2 | Abnormalities absent | Perfused recesses in color Doppler | End systole | Short axis view |
| Stöllberger 2004; [17] | Vienna | >3 prominent trabeculations | Intertrabecular spaces | Trabeculations as part of noncompacted layer | No exact cut-off value | Perfusion of intertrabecular spaces by color Doppler | Trabeculations in end diastole; two-layered myocardium in end systole | Parasternal short axis and apical level; atypical apical 2-Ch view | |
| Engberding 2007; [62] | Germany | At least 4 prominent trabeculations | Deep intertrabecular recesses | Bilaminar structure | NC/C ≥ 2 | No other cardiac abnormalities | Blood flow in recesses in color Doppler or with echo contrast | Systole | |
| Belanger 2008; [72] | New York | Trabeculations | Recesses | NC/C | Absence of cardiomyopathy, congenital HD or coronary HD | Planimetered area of noncompacted myocardium | Systole | All standard views | |
| Paterick 2012; [70] | Milwaukee | Trabeculations | NC/C > 2 | Abnormal ventricular function | Total cardiac cycle; NC/C ratio end diastole | Multiple imaging windows | |||
| Van Dalen 2008; [75] | Rotterdam | Absence of LV twist | |||||||
| Gebhard 2012; [73] | Additional | Compacta thickness < 8 mm |
| Author Year; [ref.] | Used Criteria | Cardiac Phase Used for Measurement | Recommended Views | ||||
|---|---|---|---|---|---|---|---|
| Trabeculations | Recesses | Two-layered structure | NC/C ratio | Additional criteria | |||
| Petersen 2005; [85] | Trabecular layering | Compacted epicardial and noncompacted endocardial layer | NC/C > 2.3 | True apex excluded | End diastole | Long axis | |
| Jacquier 2010; [87] | Trabeculated LV mass | Perfused, deep recesses | Jenni echo criteria | Trabeculated mass > 20% | End diastole | Short axis | |
| Stacey 2012; [86] | Trabeculation | Flow in the recesses | Noncompacted and compacted layer | NC/C > 2.0 | 16–24 mm from the true apex | End systole | Short axis |
| Captur 2015; [88] | Abnormal trabecular pattern | Jenni echo criteria and # | Maximum apical fractal dimension > 1.3; global fractal dimension > 1.26 | End diastole | Short axis | ||
| Grothoff 2012; [89] | Trabeculations | Recesses communicating with the left ventricular cavity | Noncompacted/compacted myocardium ratio | NC/C > 2 (segments 4–6) NC/C > 3 (segments 1–3, 7–16) * | Trabeculated mass > 25% of total LV mass; Trabeculated LV mass/BSA > 15 g/m2 | End diastole | Short axis |
| Choi 2016; [92] | Trabeculated mass | Most prominent noncompacted to compacted ratio | NC/C > 3.15 apical | Trabeculated mass > 35% of total LV volume | End diastole | Short axis |
| Genes | Mutations in Gen: |
|---|---|
| Sarcomere genes | MYH7; MYBPC3; ACTC1; TNT; |
| (Contractile and non-contractile | TPM1; AN2; ACTN2; DES; LDB3; |
| Structures) | MYL2; NEBL; OBSCN; TNNC1; TNNI3 |
| Arrhythmia genes | HCN4; RYR2; SCN5A; ABCC9; |
| ANK2; CACNA2D1; CASQ2; KCNE3 | |
| KCNH2; KCNQ1 | |
| Non-sarcomere/non-arrhythmia | MMPK; DSP; DTNA; FKTN; HFE; JUP; |
| Cardiomyopathy genes | LMNA (Lamin A/C); PKP2; PLEC; PLN; |
| PRDM16; RBM20; SGCD | |
| X-linked genes | G4.5 (TAZ); DMD; FHL1; GLA; |
| LAMP2: RPS6KA3 | |
| Genes associated with | MIB1; MIB2; NKX2.5; NOTCH1; |
| congenital heart disease | NSD1; PTPN11; TXB20; TBX5 |
| Mitochondrial dysfunction genes | HADHB; HMGCL; MIPEP; MLYCD |
| MT-ATP6; MT-CO3; MTFMT; MT-ND1; | |
| MT-ND2; SDHA; SDHD; TMEM70; VARS2 | |
| Complex genotypes | Multiple mutations in one patient. Complex MYBPC3 mutations with severe clinical phenotype, observed only in children |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerecke, B.J.; Engberding, R. Noncompaction Cardiomyopathy—History and Current Knowledge for Clinical Practice. J. Clin. Med. 2021, 10, 2457. https://doi.org/10.3390/jcm10112457
Gerecke BJ, Engberding R. Noncompaction Cardiomyopathy—History and Current Knowledge for Clinical Practice. Journal of Clinical Medicine. 2021; 10(11):2457. https://doi.org/10.3390/jcm10112457
Chicago/Turabian StyleGerecke, Birgit J., and Rolf Engberding. 2021. "Noncompaction Cardiomyopathy—History and Current Knowledge for Clinical Practice" Journal of Clinical Medicine 10, no. 11: 2457. https://doi.org/10.3390/jcm10112457
APA StyleGerecke, B. J., & Engberding, R. (2021). Noncompaction Cardiomyopathy—History and Current Knowledge for Clinical Practice. Journal of Clinical Medicine, 10(11), 2457. https://doi.org/10.3390/jcm10112457





