Measuring the Contrast Sensitivity Function in Non-Neovascular and Neovascular Age-Related Macular Degeneration: The Quantitative Contrast Sensitivity Function Test
Abstract
1. Introduction
1.1. Limitations of Visual Acuity Testing
1.2. The Value of Contrast Sensitivity Testing in AMD
2. Discussion
2.1. Currently Available Contrast Sensitivity Tests
2.1.1. Conventional Laboratory Contrast Sensitivity Testing
2.1.2. The Pelli-Robson Chart
2.1.3. Vistech Testing and Related Charts
2.1.4. The Spaeth/Richman Contrast Sensitivity Test
2.2. The Quantitative Contrast Sensitivity Function (qCSF) Method
2.2.1. Introduction on the qCSF Method
2.2.2. The qCSF Testing Method
2.2.3. Current Applications of the qCSF Method in Retinal Diseases
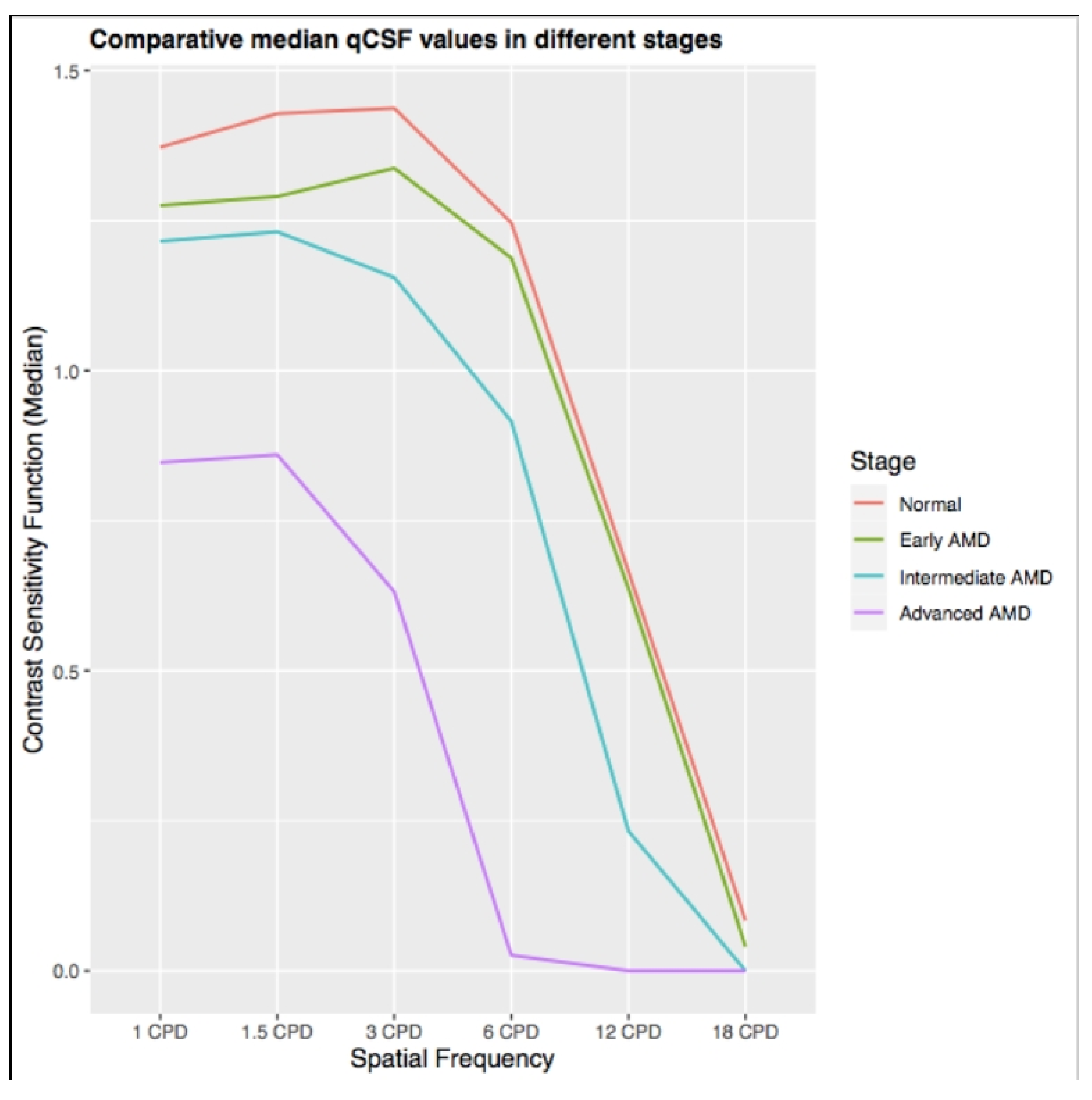
2.2.4. The Premise of the qCSF in Non-Neovascular Age-Relate Macular Degeneration
2.2.5. The Premise of the qCSF Method in Neovascular Age-Related Macular Degeneration
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef]
- Colijn, J.M.; Buitendijk, G.H.S.; Prokofyeva, E.; Alves, D.; Cachulo, M.L.; Khawaja, A.P.; Cougnard-Gregoire, A.; Merle, B.M.; Korb, C.; Erke, M.G.; et al. Prevalence of Age-Related Macular Degeneration in Europe: The Past and the Future. Ophthalmology 2017, 124, 1753–1763. [Google Scholar] [CrossRef] [PubMed]
- Owsley, C.; Huisingh, C.; Clark, M.E.; Jackson, G.R.; McGwin, G. Comparison of visual function in older eyes in the earliest stages of age-related macular degeneration to those in normal macular health. Curr. Eye Res. 2016, 41, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Sunness, J.S.; Rubin, G.S.; Broman, A.; Applegate, C.A.; Bressler, N.M.; Hawkins, B.S. Low luminance visual dysfunction as a predictor of subsequent visual acuity loss from geographic atrophy in age-related macular degeneration. Ophthalmology 2008, 115, 1480–1488. [Google Scholar] [CrossRef] [PubMed]
- Cocce, K.J.; Stinnett, S.S.; Luhmann, U.F.; Vajzovic, L.; Horne, A.; Schuman, S.G.; Toth, C.A.; Cousins, S.W.; Lad, E.M. Visual Function Metrics in Early and Intermediate Dry Age-related Macular Degeneration for Use as Clinical Trial Endpoints. Am. J. Ophthalmol. 2018, 189, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Owsley, C.; McGwin, G., Jr.; Scilley, K.; Kallies, K. Development of a questionnaire to assess vision problems under low luminance in age-related maculopathy. Investig. Ophthalmol. Vis. Sci. 2006, 47, 528–535. [Google Scholar] [CrossRef]
- Scilley, K.; Jackson, G.R.; Cideciyan, A.V.; Maguire, M.G.; Jacobson, S.G.; Owsley, C. Early age-related maculopathy and self-reported visual di culty in daily life. Ophthalmology 2002, 109, 1235–1242. [Google Scholar] [CrossRef]
- Klein, R.; Wang, Q.; Klein, B.E.; Moss, S.E.; Meuer, S.M. The relationship of age-related maculopathy, cataract, and glaucoma to visual acuity. Investig. Ophthalmol. Vis Sci. 1995, 36, 182–191. [Google Scholar]
- Siderov, J.; Tiu, A.L. Variability of measurements of visual acuity in a large eye clinic. Acta Ophthalmol. Scand. 1999, 77, 673–676. [Google Scholar] [CrossRef]
- Yu, H.J.; Kaiser, P.K.; Zamora, D.; Bocanegra, M.; Cone, C.; Brown, D.M.; Sadda, S.R.; Wykoff, C.C. Visual Acuity Variability: Comparing Discrepancies between Snellen and ETDRS Measurements among Subjects Entering Prospective Trials. Ophthalmol. Retina 2020. [Google Scholar] [CrossRef]
- Laíns, I.; Miller, J.B.; Park, D.H.; Tsikata, E.; Davoudi, S.; Rahmani, S.; Pierce, J.; Silva, R.; Chen, T.C.; Kim, I.K.; et al. Structural Changes Associated with Delayed Dark Adaptation in Age-Related Macular Degeneration. Ophthalmology 2017, 124, 1340–1352. [Google Scholar] [CrossRef] [PubMed]
- Roh, M.; Laíns, I.; Shin, H.J.; Park, D.H.; Mach, S.; Vavvas, D.G.; Kim IKv Miller, J.W.; Husain, D.; Miller, J.B. Microperimetry in age-related macular degeneration: Association with macular morphology assessed by optical coherence tomography. Br. J. Ophthalmol. 2019, 103, 1769–1776. [Google Scholar] [CrossRef] [PubMed]
- Tran, B.K.; Herbort, C.P. Discrepancy between Visual Acuity and Microperimetry in AMD Patients: Visual Acuity Appears as an Inadequate Parameter to Test Macular Function. Klin. Mon. Augenheilkd. 2015, 232, 529–532. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Ayton, L.N.; Guymer, R.H.; Luu, C.D. Low-luminance visual acuity and microperimetry in age-related macular degeneration. Ophthalmology 2014, 121, 1612–1619. [Google Scholar] [CrossRef]
- Moschos, M.M.; Nitoda, E. The Role of mf-ERG in the Diagnosis and Treatment of Age-Related Macular Degeneration: Electrophysiological Features of AMD. Semin. Ophthalmol. 2018, 33, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Wolffsohn, J.S.; Anderson, S.J.; Mitchell, J.; Woodcock, A.; Rubinstein, M.; Ffytche, T.; Browning, A.; Willbond, K.; Amoaku, W.M.; Bradley, C. Effect of age related macular degeneration on the Eger macular stressometer photostress recovery time. Br. J. Ophthalmol. 2006, 90, 432–434. [Google Scholar] [CrossRef] [PubMed]
- Falsini, B.; Fadda, A.; Iarossi, G.; Piccardi, M.; Canu, D.; Minnella, A.; Serrao, S.; Scullica, L. Retinal sensitivity to icker modulation: Reduced by early age-related maculopathy. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1498–1506. [Google Scholar]
- Hogg, R.E.; Chakravarthy, U. Visual function and dysfunction in early and late age-related maculopathy. Prog. Retin. Eye Res. 2006, 25, 249–276. [Google Scholar] [CrossRef]
- Vujosevic, S.; Smolek, M.K.; Lebow, K.A.; Notaroberto, N.; Pallikaris, A.; Casciano, M. Detection of macular function changes in early (AREDS 2) and interme- diate (AREDS 3) age-related macular degeneration. Ophthalmologica 2011, 225, 155–160. [Google Scholar] [CrossRef]
- Shah, N.; Dakin, S.C.; Dobinson, S.; Tufail, A.; Egan, C.A.; Anderson, R.S. Visual acuity loss in patients with age-related macular degeneration measured using a novel high-pass letter chart. Br. J. Ophthalmol. 2016, 100, 1346–1352. [Google Scholar] [CrossRef]
- Mones, J.; Rubin, G.S. Contrast sensitivity as an outcome measure in patients with subfoveal choroidal neovascularisation due to age-related macular degeneration. Eye 2005, 19, 1142–1150. [Google Scholar] [CrossRef] [PubMed]
- Nixon, D.R.; Flinn, N.A. Evaluation of contrast sensitivity and other visual function outcomes in neovascular age-related macular degeneration patients after treatment switch to aflibercept from ranibizumab. Clin. Ophthalmol. 2017, 11, 715–721. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Neely, D.; Zarubina, A.V.; Clark, M.E.; Huisingh, C.E.; Jackson, G.R.; Zhang, Y.; McGwin, G., Jr.; Curcio, C.A.; Owsley, C. Association between Visual Function and Subretinal Drusenoid Deposits in Normal and Early Age-Related Macular Degeneration Eyes. Retina 2017, 37, 1329–1336. [Google Scholar] [CrossRef] [PubMed]
- Pondorfer, S.G.; Terheyden, J.H.; Heinemann, M.; Wintergerst, M.W.M.; Holz, F.G.; Finger, R.P. Association of Vision-related Quality of Life with Visual Function in Age-Related Macular Degeneration. Sci. Rep. 2019, 9, 15326. [Google Scholar] [CrossRef] [PubMed]
- Ivers, R.Q.; Mitchell, P.; Cumming, R.G. Visual function tests, eye disease and symptoms of visual disability: A population-based assessment. Clin. Exp. Ophthalmol. 2000, 28, 41–47. [Google Scholar] [CrossRef]
- Rubin, G.S.; Bandeen-Roche, K.; Huang, G.H.; Munoz, B.; Schein, O.D.; Fried, L.P.; West, S.K. The association of multiple visual impairments with self-reported visual disability: SEE project. Investig. Ophthalmol. Vis. Sci. 2001, 42, 64–72. [Google Scholar]
- Lennerstrand, G.; Ahlstrom, C. Contrast sensitivity in macular degeneration and the relation to subjective visual impairment. Acta Ophthalmol. 1989, 67, 225–233. [Google Scholar] [CrossRef]
- Kleiner, R.; Enger, C.; Alexander, M.; Fine, S. Contrast sensitivity in age-related macular degeneration. Arch. Ophthalmol. 1988, 106, 55–57. [Google Scholar] [CrossRef]
- Murugappan, M.; Vayalil, J.; Bade, A.; Bittner, A.K. Reliability of Quick Contrast Sensitivity Function Testing in Adults without Ocular Disease and Patients with Retinitis Pigmentosa. Investig. Ophthalmol. Vis. Sci. 2016, 57, 616. [Google Scholar]
- Ramulu, P.Y.; Dave, P.; Friedman, D.S. Precision of contrast sensitivity testing in glaucoma. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2225. [Google Scholar]
- Preti, R.C.; Ramirez, L.M.; Pimentel, S.L.; Nakashima, Y.; Machado, C.G.; Pelayes, D.E.; Monteiro, M.L.; Takahashi, W.Y. Effect of a single intravitreal bevacizumab injection on contrast sensitivity and macular thickness in eyes with macular edema from central retinal vein occlusion: A prospective, nonrandomized, three-month follow-up study. Ophthalmic Res. 2014, 51, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Preti, R.C.; Ramirez, L.M.; Pimentel, S.L.; Motta, A.A.; Machado, C.G.; Monteiro, M.L.; Takahashi, W.Y. Single intravitreal bevacizumab injection effects on contrast sensitivity in macular edema from branch retinal vein occlusion. Arq. Bras. Oftalmol. 2012, 75, 29–32. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Finger, R.P.; Fenwick, E.; Marella, M.; Dirani, M.; Holz, F.G.; Chiang, P.P.-C.; Lamoureux, E.L. The impact of vision impairment on vision-speci c quality of life in Germany. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3613–3619. [Google Scholar] [CrossRef]
- Weih, L.M.; Hassell, J.B.; Kee, E.J. Assessment of the impact of vision impairment. Investig. Ophthalmol. Vis. Sci. 2002, 43, 927–935. [Google Scholar]
- Roh, M.; Selivanova, A.; Shin, H.J.; Miller, J.W.; Jackson, M.L. Visual acuity and contrast sensitivity are two important factors affecting vision-related quality of life in advanced age-related macular degeneration. PLoS ONE 2018, 13, e0196481. [Google Scholar] [CrossRef] [PubMed]
- Bansback, N.; Czoski-Murray, C.; Carlton, J.; Lewis, G.; Hughes, L.; Espallargues, M.; Brand, C.; Brazier, J. Determinants of health related quality of life and health state utility in patients with age related macular degeneration: The association of contrast sensitivity and visual acuity. Qual. Life Res. 2007, 16, 533–543. [Google Scholar] [CrossRef]
- Kelly, D.H.; Savoie, R.E. A study of sine-wave contrast sensitivity by two psychophysical methods. Percept. Psychophys. 1973, 14, 313–318. [Google Scholar] [CrossRef]
- Harvey, L.O., Jr. Efficient estimation of sensory thresholds with ML-PEST. Spat. Vis. 1997, 11, 121–128. [Google Scholar] [CrossRef]
- Sjöstrand, J.; Frisén, L. Contrast sensitivity in macular disease. A preliminary report. Acta Ophthalmol. 1977, 55, 507–514. [Google Scholar] [CrossRef]
- Pelli, D.G.; Robson, J.G.; Wilkins, A.J. The design of a new letter chart for measuring contrast sensitivity. Clin. Vis. Sci. 1988, 2, 187–199. [Google Scholar]
- Rubin, G.S. Reliability and sensitivity of clinical contrast sensitivity tests. Clin. Vis. Sci. 1987, 88, 169–177. [Google Scholar]
- Richman, J.; Spaeth, G.L.; Wirostko, B. Contrast sensitivity basics and a critique of currently available tests. J. Cataract. Refract. Surg. 2013, 39, 1100–1106. [Google Scholar] [CrossRef] [PubMed]
- Dorr, M.; Lesmes, L.A.; Elze, T.; Wang, H.; Lu, Z.L.; Bex, P.J. Evaluation of the precision of contrast sensitivity function assessment on a tablet device. Sci. Rep. 2017, 7, 46706. [Google Scholar] [CrossRef] [PubMed]
- Owsley, C. Contrast sensitivity. Ophthalmol. Clin. N. Am. 2003, 16, 171–177. [Google Scholar] [CrossRef]
- Ginsburg, A.P. A new contrast sensitivity vision test chart. Am. J. Optom. Physiol. Opt. 1984, 61, 403–407. [Google Scholar] [CrossRef]
- Pomerance, G.N.; Evans, D.W. Test-retest reliability of the CSV-1000 contrast test and its relationship to glaucoma therapy. Investig. Ophthalmol. Vis. Sci. 1994, 35, 3357–3361. [Google Scholar]
- Ginsburg, A.P. Next generation contrast sensitivity testing. In Functional Assessment of Low Vision; Rosenthal, B.P., Cole, R.G., Eds.; Mosby-Year Book Inc.: St. Louis, MO, USA, 1996; pp. 77–88. [Google Scholar]
- Kennedy, R.S.; Dunlap, W.P. Assessment of the Vistech contrast sensitivity test for repeated measures applications. Optom. Vis. Sci. 1990, 67, 248–251. [Google Scholar] [CrossRef]
- Long, G.M.; Tuck, J.P. Reliabilities of alternate measures of contrast sensitivity functions. Am. J. Optom. Physiol. Opt. 1988, 65, 37–48. [Google Scholar] [CrossRef]
- Reeves, B.C.; Wood, J.M.; Hill, A.R. Vistech VCTS 6500 charts—Within- and between-session reliability. Optom. Vis. Sci. 1991, 68, 728–737. [Google Scholar] [CrossRef]
- Kelly, S.A.; Pang, Y.; Klemencic, S. Reliability of the CSV-1000 in adults and children. Optom. Vis. Sci. 2012, 89, 1172–1181. [Google Scholar] [CrossRef]
- Elliott, D.B.; Bullimore, M.A. Assessing the reliability, discriminative ability, and validity of disability glare tests. Investig. Ophthalmol. Vis. Sci. 1993, 34, 108–119. [Google Scholar]
- Hohberger, B.; Laemmer, R.; Adler, W.; Juenemann, A.G.; Horn, F.K. Measuring contrast sensitivity in normal subjects with OPTEC 6500: Influence of age and glare. Graefes Arch. Clin. Exp. Ophthalmol. 2007, 245, 1805–1814. [Google Scholar] [CrossRef] [PubMed]
- Pesudovs, K.; Hazel, C.A.; Doran, R.M.; Elliott, D.B. The usefulness of Vistech and FACT contrast sensitivity charts for cataract and refractive surgery outcomes research. Br. J. Ophthalmol. 2004, 88, 11–16. [Google Scholar] [CrossRef]
- Bühren, J.; Terzi, E.; Bach, M.; Wesemann, W.; Kohnen, T. Measuring contrast sensitivity under different lighting conditions: Comparison of three tests. Optom. Vis. Sci. 2006, 83, 290–298. [Google Scholar] [CrossRef]
- Richman, J.; Zangalli, C.; Lu, L.; Wizov, S.S.; Spaeth, E.; Spaeth, G.L. The Spaeth/Richman contrast sensitivity test (SPARCS): Design, reproducibility and ability to identify patients with glaucoma. Br. J. Ophthalmol. 2015, 99, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Faria, B.M.; Duman, F.; Zheng, C.X.; Waisbourd, M.; Gupta, L.; Lalita, G.; Zangalli, C.; Lu, L.; Wizov, S.S.; Spaeth, E.; et al. Evaluating contrast sensitivity in age-related macular degeneration using a novel computer-based test, the spaeth/richman contrast sensitivity test. Retina 2015, 35, 1465–1473. [Google Scholar] [CrossRef]
- Lesmes, L.A.; Lu, Z.-L.; Baek, J.; Albright, T.D. Bayesian adaptive estimation of the contrast sensitivity function: The quick CSF method. J. Vis. 2010, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Dorr, M.; Wille, M.; Viulet, T.; Sanchez, E.; Bex, P.J.; Lu, Z. Next-generation vision testing: The quick CSF. Curr. Dir. Biomed. Eng. 2015, 1, 1–4. [Google Scholar] [CrossRef]
- Kim, Y.J.; Reynaud, A.; Hess, R.F.; Mullen, K.T. A normative data set for the clinical assessment of achromatic and chromatic contrast sensitivity using a qCSF approach. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3628–3636. [Google Scholar] [CrossRef][Green Version]
- Hou, F.; Lesmes, L.A.; Kim, W.; Gu, H.; Pitt, M.A.; Myung, J.I.; Lu, Z.-L. Evaluating the performance of the quick CSF method in detecting contrast sensitivity function changes. J. Vis. 2016, 16, 18. [Google Scholar] [CrossRef]
- Thayaparan, K.; Crossland, M.D.; Rubin, G.S. Clinical assessment of two new contrast sensitivity charts. Br. J. Ophthalmol. 2007, 91, 749–752. [Google Scholar] [CrossRef] [PubMed]
- Van Gaalen, K.W.; Jansonius, N.M.; Koopmans, S.A.; Terwee, T.; Kooijman, A.C. Relationship between contrast sensitivity and spherical aberration: Comparison of 7 contrast sensitivity tests with natural and artificial pupils in healthy eyes. J. Cataract. Refract. Surg. 2009, 35, 47–56. [Google Scholar] [CrossRef]
- Kalia, A.; Lesmes, L.A.; Dorr, M.; Gandhi, T.; Chatterjee, G.; Ganesh, S.; Bex, P.J.; Sinha, P. Development of pattern vision following early and extended blindness. Proc. Natl. Acad. Sci. USA 2014, 111, 2035–2039. [Google Scholar] [CrossRef] [PubMed]
- Gepshtein, S.; Lesmes, L.A.; Albright, T.D. Sensory adaptation as optimal resource allocation. Proc. Natl. Acad. Sci. USA 2013, 110, 4368–4373. [Google Scholar] [CrossRef]
- American National Standards Institute Committee. American National Standard for Ophthalmics: Multifocal Intraocular Lenses; Optical Laboratories Association: Fairfax, VA, USA, 2007. [Google Scholar]
- Bangor, A.; Kortum, P.; Miller, J. Determining what individual SUS scores mean: Adding an adjective rating scale. J. Usability Stud. 2009, 4, 114–123. [Google Scholar]
- Lesmes, L.A.; Jeon, S.T.; Lu, Z.L.; Dosher, B.A. Bayesian adaptive estimation of threshold versus contrast external noise functions: The quick TvC method. Vis. Res. 2006, 46, 3160–3176. [Google Scholar] [CrossRef]
- Hou, F.; Huang, C.B.; Lesmes, L.; Feng, L.X.; Tao, L.; Zhou, Y.F.; Lu, Z.L. qCSF in clinical application: Efficient characterization and classification of contrast sensitivity functions in amblyopia. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5365–5377. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Zhou, J.; Lu, Z.L.; Lesmes, L.A.; Huang, C.B. Discriminating anisometropic amblyopia from myopia based on interocular inhibition. Vis. Res. 2015, 114, 135–141. [Google Scholar] [CrossRef]
- Stellmann, J.P.; Young, K.L.; Pöttgen, J.; Dorr, M.; Heesen, C. Introducing a new method to assess vision: Computer-adaptive contrast sensitivity testing predicts visual functioning better than charts in multiple sclerosis patients. Mult. Scler. J. Exp. Transl. Clin. 2015, 1, 1–8. [Google Scholar] [CrossRef]
- Lesmes, L.A.; Jackson, M.L.; Bex, P. Visual function endpoints to enable dry AMD clinical trials. Drug Discov. Today 2013, 10, e43–e50. [Google Scholar] [CrossRef]
- Mihailovic, A.; West, S.K.; Johnson, C.; Friedman, D.S.; Kong, X.; Ramulu, P.Y. Predicting Visual Disability in Glaucoma with Combinations of Vision Measures. Transl. Vis. Sci. Technol. 2018, 7, 22. [Google Scholar]
- Thomas, M.; Silverman, R.F.; Vingopoulos, F.; Kasetty, M.; Yu, G.; Kim, E.L.; Omari, A.A.; Joltikov, K.A.; Choi, E.Y.; Kim, L.A.; et al. Active Learning of Contrast Sensitivity to Assess Visual Function in Macula-Off Retinal Detachment. J. VitreoRetinal Dis. 2020. [Google Scholar] [CrossRef]
- Silverman, R.F.; Kasetty, M.; Vingopoulos, F.; Katz, R.; Cho, J.; Lesmes, L.A.; Zacks, D.N.; Kim, L.A.; Miller, J.B. Measuring Contrast Sensitivity Function With Active Learning in Retinal Vein Occlusion: A New Endpoint of Visual Function. Ophthalmic Surg. Lasers Imaging Retina 2020, 51, 392–400. [Google Scholar] [CrossRef]
- Wang, J.; Cui, Y.; Vingopoulos, F.; Kasetty, M.; Silverman, R.F.; Katz, R.; Kim, L.; Miller, J.B. Disorganisation of retinal inner layers is associated with reduced contrast sensitivity in retinal vein occlusion. Br. J. Ophthalmol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Joltikov, K.A.; de Castro, V.M.; Davila, J.R.; Anand, R.; Khan, S.M.; Farbman, N.; Jackson, G.R.; Johnson, C.; Gardner, T.W. Multidimensional functional and structural evaluation reveals neuroretinal impairment in early diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2017, 58, BIO277–BIO290. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.F.; Hou, F.; Lu, Z.L.; Hu, X.; Huang, C.B. Efficient characterization and classification of contrast sensitivity functions in aging. Sci. Rep. 2017, 7, 5045. [Google Scholar] [CrossRef]
- Wai, K.M.; Vingopoulos, F.; Garg, I.; Kasetty, M.; Silverman, R.F.; Katz, R.; Laíns, I.; Miller, J.W.; Husain, D.; Vavvas, D.G.; et al. Contrast sensitivity function in patients with macular disease and good visual acuity. Br. J. Ophthalmol. 2021, 318494. [Google Scholar] [CrossRef]
- Terheyden, J.H.; Holz, F.G.; Schmitz-Valckenberg, S.; Lüning, A.; Schmid, M.; Rubin, G.S.; Dunbar, H.; Tufail, A.; Crabb, D.P.; Binns, A.; et al. Clinical study protocol for a low-interventional study in intermediate age-related macular degeneration developing novel clinical endpoints for interventional clinical trials with a regulatory and patient access intention—MACUSTAR. Trials 2020, 21, 659. [Google Scholar] [CrossRef]
- Csaky, K.G.; Richman, E.A.; Ferris, F.L. Report from the NEI/ FDA ophthalmic clinical trial design and endpoints symposium. Investig. Ophthalmol. Vis. Sci. 2008, 49, 479–489. [Google Scholar] [CrossRef]
- Csaky, K.; Ferris, F.; Chew, E.Y.; Nair, P.; Cheetham, J.K.; Duncan, J.L. Report From the NEI/FDA Endpoints Workshop on Age-Related Macular Degeneration and Inherited Retinal Diseases. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3456–3463. [Google Scholar] [CrossRef]
- Chandramohan, A.; Stinnett, S.S.; Petrowski, J.T.; Schuman, S.G.; Toth, C.A.; Cousins, S.W.; Lad, E.M. Visual Function Measures in Early and Intermediate Age-Related Macular Degeneration. Retina 2016, 36, 1021–1031. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vingopoulos, F.; Wai, K.M.; Katz, R.; Vavvas, D.G.; Kim, L.A.; Miller, J.B. Measuring the Contrast Sensitivity Function in Non-Neovascular and Neovascular Age-Related Macular Degeneration: The Quantitative Contrast Sensitivity Function Test. J. Clin. Med. 2021, 10, 2768. https://doi.org/10.3390/jcm10132768
Vingopoulos F, Wai KM, Katz R, Vavvas DG, Kim LA, Miller JB. Measuring the Contrast Sensitivity Function in Non-Neovascular and Neovascular Age-Related Macular Degeneration: The Quantitative Contrast Sensitivity Function Test. Journal of Clinical Medicine. 2021; 10(13):2768. https://doi.org/10.3390/jcm10132768
Chicago/Turabian StyleVingopoulos, Filippos, Karen M. Wai, Raviv Katz, Demetrios G. Vavvas, Leo A. Kim, and John B. Miller. 2021. "Measuring the Contrast Sensitivity Function in Non-Neovascular and Neovascular Age-Related Macular Degeneration: The Quantitative Contrast Sensitivity Function Test" Journal of Clinical Medicine 10, no. 13: 2768. https://doi.org/10.3390/jcm10132768
APA StyleVingopoulos, F., Wai, K. M., Katz, R., Vavvas, D. G., Kim, L. A., & Miller, J. B. (2021). Measuring the Contrast Sensitivity Function in Non-Neovascular and Neovascular Age-Related Macular Degeneration: The Quantitative Contrast Sensitivity Function Test. Journal of Clinical Medicine, 10(13), 2768. https://doi.org/10.3390/jcm10132768




