Complement Inhibition for Geographic Atrophy: A Tempting Target with Mixed Results
Abstract
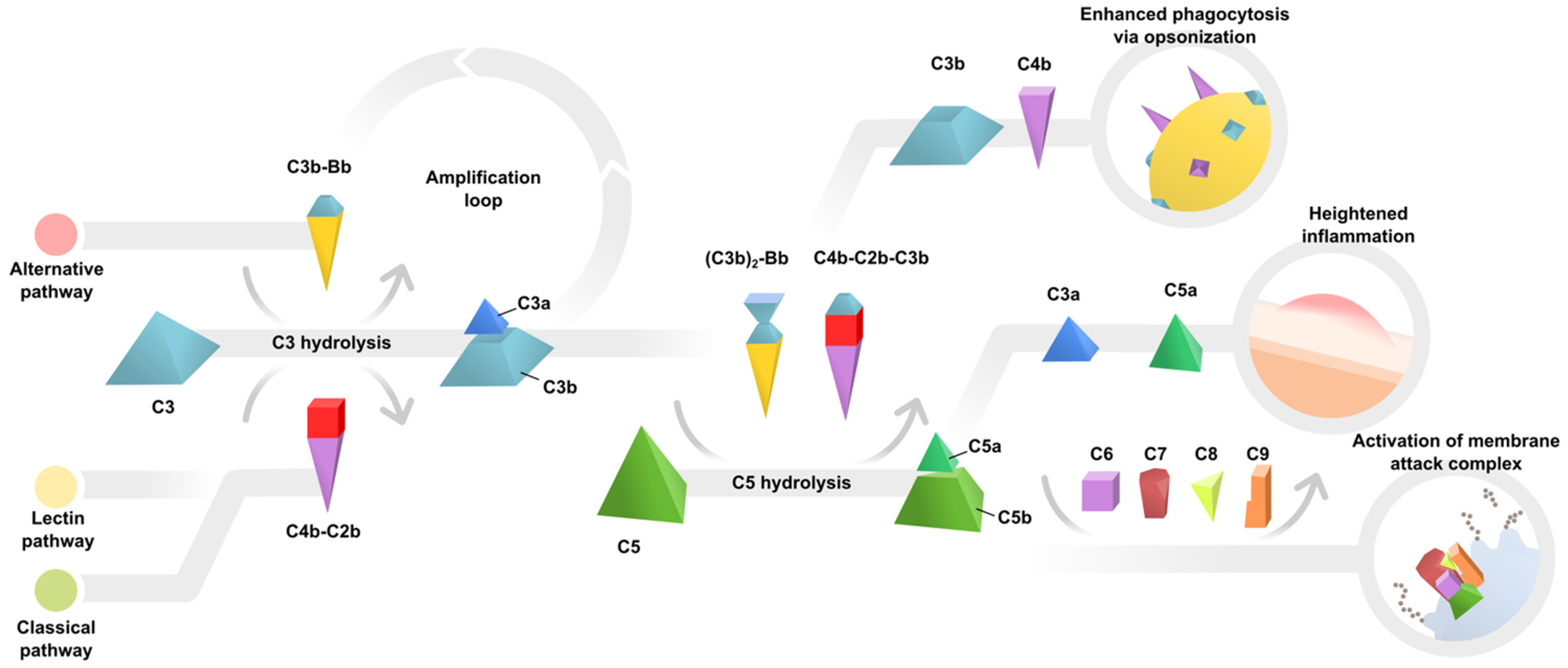
:1. Introduction
2. Evidence of Complement Dysregulation in AMD Pathogenesis
3. Complement Inhibition for Geographic Atrophy
3.1. Studies Reporting Null Results
3.2. Studies Reporting Statistically Significant Results
3.3. Some Notable Observations
3.4. The Right Drug for the Wrong Patient?
3.5. Should We Pause or Proceed?
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wong, W.L.; Su, X.; Li, B.X.; Cheung, C.M.G.; Klein, B.E.; Cheng, C.-Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [Green Version]
- Amoaku, W.M.; Chakravarthy, U.; Gale, R.; Gavin, M.; Ghanchi, F.; Gibson, J.M.; Harding, S.P.; Johnston, R.L.; Kelly, S.P.; Lotery, A.J.; et al. Defining response to anti-VEGF therapies in neovascular AMD. Eye 2015, 29, 721–731. [Google Scholar] [CrossRef]
- Age-Related Eye Disease Study 2 Research Group. Lutein + Zeaxanthin and Omega-3 Fatty Acids for Age-Related Macular Degeneration. JAMA 2013, 309, 2005–2015. [Google Scholar] [CrossRef]
- Chew, E.Y.; Clemons, T.E.; SanGiovanni, J.P.; Danis, R.P.; Ferris, F.L.; Elman, M.J.; Antoszyk, A.N.; Ruby, A.J.; Orth, D.; Bressler, S.B.; et al. Secondary Analyses of the Effects of Lutein/Zeaxanthin on Age-Related Macular Degeneration Progression: AREDS2 Report No. JAMA Ophthalmol. 2014, 132, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Chew, E.Y.; Clemons, T.E.; Agrón, E.; Sperduto, R.D.; SanGiovanni, J.P.; Kurinij, N.; Davis, M.D. Long-Term Effects of Vitamins C and E, β-Carotene, and Zinc on Age-related Macular Degeneration: AREDS Report No. Ophthalmology 2013, 120, 1604–1611.e4. [Google Scholar] [CrossRef] [Green Version]
- Vavvas, D.; Small, K.W.; Awh, C.C.; Zanke, B.W.; Tibshirani, R.J.; Kustra, R. CFH and ARMS2 genetic risk determines progression to neovascular age-related macular degeneration after antioxidant and zinc supplementation. Proc. Natl. Acad. Sci. USA 2018, 115, E696–E704. [Google Scholar] [CrossRef] [Green Version]
- Age-Related Eye Disease Study Research Group. A Randomized, Placebo-Controlled, Clinical Trial of High-Dose Supplementation With Vitamins C and E, Beta Carotene, and Zinc for Age-Related Macular Degeneration and Vision Loss: AREDS Report No. Arch. Ophthalmol. 2001, 119, 1417–1436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reis, E.S.; Mastellos, D.; Hajishengallis, G.; Lambris, J.D. New insights into the immune functions of complement. Nat. Rev. Immunol. 2019, 19, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Holers, V.M. Complement and Its Receptors: New Insights into Human Disease. Annu. Rev. Immunol. 2014, 32, 433–459. [Google Scholar] [CrossRef] [PubMed]
- Haines, J.L.; Hauser, M.A.; Schmidt, S.; Scott, W.K.; Olson, L.M.; Gallins, P.; Spencer, K.L.; Kwan, S.Y.; Noureddine, M.; Gilbert, J.R.; et al. Complement Factor H Variant Increases the Risk of Age-Related Macular Degeneration. Science 2005, 308, 419–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, R.J.; Zeiss, C.; Chew, E.Y.; Tsai, J.-Y.; Sackler, R.S.; Haynes, C.; Henning, A.K.; SanGiovanni, J.P.; Mane, S.M.; Mayne, S.T.; et al. Complement Factor H Polymorphism in Age-Related Macular Degeneration. Science 2005, 308, 385–389. [Google Scholar] [CrossRef]
- Edwards, A.O.; Iii, R.R.; Abel, K.J.; Manning, A.; Panhuysen, C.; Farrer, L. Complement Factor H Polymorphism and Age-Related Macular Degeneration. Science 2005, 308, 421–424. [Google Scholar] [CrossRef] [Green Version]
- Ormsby, R.J.; Ranganathan, S.; Tong, J.C.; Griggs, K.M.; DiMasi, D.P.; Hewitt, A.W.; Burdon, K.; Craig, J.E.; Hoh, J.; Gordon, D.L. Functional and Structural Implications of the Complement Factor H Y402H Polymorphism Associated with Age-Related Macular Degeneration. Investig. Opthalmol. Vis. Sci. 2008, 49, 1763–1770. [Google Scholar] [CrossRef] [PubMed]
- Skerka, C.; Lauer, N.; Weinberger, A.A.; Keilhauer, C.N.; Sühnel, J.; Smith, R.; Schlötzer–Schrehardt, U.; Fritsche, L.; Heinen, S.; Hartmann, A. Defective complement control of Factor H (Y402H) and FHL-1 in age-related macular degeneration. Mol. Immunol. 2007, 44, 3398–3406. [Google Scholar] [CrossRef]
- Yu, J.; Wiita, P.; Kawaguchi, R.; Honda, J.; Jorgensen, A.; Zhang, K.; Fischetti, A.V.A.; Sun, H. Biochemical Analysis of a Common Human Polymorphism Associated with Age-Related Macular Degeneration. Biochemistry 2007, 46, 8451–8461. [Google Scholar] [CrossRef]
- Shaw, P.X.; Zhang, L.; Zhang, M.; Du, H.; Zhao, L.; Lee, C.; Grob, S.; Lim, S.L.; Hughes, G.; Lee, J.; et al. Complement factor H genotypes impact risk of age-related macular degeneration by interaction with oxidized phospholipids. Proc. Natl. Acad. Sci. USA 2012, 109, 13757–13762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van De Ven, J.P.H.; Nilsson, S.C.; Tan, P.L.; Buitendijk, G.H.S.; Ristau, T.; Mohlin, F.C.; Nabuurs, S.B.; Schoenmaker-Koller, F.E.; Smailhodzic, D.; Campochiaro, P.A.; et al. A functional variant in the CFI gene confers a high risk of age-related macular degeneration. Nat. Genet. 2013, 45, 813–817. [Google Scholar] [CrossRef]
- The AMD Genetics Clinical Study Group; Gold, B.; Merriam, J.E.; Zernant, J.; Hancox, L.S.; Taiber, A.J.; Gehrs, K.; Cramer, K.; Neel, J.; Bergeron, J.; et al. Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nat. Genet. 2006, 38, 458–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanton, C.M.; Yates, J.R.; Hollander, A.I.D.; Seddon, J.M.; Swaroop, A.; Stambolian, D.; Fauser, S.; Hoyng, C.; Yu, Y.; Atsuhiro, K.; et al. Complement Factor D in Age-Related Macular Degeneration. Investig. Opthalmol. Vis. Sci. 2011, 52, 8828–8834. [Google Scholar] [CrossRef]
- Johnson, L.V.; Ozaki, S.; Staples, M.K.; Erickson, P.A.; Anderson, D.H. A Potential Role for Immune Complex Pathogenesis in Drusen Formation. Exp. Eye Res. 2000, 70, 441–449. [Google Scholar] [CrossRef]
- Johnson, L.V.; Leitner, W.P.; Staples, M.K.; Anderson, D.H. Complement Activation and Inflammatory Processes in Drusen Formation and Age Related Macular Degeneration. Exp. Eye Res. 2001, 73, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.H.; Mullins, R.F.; Hageman, G.S.; Johnson, L.V. A role for local inflammation in the formation of drusen in the aging eye. Am. J. Ophthalmol. 2002, 134, 411–431. [Google Scholar] [CrossRef]
- Heesterbeek, T.J.; Lechanteur, Y.T.E.; Lorés-Motta, L.; Schick, T.; Daha, M.R.; Altay, L.; Liakopoulos, S.; Smailhodzic, D.; den Hollander, A.I.; Hoyng, C.B.; et al. Complement Activation Levels Are Related to Disease Stage in AMD. Investig. Opthalmol. Vis. Sci. 2020, 61, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yehoshua, Z.; de Amorim Garcia Filho, C.A.; Nunes, R.P.; Gregori, G.; Penha, F.M.; Moshfeghi, A.A.; Zhang, K.; Sadda, S.; Feuer, W.; Rosenfeld, P.J. Systemic Complement Inhibition with Eculizumab for Geographic Atrophy in Age-Related Macular Degeneration. Ophthalmology 2014, 121, 693–701. [Google Scholar] [CrossRef] [Green Version]
- Holz, F.G.; Sadda, S.R.; Busbee, B.; Chew, E.Y.; Mitchell, P.; Tufail, A.; Brittain, C.; Ferrara, D.; Gray, S.; Honigberg, L.; et al. Efficacy and Safety of Lampalizumab for Geographic Atrophy Due to Age-Related Macular Degeneration: Chroma and Spectri Phase 3 Randomized Clinical Trials. JAMA Ophthalmol. 2018, 136, 666–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaspan, B.L.; Williams, D.F.; Holz, F.G.; Regillo, C.D.; Li, Z.; Dressen, A.; van Lookeren Campagne, M.; Le, K.N.; Graham, R.R.; Beres, T.; et al. Targeting factor D of the alternative complement pathway reduces geographic atrophy progression secondary to age-related macular degeneration. Sci. Transl. Med. 2017, 9, eaaf1443. [Google Scholar] [CrossRef] [Green Version]
- Liao, D.S.; Grossi, F.V.; El Mehdi, D.; Gerber, M.R.; Brown, D.M.; Heier, J.S.; Wykoff, C.C.; Singerman, L.J.; Abraham, P.; Grassmann, F.; et al. Complement C3 Inhibitor Pegcetacoplan for Geographic Atrophy Secondary to Age-Related Macular Degeneration. Ophthalmology 2020, 127, 186–195. [Google Scholar] [CrossRef] [Green Version]
- Jaffe, G.J.; Westby, K.; Csaky, K.G.; Monés, J.; Pearlman, J.A.; Patel, S.S.; Joondeph, B.C.; Randolph, J.; Masonson, H.; Rezaei, K.A. C5 Inhibitor Avacincaptad Pegol for Geographic Atrophy Due to Age-Related Macular Degeneration. Ophthalmology 2021, 128, 576–586. [Google Scholar] [CrossRef]
- Poor, S.H.; Qiu, Y.; Fassbender, E.S.; Shen, S.; Woolfenden, A.; Delpero, A.; Kim, Y.; Buchanan, N.; Gebuhr, T.C.; Hanks, S.M.; et al. Reliability of the Mouse Model of Choroidal Neovascularization Induced by Laser Photocoagulation. Investig. Opthalmol. Vis. Sci. 2014, 55, 6525–6534. [Google Scholar] [CrossRef]
- Wykoff, C.C.; Rosenfeld, P.J.; Waheed, N.K.; Singh, R.P.; Ronca, N.; Slakter, J.S.; Staurenghi, G.; Monés, J.; Baumal, C.R.; Saroj, N.; et al. Characterizing New-Onset Exudation in the Randomized Phase 2 FILLY Trial of Complement Inhibitor Pegcetacoplan for Geographic Atrophy. Ophthalmology 2021. [Google Scholar] [CrossRef]
- Yu, M.; Zou, W.; Peachey, N.S.; McIntyre, T.M.; Liu, J. A Novel Role of Complement in Retinal Degeneration. Investig. Opthalmol. Vis. Sci. 2012, 53, 7684–7692. [Google Scholar] [CrossRef] [Green Version]
- Hoh Kam, J.; Lenassi, E.; Malik, T.H.; Pickering, M.; Jeffery, G. Complement Component C3 Plays a Critical Role in Protecting the Aging Retina in a Murine Model of Age-Related Macular Degeneration. Am. J. Pathol. 2013, 183, 480–492. [Google Scholar] [CrossRef] [PubMed]
- Mukai, R.; Okunuki, Y.; Husain, D.; Kim, C.B.; Lambris, J.D.; Connor, K.M. The Complement System Is Critical in Maintaining Retinal Integrity during Aging. Front. Aging Neurosci. 2018, 10, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silverman, S.M.; Ma, W.; Wang, X.; Zhao, L.; Wong, W.T. C3- and CR3-dependent microglial clearance protects photoreceptors in retinitis pigmentosa. J. Exp. Med. 2019, 216, 1925–1943. [Google Scholar] [CrossRef] [Green Version]
- De Jong, S.; Gagliardi, G.; Garanto, A.; de Breuk, A.; Lechanteur, Y.T.E.; Katti, S.; van den Heuvel, L.P.; Volokhina, E.B.; den Hollander, A.I. Implications of genetic variation in the complement system in age-related macular degeneration. Prog. Retin. Eye Res. 2021, 100952. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Mastellos, D.C.; Li, Y.; Dunaief, J.L.; Lambris, J.D. Targeting complement components C3 and C5 for the retina: Key concepts and lingering questions. Prog. Retin. Eye Res. 2020, 100936. [Google Scholar] [CrossRef] [PubMed]
- Scholl, H.P.N.; Fleckenstein, M.; Fritsche, L.G.; Schmitz-Valckenberg, S.; Göbel, A.; Adrion, C.; Herold, C.; Keilhauer, C.N.; Mackensen, F.; Mößner, A.; et al. CFH, C3 and ARMS2 Are Significant Risk Loci for Susceptibility but Not for Disease Progression of Geographic Atrophy Due to AMD. PLoS ONE 2009, 4, e7418. [Google Scholar] [CrossRef] [Green Version]
- Klein, M.L.; Ferris, F.L.; Francis, P.J.; Lindblad, A.S.; Chew, E.Y.; Hamon, S.C.; Ott, J. Progression of Geographic Atrophy and Genotype in Age-Related Macular Degeneration. Ophthalmology 2010, 117, 1554–1559.e1. [Google Scholar] [CrossRef]
- Grassmann, F.; Harsch, S.; Brandl, C.; Kiel, C.; Nürnberg, P.; Toliat, M.R.; Fleckenstein, M.; Pfau, M.; Schmitz-Valckenberg, S.; Holz, F.G.; et al. Assessment of Novel Genome-Wide Significant Gene Loci and Lesion Growth in Geographic Atrophy Secondary to Age-Related Macular Degeneration. JAMA Ophthalmol. 2019, 137, 867. [Google Scholar] [CrossRef]
- Grassmann, F.; Fleckenstein, M.; Chew, E.Y.; Strunz, T.; Schmitz-Valckenberg, S.; Göbel, A.P.; Klein, M.L.; Ratnapriya, R.; Swaroop, A.; Holz, F.G.; et al. Clinical and Genetic Factors Associated with Progression of Geographic Atrophy Lesions in Age-Related Macular Degeneration. PLoS ONE 2015, 10, e0126636. [Google Scholar] [CrossRef]
- Keenan, T.D.; Agrón, E.; Domalpally, A.; Clemons, T.E.; van Asten, F.; Wong, W.T.; Danis, R.G.; Sadda, S.; Rosenfeld, P.J.; Klein, M.L.; et al. Progression of Geographic Atrophy in Age-related Macular Degeneration: AREDS2 Report Number. Ophthalmology 2018, 125, 1913–1928. [Google Scholar] [CrossRef]
- Hagstrom, S.A.; Ying, G.-S.; Pauer, G.J.; Sturgill-Short, G.M.; Huang, J.; Callanan, D.G.; Kim, I.L.; Klein, M.L.; Maguire, M.G.; Martin, D.F.; et al. Pharmacogenetics for Genes Associated with Age-related Macular Degeneration in the Comparison of AMD Treatments Trials (CATT). Ophthalmology 2013, 120, 593–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smailhodzic, D.; Muether, P.S.; Chen, J.; Kwestro, A.; Zhang, A.Y.; Omar, A.; Van De Ven, J.P.; Keunen, J.E.; Kirchhof, B.; Hoyng, C.B.; et al. Cumulative Effect of Risk Alleles in CFH, ARMS2, and VEGFA on the Response to Ranibizumab Treatment in Age-related Macular Degeneration. Ophthalmology 2012, 119, 2304–2311. [Google Scholar] [CrossRef]
- Ebrahimi, K.B.; Cano, M.; Rhee, J.; Datta, S.; Wang, L.; Handa, J.T. Oxidative Stress Induces an Interactive Decline in Wnt and Nrf2 Signaling in Degenerating Retinal Pigment Epithelium. Antioxid. Redox Signal. 2018, 29, 389–407. [Google Scholar] [CrossRef]
- Cheng, S.-Y.; Cipi, J.; Ma, S.; Hafler, B.P.; Kanadia, R.N.; Brush, R.S.; Agbaga, M.-P.; Punzo, C. Altered photoreceptor metabolism in mouse causes late stage age-related macular degeneration-like pathologies. Proc. Natl. Acad. Sci. USA 2020, 117, 13094–13104. [Google Scholar] [CrossRef]
- Lin, J.B.; Apte, R.S. NAD+ and sirtuins in retinal degenerative diseases: A look at future therapies. Prog. Retin. Eye Res. 2018, 67, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.B.; Kubota, S.; Ban, N.; Yoshida, M.; Santeford, A.; Sene, A.; Nakamura, R.; Zapata, N.; Kubota, M.; Tsubota, K.; et al. NAMPT-Mediated NAD+ Biosynthesis Is Essential for Vision In Mice. Cell Rep. 2016, 17, 69–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Study (Phase) | Drug (Target) | Group 1 | Group 2 | Sham | Group 1 | Group 2 | Time b | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | GA Growth | N | GA Growth | P a | N | GA Growth | P a | |||||
| COMPLETE (2) | Eculizumab (C5) | Low dose | High dose | 10 | 0.37 c | 10 | 0.35 c | N.S. | 10 | 0.40 c | N.S. | 52 W |
| MAHALO (2) | Lampalizumab (CFD) | q2M | q1M | 40 | 0.30 [2.92] d | 41 | 0.33 [3.15] d | 0.552 | 42 | 0.25 [2.33] d | 0.117 | 18 M |
| CHROMA/SPECTRI (3) | Lampalizumab (CFD) | q6W | q4W | 598 | 0.36 [1.98] d | 603 | 0.36 [2.05] d | N.S. | 596 | 0.37 [2.06] d | N.S. | 48 W |
| FILLY (2) | APL-2 (C3) | q2M | q1M | 81 | 0.35 c | 79 | 0.28 c | 0.067 | 86 | 0.26 c | 0.008 | 12 M |
| GATHER1 (2/3) | ARC1905 (C5) | Low dose | High dose | 194 | 0.42 e | 67 | 0.29 c | 0.007 | 83 | 0.32 c | 0.005 | 12 M |
| Study Authors | Year | Key Finding |
|---|---|---|
| Yu et al. [31] | 2012 | Mice lacking receptors for C3a and C5a develop retinal degeneration |
| Hoh Kam et al. [32] | 2013 | Mice lacking C3, CFH, or both develop retinal degeneration during aging |
| Mukai et al. [33] | 2018 | Mice lacking C1q, Mbl, Fb, C3, and C5 have accelerated retinal degeneration with aging |
| Silverman et al. [34] | 2019 | C3-CR3 signaling protects against degeneration in a mouse model of retinitis pigmentosa |
| Supporting Evidence | Opposing Evidence |
|---|---|
|
|
|
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, J.B.; Halawa, O.A.; Miller, J.W.; Vavvas, D.G. Complement Inhibition for Geographic Atrophy: A Tempting Target with Mixed Results. J. Clin. Med. 2021, 10, 2890. https://doi.org/10.3390/jcm10132890
Lin JB, Halawa OA, Miller JW, Vavvas DG. Complement Inhibition for Geographic Atrophy: A Tempting Target with Mixed Results. Journal of Clinical Medicine. 2021; 10(13):2890. https://doi.org/10.3390/jcm10132890
Chicago/Turabian StyleLin, Jonathan B., Omar A. Halawa, Joan W. Miller, and Demetrios G. Vavvas. 2021. "Complement Inhibition for Geographic Atrophy: A Tempting Target with Mixed Results" Journal of Clinical Medicine 10, no. 13: 2890. https://doi.org/10.3390/jcm10132890
APA StyleLin, J. B., Halawa, O. A., Miller, J. W., & Vavvas, D. G. (2021). Complement Inhibition for Geographic Atrophy: A Tempting Target with Mixed Results. Journal of Clinical Medicine, 10(13), 2890. https://doi.org/10.3390/jcm10132890






