Role of Concomitant Coronary Artery Bypass Grafting in Valve Surgery for Infective Endocarditis
Abstract
:1. Introduction
2. Methods
2.1. Patient Population
2.2. Data Collection
2.3. Outcome Definitions
2.4. Statistical Analysis
3. Results
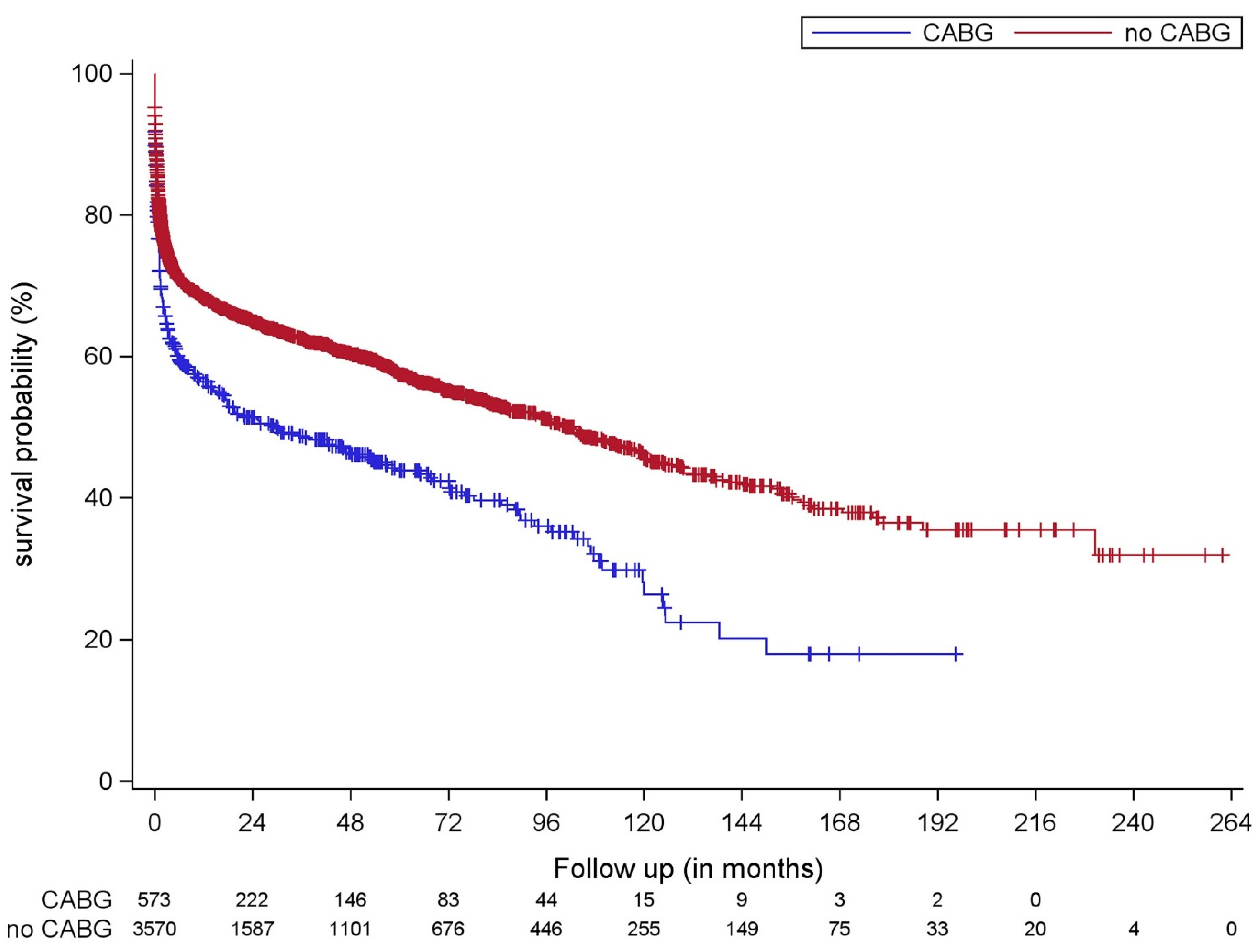
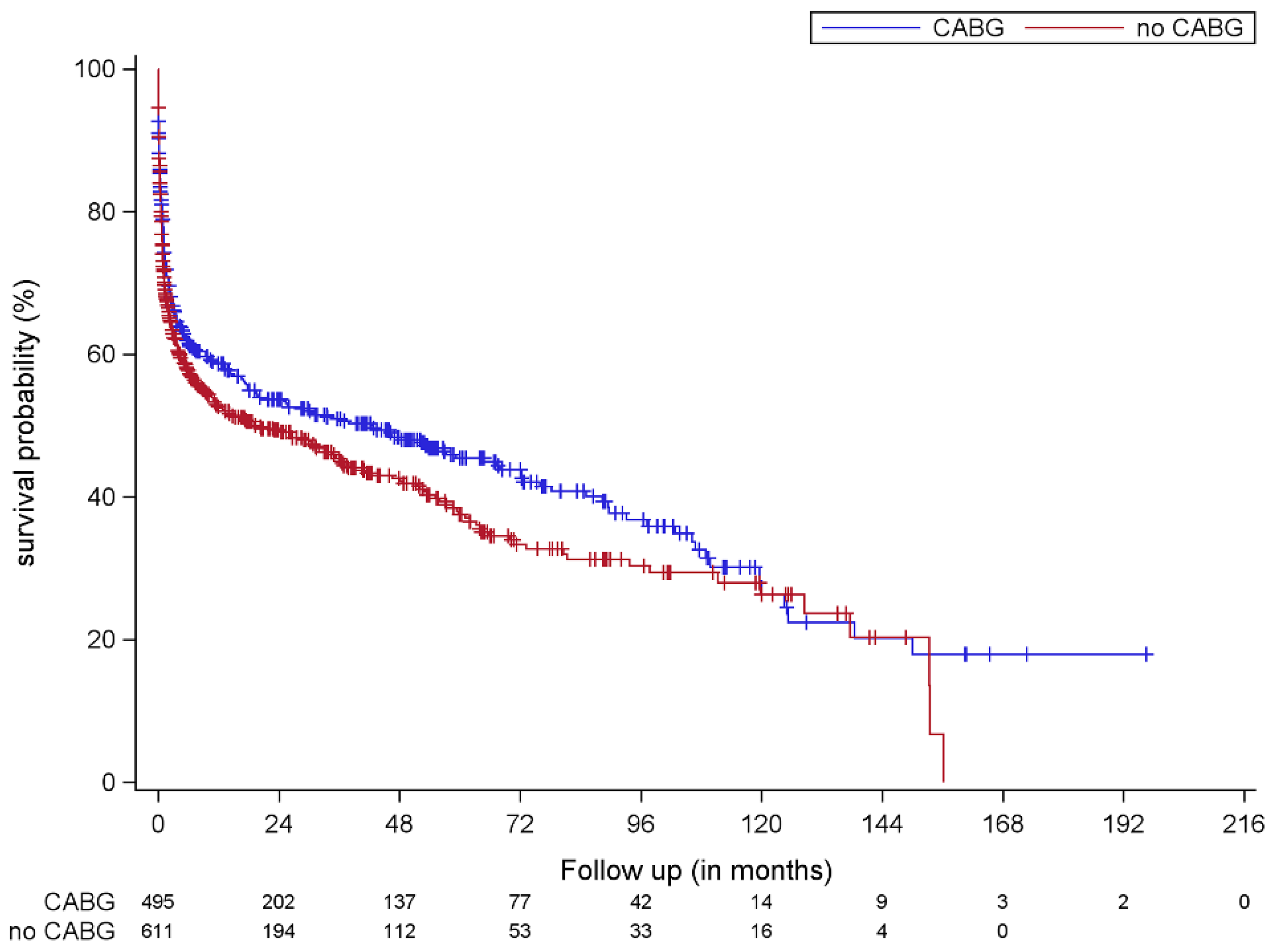
4. Discussion
5. Limitations of the Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murdoch, D.R.; Corey, G.R.; Hoen, B.; Miro, J.M.; Fowler, V.G., Jr.; Bayer, A.S.; Karchmer, A.W.; Olaison, L.; Pappas, P.A.; Moreillon, P.; et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: The International Collaboration on Endocarditis-Prospective Cohort Study. Arch. Intern. Med. 2009, 169, 463–473. [Google Scholar] [CrossRef] [Green Version]
- Pericart, L.; Fauchier, L.; Bourguignon, T.; Bernard, L.; Angoulvant, D.; Delahaye, F.; Babuty, D.; Bernard, A. Long-Term Outcome and Valve Surgery for Infective Endocarditis in the Systematic Analysis of a Community Study. Ann. Thorac. Surg. 2016, 102, 496–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.P.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.A.; Iung, B.; et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC) Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur. Heart J. 2015, 36, 3075–3128. [Google Scholar] [CrossRef] [PubMed]
- Bannay, A.; Hoen, B.; Duval, X.; Obadia, J.F.; Selton-Suty, C.; Le Moing, V.; Tattevin, P.; Iung, B.; Delahaye, F.; Alla, F.; et al. The impact of valve surgery on short- and long-term mortality in left-sided infective endocarditis: Do differences in methodological approaches explain previous conflicting results? Eur. Heart J. 2011, 32, 2003–2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sims, J.R.; Anavekar, N.S.; Chandrasekaran, K.; Steckelberg, J.M.; Wilson, W.R.; Gersh, B.J.; Baddour, L.M.; DeSimone, D.C. Utility of cardiac computed tomography scanning in the diagnosis and pre-operative evaluation of patients with infective endocarditis. Int. J. Cardiovasc. Imaging 2018, 34, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines onmyocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef] [PubMed]
- Mirabel, M.; Sonneville, R.; Hajage, D.; Novy, E.; Tubach, F.; Vignon, P.; Perez, P.; Lavoue, S.; Kouatchet, A.; Pajot, O.; et al. Long-term outcomes and cardiac surgery in critically ill patients with infective endocarditis. Eur. Heart J. 2014, 35, 1195–1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diab, M.; Tasar, R.; Sponholz, C.; Lehmann, T.; Pletz, M.W.; Bauer, M.; Brunkhorst, F.M.; Doenst, T. Changes in inflammatory and vasoactive mediator profiles during valvular surgery with or without infective endocarditis: A case control pilot study. PLoS ONE 2020, 15, e0228286. [Google Scholar] [CrossRef]
- Galvez-Acebal, J.; Rodriguez-Bano, J.; Martinez-Marcos, F.J.; Reguera, J.M.; Plata, A.; Ruiz, J.; Marquez, M.; Lomas, J.M.; de la Torre-Lima, J.; Hidalgo-Tenorio, C.; et al. Prognostic factors in left-sided endocarditis: Results from the andalusian multicenter cohort. BMC Infec. Dis. 2010, 10, 17. [Google Scholar] [CrossRef] [Green Version]
- Doenst, T.; Borger, M.A.; Weisel, R.D.; Yau, T.M.; Maganti, M.; Rao, V. Relation between aortic cross-clamp time and mortality—Not as straightforward as expected. Eur. J. Cardio-Thorac. 2008, 33, 660–665. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Anstey, C.; Tesar, P.; Shekar, K. Risk Factors for Mortality in Patients Undergoing Cardiothoracic Surgery for Infective Endocarditis. Ann. Thorac. Surg. 2019, 108, 1101–1106. [Google Scholar] [CrossRef] [Green Version]
- Diab, M.; Sponholz, C.; von Loeffelholz, C.; Scheffel, P.; Bauer, M.; Kortgen, A.; Lehmann, T.; Farber, G.; Pletz, M.W.; Doenst, T. Impact of perioperative liver dysfunction on in-hospital mortality and long-term survival in infective endocarditis patients. Infection 2017, 45, 857–866. [Google Scholar] [CrossRef]
- Gorjipour, F.; Totonchi, Z.; Gholampour Dehaki, M.; Hosseini, S.; Tirgarfakheri, K.; Mehrabanian, M.; Mortazian, M.; Arasteh Manesh, S.; Rahab, M.; Shafighnia, S.; et al. Serum levels of interleukin-6, interleukin-8, interleukin-10, and tumor necrosis factor-alpha, renal function biochemical parameters and clinical outcomes in pediatric cardiopulmonary bypass surgery. Perfusion 2019, 34, 651–659. [Google Scholar] [CrossRef]
- Malmberg, M.; Gunn, J.; Sipila, J.; Pikkarainen, E.; Rautava, P.; Kyto, V. Comparison of Long-Term Outcomes of Patients Having Surgical Aortic Valve Replacement with Versus without Simultaneous Coronary Artery Bypass Grafting. Am. J. Cardiol. 2020, 125, 964–969. [Google Scholar] [CrossRef]
- Beach, J.M.; Mihaljevic, T.; Svensson, L.G.; Rajeswaran, J.; Marwick, T.; Griffin, B.; Johnston, D.R.; Sabik, J.F., 3rd; Blackstone, E.H. Coronary artery disease and outcomes of aortic valve replacement for severe aortic stenosis. J. Am. Coll. Cardiol. 2013, 61, 837–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flury, B.K.; Riedwyl, H. Standard Distance in Univariate and Multivariate-Analysis. Am. Stat. 1986, 40, 249–251. [Google Scholar] [CrossRef]
- Gelsomino, S.; Maessen, J.G.; van der Veen, F.; Livi, U.; Renzulli, A.; Luca, F.; Carella, R.; Crudeli, E.; Rubino, A.; Rostagno, C.; et al. Emergency surgery for native mitral valve endocarditis: The impact of septic and cardiogenic shock. Ann. Thorac. Surg. 2012, 93, 1469–1476. [Google Scholar] [CrossRef]
- Werdan, K.; Oelke, A.; Hettwer, S.; Nuding, S.; Bubel, S.; Hoke, R.; Russ, M.; Lautenschlager, C.; Mueller-Werdan, U.; Ebelt, H. Septic cardiomyopathy: Hemodynamic quantification, occurrence, and prognostic implications. Clin. Res. Cardiol. 2011, 100, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Diab, M.; Guenther, A.; Scheffel, P.; Sponholz, C.; Lehmann, T.; Hedderich, J.; Faerber, G.; Brunkhorst, F.; Pletz, M.W.; Doenst, T. Can radiological characteristics of preoperative cerebral lesions predict postoperative intracranial haemorrhage in endocarditis patients? Eur. J. Cardio-Thorac. Surg. 2016, 49, e119–e126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thuny, F.; Avierinos, J.F.; Tribouilloy, C.; Giorgi, R.; Casalta, J.P.; Milandre, L.; Brahim, A.; Nadji, G.; Riberi, A.; Collart, F.; et al. Impact of cerebrovascular complications on mortality and neurologic outcome during infective endocarditis: A prospective multicentre study. Eur. Heart J. 2007, 28, 1155–1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lytle, B.W.; Cosgrove, D.M.; Goormastic, M.; Loop, F.D. Aortic valve replacement and coronary bypass grafting for patients with aortic stenosis and coronary artery disease: Early and late results. Eur. Heart J. 1988, 9 (Suppl. SE), 143–147. [Google Scholar] [CrossRef]
- Czer, L.S.; Gray, R.J.; Stewart, M.E.; De Robertis, M.; Chaux, A.; Matloff, J.M. Reduction in sudden late death by concomitant revascularization with aortic valve replacement. J. Thorac. Cardiovasc. Surg. 1988, 95, 390–401. [Google Scholar] [CrossRef]
- Mullany, C.J.; Elveback, L.R.; Frye, R.L.; Pluth, J.R.; Edwards, W.D.; Orszulak, T.A.; Nassef, L.A., Jr.; Riner, R.E.; Danielson, G.K. Coronary artery disease and its management: Influence on survival in patients undergoing aortic valve replacement. J. Am. Coll. Cardiol. 1987, 10, 66–72. [Google Scholar] [CrossRef] [Green Version]
- Lund, O.; Nielsen, T.T.; Pilegaard, H.K.; Magnussen, K.; Knudsen, M.A. The influence of coronary artery disease and bypass grafting on early and late survival after valve replacement for aortic stenosis. J. Thorac. Cardiovasc. Surg. 1990, 100, 327–337. [Google Scholar] [CrossRef]
- Jamieson, W.R.E.; Ye, J.; Higgins, J.; Cheung, A.; Fradet, G.J.; Skarsgard, P.; Germann, E.; Chan, F.; Lichtenstein, S.V. Effect of Prosthesis-Patient Mismatch on Long-Term Survival with Aortic Valve Replacement: Assessment to 15 Years. Ann. Thorac. Surg. 2010, 89, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Maron, D.J.; Hochman, J.S.; Reynolds, H.R.; Bangalore, S.; O’Brien, S.M.; Boden, W.E.; Chaitman, B.R.; Senior, R.; Lopez-Sendon, J.; Alexander, K.P.; et al. Initial Invasive or Conservative Strategy for Stable Coronary Disease. N. Engl. J. Med. 2020, 382, 1395–1407. [Google Scholar] [CrossRef] [PubMed]
- De Luca, L.; Uguccioni, M.; Meessen, J.; Temporelli, P.L.; Tomai, F.; De Rosa, F.M.; Passamonti, E.; Formigli, D.; Riccio, C.; Gabrielli, D.; et al. External applicability of the ISCHEMIA trial: An analysis of a prospective, nationwide registry of patients with stable coronary artery disease. Eurointervention 2020, 16, e966–e973. [Google Scholar] [CrossRef]
- Navarese, E.P.; Lansky, A.J.; Kereiakes, D.J.; Kubica, J.; Gurbel, P.A.; Gorog, D.A.; Valgimigli, M.; Curzen, N.; Kandzari, D.E.; Bonaca, M.P.; et al. Cardiac mortality in patients randomised to elective coronary revascularisation plus medical therapy or medical therapy alone: A systematic review and meta-analysis. Eur. Heart J. 2021, ehab246. [Google Scholar] [CrossRef] [PubMed]
- Doenst, T.; Haverich, A.; Serruys, P.; Bonow, R.O.; Kappetein, P.; Falk, V.; Velazquez, E.; Diegeler, A.; Sigusch, H. PCI and CABG for Treating Stable Coronary Artery Disease: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 73, 964–976. [Google Scholar] [CrossRef] [PubMed]
- Doenst, T.; Sigusch, H. Surgical collateralization: The hidden mechanism for improving prognosis in chronic coronary syndromes. J. Thorac. Cardiovasc. Surg. 2020. [Google Scholar] [CrossRef]
| Variables | CABG (n = 615) | No-CABG (n = 4302) | p |
|---|---|---|---|
| Age (year) | 68.5 ± 9.5 | 61.3 ± 15.0 | <0.001 |
| Male sex | 453 (74) | 3103 (72) | 0.441 |
| BMI | 27.3 ± 10.5 | 26.5 ± 5.8 | 0.012 |
| EuroSCORE | 21.1 ± 20.8 | 15.4 ± 16.6 | <0.001 |
| LVEF (%) | 0.078 | ||
| ≥50 | 429 (70) | 3065 (74) | |
| 30–50 | 158 (26) | 939 (23) | |
| <30 | 28 (4.6) | 146 (3.5) | |
| NYHA ≥ III | 356 (58) | 1954 (57) | 0.627 |
| Diabetes | 227(37) | 1285 (26) | <0.001 |
| Hypertension | 445 (72) | 2047 (48) | <0.001 |
| COPD | 87 (14) | 428 (10) | 0.002 |
| Hyperlipidemia | 215 (38) | 739 (18) | <0.001 |
| PAD | 117 (19.0) | 260 (6.0) | <0.001 |
| CAD | 527 (86) | 715 (17) | <0.001 |
| 1-vessel CAD | 182 (30) | 296 (7) | |
| 2-vessel CAD | 158 (26) | 182 (4) | |
| 3-vessel CAD | 141 (23) | 180 (4) | |
| unclassified | 45 (7) | 57 (1) | |
| Pre-operative Stroke | 175 (29) | 913 (21) | <0.001 |
| Renal insufficiency | 284 (46) | 1600 (37) | <0.001 |
| Prosthetic IE | 136 (22) | 118 (28) | 0.005 |
| Previous cardiac surgery | 157 (26) | 1340 (31) | 0.005 |
| IE localization | |||
| Aortic | 392 (64) | 2759 (64) | 0.857 |
| Mitral | 299 (49) | 1879 (44) | 0.021 |
| Tricuspid | 23 (4) | 256 (6) | 0.025 |
| Microbiological findings | <0.001 | ||
| Staphylococcus | 209 (44) | 1051 (41) | |
| Streptococcus | 88 (18) | 704 (27) | |
| Enterococcus | 101 (21) | 440 (17) | |
| Other | 83 (17) | 371 (15) |
| Variables | CABG | No-CABG | p |
|---|---|---|---|
| (n = 527) | (n = 715) | ||
| Age (yr) | 68.8 ± 9.0 | 69.3 ± 9.5 | 0.342 |
| Male sex | 396 (75%) | 599 (78%) | 0.221 |
| BMI | 27.3 ± 11.1 | 26.9 ± 4.8 | 0.371 |
| EuroSCORE | 21.4 ± 20.6 | 22.0 ± 21.5 | 0.519 |
| LVEF (%) | 0.101 | ||
| ≥50 | 364 (70%) | 448 (64%) | |
| 30–50 | 139 (26%) | 203 (29%) | |
| <30 | 24 (5%) | 48 (7%) | |
| NYHA ≥ III | 316 (60%) | 366 (63%) | 0.239 |
| Diabetes | 196 (37%) | 294 (41%) | 0.177 |
| Hypertension | 384 (73%) | 457 (68%) | 0.001 |
| COPD | 78 (15%) | 98 (14%) | 0.621 |
| HYPERLIPIDEMIA | 185 (38%) | 212 (32%) | 0.044 |
| PAD | 111 (21%) | 102 (14%) | 0.002 |
| Pre-operative Stroke | 145 (28%) | 156 (22%) | 0.023 |
| Renal insufficiency | 249 (47%) | 360 (50%) | 0.301 |
| Prosthetic IE | 89 (17%) | 330 (46%) | <0.001 |
| Previous cardiac surgery | 108 (21%) | 386 (54%) | <0.001 |
| IE localization | |||
| Aortic | 319 (61%) | 464 (65%) | 0.122 |
| Mitral | 265 (50%) | 306 (43%) | 0.010 |
| Tricuspid | 19 (4%) | 41 (6%) | 0.107 |
| Microbiological findings | 0.091 | ||
| Staphylococcus | 176 (43%) | 192 (46%) | |
| Streptococcus | 74 (18%) | 85 (20%) | |
| Enterococcus | 90 (22%) | 94 (23%) | |
| Other | 70 (17%) | 46 (11%) | |
| Aortic valve surgery | 334 (63%) | 481 (67%) | 0.165 |
| Mitral valve surgery | 279 (53%) | 339 (48%) | 0.058 |
| Tricuspid Valve surgery | 35 (7%) | 73 (10%) | 0.032 |
| Number of valves | 0.041 | ||
| Single-valve surgery | 396 (76%) | 533 (76%) | |
| Double-valve surgery | 120 (23%) | 153 (22%) | |
| Triple-valve surgery | 4 (1%) | 19 (3%) | |
| Ascending or aortic root | 81 (15%) | 128 (18%) | 0.250 |
| Cross-clamp time (min) | 100.29 ± 43.94 | 87.83 ± 11.19 | <0.001 |
| CPB time (min) | 153.28 ± 11.12 | 140.38 ± 72.17 | 0.003 |
| Length of ventilation (h) | 140.29 ± 233.28 | 146.74 ± 256.28 | 0.670 |
| ICU stay (d) | 8.23 ± 12.39 | 8.06 ± 11.19 | 0.800 |
| Hospital stay | 16.82 ± 14.21 | 18.02 ± 17.39 | 0.227 |
| Postop. Hemodialysis | 145 (28%) | 164 (24%) | 0.125 |
| Postoperative stroke | 123 (27%) | 124 (20%) | 0.003 |
| Re-exploration | 87 (17%) | 111 (16%) | 0.348 |
| 30-d mortality | 110 (21%) | 168 (24%) | 0.163 |
| Variables | CABG | No-CABG | p | SMD |
|---|---|---|---|---|
| (n = 527) | (n = 715) | |||
| Age (yr) | 69.0 | 69.1 | 0.822 | −0.011 |
| Male sex | 76% | 77% | 0.882 | 0.024 |
| BMI | 26.8 | 26.9 | 0.636 | −0.021 |
| EuroSCORE | 24.74 | 23.00 | 0.043 | 0.092 |
| LVEF (%) | 0.252 | |||
| ≥50 | 68% | 66% | 0.002 | |
| 30–50 | 27% | 28% | −0.022 | |
| <30 | 5% | 6% | −0.044 | |
| NYHA ≥ III | 60% | 63% | 0.085 | −0.004 |
| Diabetes | 35% | 40% | 0.007 | −0.008 |
| Hypertension | 68% | 67% | 0.421 | 0.001 |
| COPD | 14% | 14% | 0.903 | 0.000 |
| HYPERLIPIDEMIA | 38% | 36% | 0.572 | 0.003 |
| PAD | 17% | 17% | 1.00 | 0.000 |
| Pre-operative Stroke | 26% | 25% | 0.810 | 0.002 |
| Renal insufficiency | 44% | 49% | 0.016 | −0.007 |
| Prosthetic IE | 32% | 33% | 0.653 | −0.002 |
| Previous cardiac surgery | 38% | 39% | 0.730 | −0.002 |
| IE localization | ||||
| Aortic | 66% | 61% | 0.03 | 0.006 |
| Mitral | 46% | 46% | 0.866 | 0.000 |
| Tricuspid | 3% | 7% | <0.001 | −0.018 |
| Microbiological findings | <0.001 | |||
| Staphylococcus | 39% | 45% | −0.009 | |
| Streptococcus | 19% | 23% | −0.009 | |
| Enterococcus | 23% | 23% | 0.000 | |
| Other | 19 | 10 | <0.001 | 0.258 |
| Variables | CABG | No-CABG | p |
|---|---|---|---|
| (n = 527) | (n = 715) | ||
| Mitral valve surgery | 51% | 49% | 0.557 |
| Aortic valve surgery | 69% | 64% | 0.011 |
| Tricuspid Valve surgery | 6% | 9% | 0.008 |
| Number of valves | <0.001 | ||
| Single-valve surgery | 74% | 77% | |
| Double-valve surgery | 26% | 21% | |
| Triple-valve surgery | 1% | 2% | |
| Ascending or aortic root | 22% | 15% | <0.001 |
| Cross-clamp time (min) | 106.1 | 85.3 | <0.001 |
| CPB time (min) | 165.9 | 133.4 | <0.001 |
| Length of ventilation (h) | 147.2 | 146.2 | 0.931 |
| ICU stay (d) | 8.3 | 8.0 | 0.458 |
| Hospital stay | 16.5 | 17.3 | 0.266 |
| Postop. Hemodialysis | 29% | 25% | 0.052 |
| Postoperative stroke | 26% | 21% | 0.003 |
| Re-exploration | 17% | 15% | 0.228 |
| 30-d mortality | 24% | 23% | 0.370 |
| Variables | Adjusted HR | 95% CI | p Value |
|---|---|---|---|
| Age | 1.010 | 0.998–1.022 | 0.111 |
| BMI | 1.026 | 1.008–1.045 | 0.005 |
| Hypertension | 1.223 | 0.906–1.651 | 0.189 |
| Hyperlipidaemia | 0.861 | 0.693–1.070 | 0.176 |
| Preop. Stroke | 1.157 | 0.915–1.462 | 0.223 |
| Prosthetic IE | 1.339 | 1.042–1.719 | 0.023 |
| Staphylococcus | 1.257 | 1.014–1.560 | 0.037 |
| CABG | 1.000 | 0.815–1.226 | 0.998 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diab, M.; Lehmann, T.; Weber, C.; Petrov, G.; Luehr, M.; Akhyari, P.; Tugtekin, S.-M.; Schulze, P.C.; Franz, M.; Misfeld, M.; et al. Role of Concomitant Coronary Artery Bypass Grafting in Valve Surgery for Infective Endocarditis. J. Clin. Med. 2021, 10, 2867. https://doi.org/10.3390/jcm10132867
Diab M, Lehmann T, Weber C, Petrov G, Luehr M, Akhyari P, Tugtekin S-M, Schulze PC, Franz M, Misfeld M, et al. Role of Concomitant Coronary Artery Bypass Grafting in Valve Surgery for Infective Endocarditis. Journal of Clinical Medicine. 2021; 10(13):2867. https://doi.org/10.3390/jcm10132867
Chicago/Turabian StyleDiab, Mahmoud, Thomas Lehmann, Carolyn Weber, Georgi Petrov, Maximilian Luehr, Payam Akhyari, Sems-Malte Tugtekin, P. Christian Schulze, Marcus Franz, Martin Misfeld, and et al. 2021. "Role of Concomitant Coronary Artery Bypass Grafting in Valve Surgery for Infective Endocarditis" Journal of Clinical Medicine 10, no. 13: 2867. https://doi.org/10.3390/jcm10132867






