Tailored Prediction Model of Survival after Liver Transplantation for Hepatocellular Carcinoma
Abstract
:1. Introduction
2. Methods
2.1. Patients
2.1.1. Derivation Set
2.1.2. Validation Set
2.2. Demographic Characteristics and Definitions
2.3. Pre-Transplant Evaluation
2.4. Post-Transplantation Management and Follow-Up
2.5. Statistical Analyses
3. Results
3.1. Demographic and Clinical Characteristics
3.2. Derivation of Tailored Survival Calculator
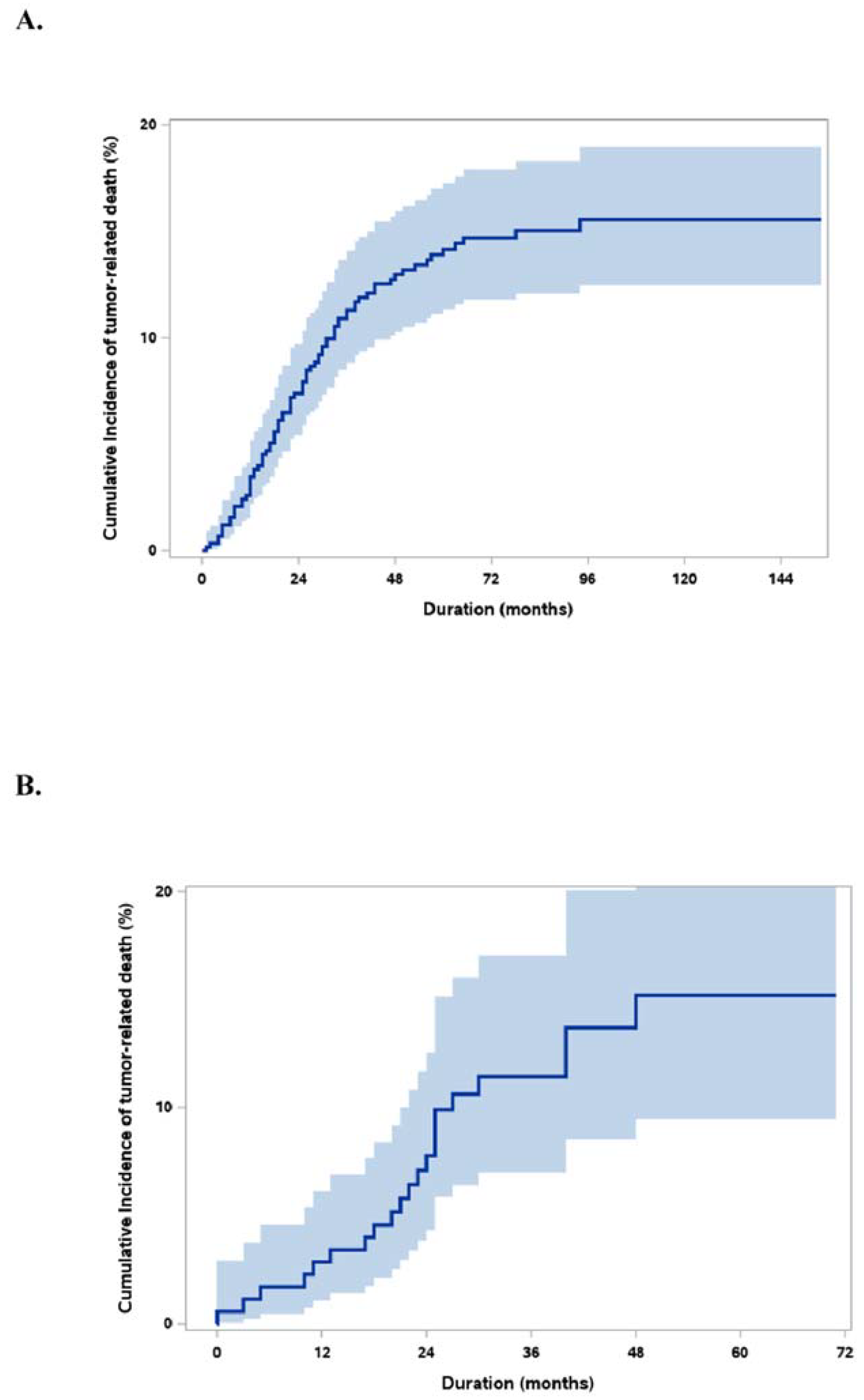
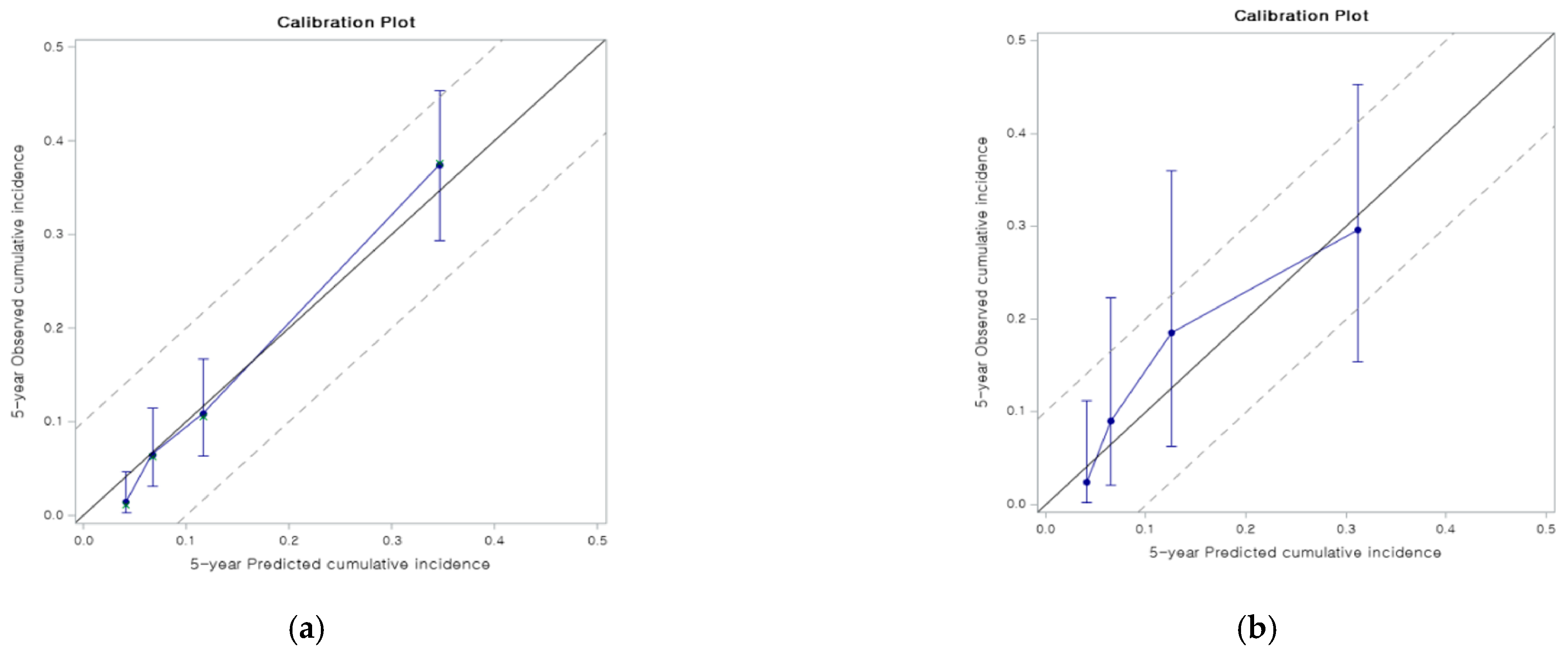
3.3. Validation
3.4. Groups by Risk Score
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| [18]-FDG PET | [18]F-fluorodeoxyglucose positron emission tomography |
| AFP | alpha-fetoprotein; CT: computed tomography |
| DDLT | deceased donor liver transplantation |
| HCC | hepatocellular carcinoma |
| HCCD | cumulative incidence of HCC-specific death |
| IP | incidence probability |
| LDLT | living donor liver transplant |
| LT | liver transplantation |
| MoRAL | model to predict tumor recurrence after LDLT |
| MRI | magnetic resonance imaging |
| mTORi | mammalian target of rapamycin inhibitors |
| NBNC | non-B non-C hepatocellular carcinoma |
| NLR | neutrophil-to-lymphocyte ratio |
| PET | positron emission tomography |
| PIVKAII | protein induced by vitamin K absence/antagonist-II |
| RS | risk score |
| SALT | survival after liver transplantation for HCC |
| SNUH | Seoul National University Hospital |
| TBS | tumor-bearing survival |
| UCSF | University of California, San Francisco |
References
- Bodzin, A.S.; Lunsford, K.E.; Markovic, D.; Harlander-Locke, M.P.; Busuttil, R.W.; Agopian, V.G. Predicting mortality in patients developing recurrent hepatocellular carcinoma after liver transplantation: Impact of treatment modality and recurrence characteristics. Ann. Surg. 2017, 266, 118–125. [Google Scholar] [CrossRef]
- Marsh, J.W.; Dvorchik, I.; Subotin, M.; Balan, V.; Rakela, J.; Popechitelev, E.P.; Subbotin, V.; Casavilla, A.; Carr, B.I.; Fung, J.J.; et al. The prediction of risk of recurrence and time to recurrence of hepatocellular carcinoma after orthotopic liver transplantation: A pilot study. Hepatology 1997, 26, 444–450. [Google Scholar] [CrossRef]
- Hong, S.K.; Lee, K.W.; Yoon, K.C.; Kim, H.S.; Ahn, S.W.; Kim, H.; Lee, J.M.; Cho, J.H.; Yi, N.J.; Suh, K.S. Different prognostic factors and strategies for early and late recurrence after adult living donor liver transplantation for hepatocellular carcinoma. Clin. Transplant. 2019, 33, e13703. [Google Scholar] [CrossRef]
- Clavien, P.A.; Lesurtel, M.; Bossuyt, P.M.; Gores, G.J.; Langer, B.; Perrier, A. Recommendations for liver transplantation for hepatocellular carcinoma: An international consensus conference report. Lancet Oncol. 2012, 13, e11–e22. [Google Scholar] [CrossRef] [Green Version]
- Mazzaferro, V.; Sposito, C.; Zhou, J.; Pinna, A.D.; De Carlis, L.; Fan, J.; Cescon, M.; Di Sandro, S.; Yi-Feng, H.; Lauterio, A.; et al. Metroticket 2.0 model for analysis of competing risks of death after liver transplantation for hepatocellular carcinoma. Gastroenterology 2018, 154, 128–139. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.K.; Lee, K.W.; Kim, H.S.; Yoon, K.C.; Yi, N.J.; Suh, K.S. Living donor liver transplantation for hepatocellular carcinoma in Seoul National University. Hepatobiliary Surg. Nutr. 2016, 5, 453–460. [Google Scholar] [CrossRef] [Green Version]
- Park, M.S.; Lee, K.W.; Yi, N.J.; Choi, Y.R.; Kim, H.; Hong, G.; Suh, K.S.; Kwon, C.H.D.; Joh, J.W.; Lee, S.K. Optimal tailored screening protocol after living donor liver transplantation for hepatocellular carcinoma. J. Korean Med. Sci. 2014, 29, 1360–1366. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Cho, Y.; Kim, H.Y.; Cho, E.J.; Lee, D.H.; Yu, S.J.; Lee, J.W.; Yi, N.J.; Lee, K.W.; Kim, S.H.; et al. Serum tumor markers provide refined prognostication in selecting liver transplantation candidate for hepatocellular carcinoma patients beyond the Milan criteria. Ann. Surg. 2016, 263, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Suh, K.S.; Suh, S.W.; Yoo, T.; Kim, H.; Park, M.S.; Choi, Y.; Paeng, J.C.; Yi, N.J.; Lee, K.W. Alpha-fetoprotein and 18F-FDG positron emission tomography predict tumor recurrence better than Milan criteria in living donor liver transplantation. J. Hepatol. 2016, 64, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, V.; Regalia, E.; Doci, R.; Andreola, S.; Pulvirenti, A.; Bozzetti, F.; Montalto, F.; Ammatuna, M.; Morabito, A.; Gennari, L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N. Engl. J. Med. 1996, 334, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.Y.; Ferrell, L.; Bass, N.M.; Watson, J.J.; Bacchetti, P.; Venook, A.; Ascher, N.L.; Roberts, J.P. Liver transplantation for hepatocellular carcinoma: Expansion of the tumor size limits does not adversely impact survival. Hepatology 2001, 33, 1394–1403. [Google Scholar] [CrossRef]
- Shah, S.A.; Tan, J.C.; McGilvray, I.D.; Cattral, M.S.; Cleary, S.P.; Levy, G.A.; Greig, P.D.; Grant, D.R. Accuracy of staging as a predictor for recurrence after liver transplantation for hepatocellular carcinoma. Transplantation 2006, 81, 1633–1639. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhu, S.; Li, X. Comparison of values of CT and MRI imaging in the diagnosis of hepatocellular carcinoma and analysis of prognostic factors. Oncol. Lett. 2019, 17, 1184–1188. [Google Scholar] [CrossRef] [Green Version]
- Suh, K.S.; Cho, E.H.; Lee, H.W.; Shin, W.Y.; Yi, N.J.; Lee, K.U. Liver transplantation for hepatocellular carcinoma in patients who do not meet the Milan criteria. Dig. Dis. 2007, 25, 329–333. [Google Scholar] [CrossRef]
- Duvoux, C.; Roudot–Thoraval, F.; Decaens, T.; Pessione, F.; Badran, H.; Piardi, T.; Francoz, C.; Compagnon, P.; Vanlemmens, C.; Dumortier, J.; et al. Liver transplantation for hepatocellular carcinoma: A model including α-fetoprotein improves the performance of Milan criteria. Gastroenterology 2012, 143, 986–994.e3. [Google Scholar] [CrossRef]
- Xu, J.F.; Liu, X.Y. PIVKA-II is an independent prognostic factor for overall survival of HCC patients and maybe associated with epithelial-mesenchymal transition. J. Hepatol. 2015, 63, 1040–1041. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.M.; Lee, J.H.; Yoon, J.H.; Kim, Y.J.; Heo, D.S.; Lee, H.S. Protein induced by vitamin K absence or antagonist-II production is a strong predictive marker for extrahepatic metastases in early hepatocellular carcinoma: A prospective evaluation. BMC Cancer 2011, 11, 435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poté, N.; Cauchy, F.; Albuquerque, M.; Voitot, H.; Belghiti, J.; Castera, L.; Puy, H.; Bedossa, P.; Paradis, V. Performance of PIVKA-II for early hepatocellular carcinoma diagnosis and prediction of microvascular invasion. J. Hepatol. 2015, 62, 848–854. [Google Scholar] [CrossRef]
- Vallabhajosula, S. 18F-labeled positron emission tomographic radiopharmaceuticals in oncology: An overview of radiochemistry and mechanisms of tumor localization. Semin. Nucl. Med. 2007, 37, 400–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.M.; Kim, H.S.; Lee, S.; Lee, J.W. Emerging role of 18F-fluorodeoxyglucose positron emission tomography for guiding management of hepatocellular carcinoma. World J. Gastroenterol. 2019, 25, 1289–1306. [Google Scholar] [CrossRef]
- Guthrie, G.J.; Charles, K.A.; Roxburgh, C.S.; Horgan, P.G.; McMillan, D.C.; Clarke, S.J. The systemic inflammation-based neutrophil-lymphocyte ratio: Experience in patients with cancer. Crit. Rev. Oncol. Hematol. 2013, 88, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Gomez, D.; Farid, S.; Malik, H.Z.; Young, A.L.; Toogood, G.J.; Lodge, J.P.A.; Prasad, K.R. Preoperative neutrophil-to-lymphocyte ratio as a prognostic predictor after curative resection for hepatocellular carcinoma. World J. Surg. 2008, 32, 1757–1762. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.K.; Chen, D.; Li, S.Q.; Fu, S.J.; Peng, B.G.; Liang, L.J. Prognostic significance of neutrophil-lymphocyte ratio in hepatocellular carcinoma: A meta-analysis. BMC Cancer 2014, 14, 117. [Google Scholar] [CrossRef] [Green Version]
- Halazun, K.J.; Hardy, M.A.; Rana, A.A.; Woodland, D.C., IV; Luyten, E.J.; Mahadev, S.; Witkowski, P.; Siegel, A.B.; Brown, R.S., Jr.; Emond, J.C. Negative impact of neutrophil-lymphocyte ratio on outcome after liver transplantation for hepatocellular carcinoma. Ann. Surg. 2009, 250, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Motomura, T.; Shirabe, K.; Mano, Y.; Muto, J.; Toshima, T.; Umemoto, Y.; Fukuhara, T.; Uchiyama, H.; Ikegami, T.; Yoshizumi, T.; et al. Neutrophil-lymphocyte ratio reflects hepatocellular carcinoma recurrence after liver transplantation via inflammatory microenvironment. J. Hepatol. 2013, 58, 58–64. [Google Scholar] [CrossRef]
- Xiao, G.Q.; Liu, C.; Liu, D.L.; Yang, J.Y.; Yan, L.N. Neutrophil-lymphocyte ratio predicts the prognosis of patients with hepatocellular carcinoma after liver transplantation. World J. Gastroenterol. 2013, 19, 8398–8407. [Google Scholar] [CrossRef]
- Park, M.S.; Lee, K.W.; Suh, S.W.; You, T.; Choi, Y.; Kim, H.; Hong, G.; Yi, N.J.; Kwon, C.H.D.; Joh, J.W.; et al. Living-donor liver transplantation associated with higher incidence of hepatocellular carcinoma recurrence than deceased-donor liver transplantation. Transplantation 2014, 97, 71–77. [Google Scholar] [CrossRef] [PubMed]

| Variables | Values | |||
|---|---|---|---|---|
| Derivation Set | Validation Set | p-Value | ||
| Sex | Male Female | 472 (81.7%) 106 (18.3%) | 153 (87.4%) 22 (12.6%) | 0.07 |
| Age (year) | Mean ± SD | 55.6 ± 8.3 | 56.7±6.3 | 0.06 |
| Primary Disease | HBV HBV + HCV HCV NBNC Alcoholic Others | 442 (76.5%) 16 (2.8%) 66 (11.4%) 18 (3.1%) 27 (4.7%) 9 (1.6%) | 144 (82.3%) 4 (2.3%) 9 (5.1%) 6 (3.4%) 11 (6.3%) 1 (0.6%) | |
| MELD score | Median (range) | 14.7 (0.9–49) | 9 (5–40) | <0.01 |
| Type of LT | DDLT LDLT | 83 (14.4%) 495 (85.6%) | 7 (4%) 168 (96%) | |
| AFP | Median (range) | 9.6 (0.8–1,708,000) | 7.2 (1.3–8367.7) | 0.10 |
| PIVKA-II | Mean ± SD (range) | 29 (2–76,000) | 27 (7–22,462) | 0.80 |
| NLR * | Median (range) | 2.3 (0.4–92) | 1.9 (0.6–34.9) | <0.01 |
| PET | Positive Negative | 212 (36.7%) 366 (63.3%) | 72 (41.1%) 103 (58.9%) | 0.28 |
| Largest tumor size | Median (range) | 26 (4–240) | 25 (5–105) | 0.16 |
| Recurrence | Yes Intrahepatic Extrahepatic Both | 102 (17.8%) 34 (5.9%) 25 (4.3%) 43 (7.4%) | 52 (29.7%) 23 (13.1%) 15 (8.6%) 14 (8%) | |
| Follow-up Period (months) | Median (range) | 65.5 (0–154) | 34 (0–71) | |
| Variable | Multivariable Analysis | |
|---|---|---|
| Shr * (95% CI) | p-Value | |
| Sex | ||
| Female | Reference | |
| Male | 2.61 (1.21–5.63) | 0.01 |
| Largest tumor size | 1.01 (1–1.01) | 0.04 |
| PET | ||
| Negative | Reference | |
| Positive | 2.41 (1.46–3.97) | <0.01 |
| Ln(AFP+1) | 1.24 (1.12–1.38) | <0.01 |
| Ln(PIVKA-II) | 1.18 (1.03–1.35) | 0.01 |
| NLR | 1.02 (1.01–1.04) | <0.01 |
| Uno’s c-Index (95% CI) | Model | 0.8 (0.75, 0.86) |
| Internal * | 0.79 | |
| external | 0.75 (0.65, 0.86) | |
| Calibration slope (95% CI) | Model | 1 (0.84, 1.16) |
| Internal * | 0.85 | |
| external | 0.9 (0.49, 1.3) |
| 1 Year | 3 Years | 5 Years | ||
|---|---|---|---|---|
| Group I (n = 74) (RS ≤ 1.91) | 0 | 0 | 2% (0.1–7.7) | |
| Group II (n = 433) (RS 1.91–4.07) | 1% (0.4–2.6) | 7.7% (5.4–10.5)% | 11% (8.3–14.6) | |
| Group III (n = 47) (RS > 4.07) | 32% (19.1–45.5) | 64% (47.7–76.9) | 64% (47.7–76.9) | p < 0.01 * |
| 1 Year | 3 Years | 5 Years | ||
|---|---|---|---|---|
| Group I (n = 74) (RS ≤ 1.91) | 0 | 0 | 5% | |
| Group II (n = 433) (RS 1.91–4.07) | 5% | 12.5% | 14% | |
| Group III (n = 47) (RS > 4.07) | 56% | 78% | 82% | p < 0.01 * |
| 1 Year | 3 Years | 5 Years | ||
|---|---|---|---|---|
| Group I (n = 74) (RS ≤ 1.91) | 0 | 0 | 2% | |
| Group II (n = 433) (RS 1.91–4.07) | 3% | 10% | 14% | |
| Group III (n = 47) (RS >4.07) | 41% | 76% | 76% | p < 0.01 * |
| Groups | Recurrence | Initial Sites of Recurrence | Recurrence-to-Death Median (Range) ** | ||||
|---|---|---|---|---|---|---|---|
| Intrahepatic ** | Extrahepatic ** | Combined ** | |||||
| Lung | Bone | Others | |||||
| Group I (n = 74) (RS ≤ 1.91) | 3 (4.1%) | 0 | 1 (33.3%) | 0 | 0 | 2 (66.7%) | 78 (0–98) |
| Group II (n = 433) (RS 1.91–4.07) | 62 (14.3%) | 21 (33.9%) | 4 (6.5%) | 3 (4.8%) | 1 (1.6%) | 33 (53.2%) | 6.5 (0–136) † |
| Group III (n = 47) (RS > 4.07) | 34 (72.3%) | 16 (47.1%) | 3 (8.8%) | 2 (5.9%) | 1 (2.9%) | 12 (35.3%) | 2.5 (0–44) † |
| Total | 99 (17.9%) | 37 (37.4%) | 8 (8.1%) | 5 (5.1%) | 2 (2%) | 47 (47.5%) | 15.20 ± 23.02 (0–136) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamtani, I.; Lee, K.-W.; Choi, Y.; Choi, Y.; Lee, J.-M.; Han, E.-S.; Hong, K.; Choi, G.-S.; Kim, J.M.; Yi, N.-J.; et al. Tailored Prediction Model of Survival after Liver Transplantation for Hepatocellular Carcinoma. J. Clin. Med. 2021, 10, 2869. https://doi.org/10.3390/jcm10132869
Jamtani I, Lee K-W, Choi Y, Choi Y, Lee J-M, Han E-S, Hong K, Choi G-S, Kim JM, Yi N-J, et al. Tailored Prediction Model of Survival after Liver Transplantation for Hepatocellular Carcinoma. Journal of Clinical Medicine. 2021; 10(13):2869. https://doi.org/10.3390/jcm10132869
Chicago/Turabian StyleJamtani, Indah, Kwang-Woong Lee, Yunhee Choi, YoungRok Choi, Jeong-Moo Lee, Eui-Soo Han, Kwangpyo Hong, Gyu-Seong Choi, Jong Man Kim, Nam-Joon Yi, and et al. 2021. "Tailored Prediction Model of Survival after Liver Transplantation for Hepatocellular Carcinoma" Journal of Clinical Medicine 10, no. 13: 2869. https://doi.org/10.3390/jcm10132869
APA StyleJamtani, I., Lee, K.-W., Choi, Y., Choi, Y., Lee, J.-M., Han, E.-S., Hong, K., Choi, G.-S., Kim, J. M., Yi, N.-J., Hong, S. K., Byun, J., Hong, S. Y., Suh, S., Joh, J.-W., & Suh, K.-S. (2021). Tailored Prediction Model of Survival after Liver Transplantation for Hepatocellular Carcinoma. Journal of Clinical Medicine, 10(13), 2869. https://doi.org/10.3390/jcm10132869






